Simple Summary
In the current clinical practice, HER2 status is tested in breast and gastroesophageal cancers to select patients eligible for anti-HER2 treatment. However, HER2 is an emerging biomarker in colorectal cancer (CRC), one of the big killers in oncology. The most frequent types of HER2 alterations in CRC include gene amplification and mutations and often involve protein overexpression. In this review, we discuss the current knowledge of HER2 testing in CRC and the immediate future perspectives for HER2 targeting in the metastatic setting.
Abstract
HER2 is an emerging biomarker in colorectal cancer (CRC). This oncogene plays an essential role in regulating cell proliferation, differentiation, migration, and, more in general, tumorigenesis and tumor progression. The most frequent types of HER2 alterations in CRC include gene amplification and missense mutations in 7–8% of CRC, often being mirrored by HER2 protein overexpression, representing founder events in solid tumors, including CRC. There are currently no approved HER2-targeted therapy guidelines for CRC; however, several studies have shown that HER2 can be effectively targeted in meta-static CRC settings. In this review, we discuss the current knowledge of HER2 testing in CRC and the immediate future perspectives for HER2 targeting in the metastatic setting.
1. Introduction
Human epidermal growth factor receptor 2 (HER2) is a proto-oncogene encoding for a transmembrane glycoprotein with a tyrosine kinase activity, a member of the ErbB receptor tyrosine kinases family, and one of the epidermal growth factor receptors (EGFRs) [1,2]. HER2 plays an essential role in normal biological and oncogenic processes, regulating cell proliferation, differentiation, and migration via numerous signaling pathways, such as mitogen-activated protein kinase/extracellular signal-regulated kinases (MAPK/ERK) and phosphoinositide 3 kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) [1,2]. The schematic overview of HER2 pathways is represented in Supplementary Figure S1. Alterations of HER2 include gene amplification and missense mutations and often lead to protein overexpression [3]. These types of molecular aberrations are considered founder events in tumorigenesis and tumor progression because of uncontrolled cell proliferation, inhibition of apoptosis, and migration [1,4,5,6].
In the current clinical practice, HER2 status is tested in breast and gastroesophageal cancers to select patients eligible for anti-HER2 treatment [5]. However, alterations in HER2 have been documented in a plethora of other solid tumors, including colorectal cancer (CRC) [2,3,4,5,6,7]. This tumor type is the second leading cause of cancer-associated deaths worldwide in both sexes [8]. In CRC, the frequency of HER2 overexpression is 5–6% with somatic HER2 gene alterations, including amplifications, reported in ~7% of patients [4,6,9,10,11]. HER2 mutations in colonic epithelial cells have been shown to be indicative of HER2 signaling pathway activations, promote independent cell growth, and potentially acquire resistance to EGFR-targeted therapies, subjecting patients to a worse prognosis [2,4,6,9,10,11,12]. There are currently no approved HER2-targeted therapy guidelines for CRC; however, several studies have shown that HER2 can be effectively targeted in metastatic CRC settings [2,12,13,14].
In this review, we discuss the current knowledge of HER2 testing in CRC and its significance in both translational research and clinical studies. Particular emphasis will be given to the immediate future perspectives for HER2 targeting in patients with CRC in the metastatic setting (mCRC).
2. HER2 Targeting in Metastatic Colorectal Cancer
The administration of anti-HER2 drugs (e.g., trastuzumab, pertuzumab) is presently a standard of care in HER2-positive (i.e., score 3+ by immunohistochemistry (IHC) or 2+/in situ hybridization (ISH)-positive) breast and gastric cancer [15,16]. For CRC, HER2 first emerged as a negative predictive biomarker. Amplification or overexpression of HER2 was associated with a lack of response to anti-EGFR treatment [17].
The novel antibody–drug conjugate trastuzumab deruxtecan (T-DXd) has been approved by the United States Food and Drug Administration (FDA) for HER2-low (i.e., score 1+ or 2+/ISH-negative) breast cancers [18], and it is currently under investigation in histology-agnostic settings in the DESTINY-PanTumor02 study (NCT04482309). The first clinical trials using trastuzumab in mCRC evaluated the combination of this monoclonal antibody with chemotherapy. Clark et al. assessed the association of FOLFOX + trastuzumab in the second- or third-line treatment of HER2-positive mCRC. A total of 5 patients out of 21 (24%) had a partial (PR) or complete response (CR), with a median duration of response of 4.5 months [19]. Another phase II study assessed the combination of trastuzumab and irinotecan in HER2-positive mCRC pretreated with one line of therapy. Objective responses were recorded in five patients (71%), and these were maintained for at least 6 months [20].
Subsequent studies evaluated different strategies of an HER2 dual blockade with significant results. HERACLES-A tested the combination of trastuzumab and lapatinib in patients with KRAS exon-2 wild-type (WT) mCRC refractory to standard treatment and with HER2 amplification and/or overexpression. A total of 914 patients were screened, of whom 48 (5%) had HER2-positive diseases [14,21]. The long-term clinical results at a follow-up of 6.7 years reported an overall response rate (ORR) of 28% with one CR and eight PRs, a disease control rate (DCR) of 69%, median progression-free survival (mPFS) of 4.7 months, and median overall survival (OS) of 10 months for 32 treated patients. Two of them (6%) reported a grade 3 decrease in left ventricular ejection fraction, while fatigue was registered in five cases (16%) [22]. Differently, HERACLES-B tested the combination of pertuzumab and the antibody–drug conjugate trastuzumab emtansine (TDM-1) in RAS and BRAF WT and HER2-positive mCRC refractory to standard therapies. The primary endpoint was not reached, with ORR below the expected rate ≥30% (9.7%). Stable disease (SD) was seen in 21 patients (67.7%), and the DCR rate was 77.4%. Treatment was well-tolerated, with two patients suffering from grade (G) 3 thrombocytopenia [23]. A median PFS of 4.2 months was similar to the HERACLES-A study, and patients with a HER2 3+ score at immunohistochemistry (IHC) had a significantly higher mPFS compared to HER2 IHC and fluorescent in situ hybridization (FISH)-amplified tumors (HR: 0.20; 95% confidence interval (CI): 0.07–0.56; p = 0.0008) [17]. The MyPathway phase II trial enrolled 57 patients with HER2-amplified mCRC receiving a combination of trastuzumab and pertuzumab. One patient had a CR while seventeen (30%) benefited from a PR. On the whole, 18 patients (32%) achieved an objective response, and, in 4 cases, it was longer than 12 months. Treatment was well-tolerated, with the most common adverse events being G 1 or 2 diarrhea, fatigue, and nausea. Patients harboring a KRAS mutation had a significantly shorter PFS and OS compared to the KRAS WT population (PFS: 1.4 months; 95% CI, 1.2–2.8 months versus 5.3 months; 95% CI: 2.7–6.1 months for mutated and WT, respectively; OS: 8.5 months; 95% CI: 3.9–not estimable versus 14.0 months; 95% CI: 8.0–not estimable for mutated and WT, respectively) [24]. Two other phase II studies (TRIUMPH and TAPUR) evaluated the combination of trastuzumab + pertuzumab. In the first study, 19 patients with RAS wt mCRC and HER2 amplification in tissue samples achieved an ORR of 35% with a complete response and five partial responses. Interestingly, the TRIUMPH trial evaluated HER2 status on circulating tumor DNA (ctDNA). Similarly, to the patients with HER2 amplification detected on tissue, 15 patients with ctDNA positivity for HER2 amplification had an ORR of 33% with one CR and four PRs. With a median follow-up of 5.4 months, mPFS was 4 months [25]. In the TAPUR basket trial, a cohort of 28 patients with HER2 amplified mCRC was treated; ORR was 14% and DCR for at least 16 weeks was 50% with an mPFS of 3.8 months [26]. A new anti-HER2 agent, trastuzumab deruxtecan, was tested in the DESTINY-CRC01 phase II trial. This antibody–drug conjugate of a humanized anti-HER2 antibody with a topoisomerase I inhibitor was tested in HER2-positive RAS-BRAF WT mCRC progressed on two or more lines of treatment, with the possibility to include patients pretreated with different anti-HER2 agents. A total of 78 patients were enrolled with 53 placed in cohort A (HER2 IHC 3+ or 2+ and positive in situ hybridization), a total of 7 in cohort B (IHC 2+ and negative in situ hybridization), and 18 in cohort C (IHC 1+). After a median follow-up of 27.1 weeks, the ORR in group A was 45.3% (95% CI, 31.6–59.6), and patients pretreated with anti-HER2 agents obtained a high ORR of 43%, as well. Differently, no responses were seen in groups B and C of treatment [12]. At a longer median follow-up of 62.4 weeks with 86 patients treated, the ORR of the group A was confirmed (45.3%). Moreover, DCR was 83%, mPFS 6.9 months, and mOS 15.5 months. Pulmonary toxicity in terms of interstitial lung disease and pneumonitis was recorded in eight patients (9.3%). Two patients died because of grade 5 lung toxicity. Interestingly, trastuzumab deruxtecan was also effective in the group of patients with RAS mutation-positive ctDNA [27]. In the ongoing MOUNTAINEER trial, trastuzumab is associated with tucatinib, a tyrosine kinase inhibitor (TKI) of the HER2 protein. Twenty-six patients with chemorefractory, RAS WT, and HER2-positive tumors have been treated so far with an ORR of 52.2%, twelve PRs, and six cases of SD. Patients experienced a prolonged median response of 10.4 months, an mPFS of 8.1 months (95% CI, 3.8–not estimable), and a median OS of 18.7 months (95% CI, 12.3–not estimable) [28]. The HER2 FUSCC-G trial is testing the combination of trastuzumab + pyrotinib, an irreversible dual pan-ErbB tyrosine kinase inhibitor (TKI) [29]. A cohort of 11 mCRC HER2-positive patients have received this combination so far, with a global ORR of 45.5% and 55.6% in RAS WT tumors. With a median follow-up of 17.7 months, mPFS was 7.8 months, while mOS 14.9 months. Patients harboring KRAS mutation had poorer outcomes compared to the KRAS WT group (PFS, 7.7 versus 9.9 months; p = 0.19; OS, 12.4 versus 20.6 months; p = 0.021) [30]. Differently, the combination of the anti-HER2 TKI neratinib and anti-EGFR agent cetuximab was not effective in terms of objective responses. A total of 16 patients with KRAS, NRAS, BRAF, and PI3KCA WT tumors were treated; 6 of them (44%) had SD with 5 harboring HER2 amplification at baseline. G 3adverse events, such as diarrhea, skin rash, and an increased level of transaminases, were registered in 67% of patients [31]. Table 1 lists the main trials with HER2-targeted therapies in mCRC that have been conducted so far.

Table 1.
Main phase II studies with HER2-targeted therapies in refractory HER2-positive mCRC.
While anti-HER2 therapy in CRC is awaiting approval, many early trials continue, demonstrating promising results [32].
Supposing that HER2 may be immunogenic and leads to T-cell activation suggests it is targetable for immunotherapy [33].
Some studies demonstrate the induction of HER2 downregulation in HER2-positive cancer cells with the immune effector cells’ engagement, revealing a new function of immune cells in trastuzumab-mediated antitumor efficacy and probably representing a novel mechanism of action of trastuzumab, predicting active immune effector cells’ recruitment in the tumor microenvironment [34].
The acquisition of drug resistance to trastuzumab has been recently explained in HER2-positive gastric cancer by vessel destabilization and activation of the glycolytic pathway inducing 6-phosphofructo-2-kinase (PFKFB3). The inhibition of PFKFB3 in patient-derived xenograft models has significantly diminished tumor proliferation and promoted vessel normalization. It has also been found that PFKFB3 promotes the interleukin-8 coding gene CXCL8 by activating the PI3K/AKT/NFκB p65 pathway, which leads to the idea that PFKFB3 inhibition might be effective in overcoming trastuzumab resistance in HER2-positive cancers [35].
HER2-activating mutations are also known for their association with microsatellite instability-high tumors, which has been observed in CRC [36]. With the evolution of immunotherapy, after the US Food and Drug Administration (FDA) approval of pembrolizumab, it has become a practice-changing treatment option for unresectable or mCRC in patients with high microsatellite instability (MSI-H) or mismatched repair deficiency (dMMR) [37].
In the metastatic settings, however, only a small proportion of CRC patients responded to immune checkpoint therapy, despite positive results in some phase I trials, and all these tumors were MSI-H/dMMR and had a high tumor mutation burden [38]. While tumor mutational burden has been associated with the immune checkpoint response rate in other tumor types, such as melanoma and non-small-cell lung cancer, the underlying mechanism is still unknown, although it might be related to immune cells’ reactivity, increasing T-cell infiltration [38,39,40].
An ongoing recruiting four-part, phase 1/2 dose-escalation/expansion study is aiming to evaluate the effects of BDC-1001 (immune stimulating antibody conjugate (ISAC), consisting of an anti-HER2 monoclonal antibody conjugated to a TLR 7/8 dual agonist) in combination with/without PD1 inhibitor pembrolizumab in patients with progressive HER2-expressing solid tumors (NCT04278144). Current results demonstrate BDC-1001 to be well tolerated and clinically efficient also in patients previously treated with anti-HER2 therapy; however, the safety and efficacy of combining with a PD1 inhibitor is yet to be studied [6,41], although the data on PD-L1 expression in CRC with its regards to microsatellite instability remain controversial [38]. Some authors advocate that HER2-targeted therapies may favorably be combined with other emerging therapeutic strategies for advanced CRC, including immune checkpoint inhibitors, increasing the tailored therapeutic approach [42,43].
3. Spectrum and Heterogeneity of HER2 Expression in Colorectal Cancer
The analysis of publicly available genomic datasets shows that the frequency of HER2 alterations in CRC ranges from ~6% of colon adenocarcinomas to ~7.5% of rectal carcinomas, with a particularly high frequency in the mucinous histological subtype (Figure 1). Of note, the frequency of alteration types, namely gene amplifications and missense mutations, is heterogeneous based on the anatomical site.
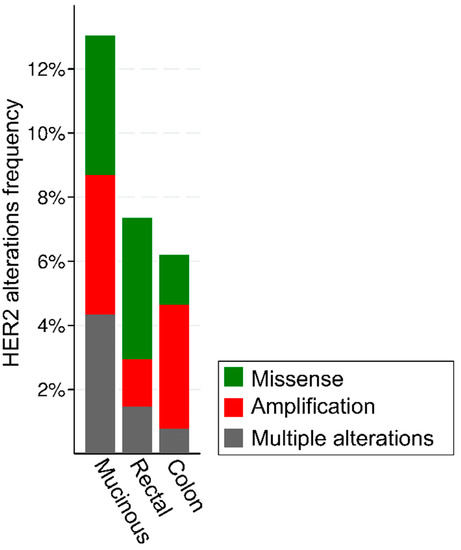
Figure 1.
Frequency and types of HER2 alterations in 636 patients with colorectal cancer from the TGCA Firehose Legacy series from https://www.cbioportal.org/, accessed on 11 August 2022.
Independent cell growth signaling pathways are activated by several types of HER2 gain-of-function mutations, including S310F, L755S, V777L, V842I, and L866M. These mutations are clinically actionable in different tumors, as summarized in Table 2.

Table 2.
Cancer type-specific HER2 S310F, L755S, V777L, V842I, and L866M alterations that may predict response to a targeted drug and the corresponding OncoKB™ level of evidence assigning their level of clinical actionability. Level 2: Standard care biomarker recommended by the NCCN or other expert panels predictive of response to an FDA-approved drug in this indication. Level 3A: Compelling clinical evidence supports the biomarker as being predictive of response to a drug in this indication but neither biomarker nor drug is standard of care.
These oncogenic mutations cause EGFR antibody resistance in CRC cell lines, and patient-derived xenografts with S310F, L755S, V777L, V842I, and L866M mutations show durable tumor regression when treated with dual HER2-targeted therapy [9]. Of note, position 842 is a hotspot in colorectal cancer, as shown in Figure 2.
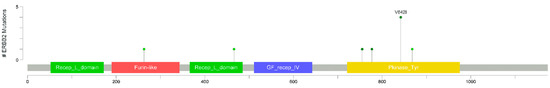
Figure 2.
Lollipop plot showing the frequency of HER2 missense mutations in 16 patients with HER2-mutated colorectal cancer from the TGCA Firehose Legacy series from https://www.cbioportal.org/, accessed on 11 August 2022.
The amplification of HER2 most frequently occurs in the rectum and then other parts of the colon, and it has been associated with acquiring resistance to EGFR-targeted therapies and shorter overall survival compared to HER2 wild-type CRC [7].
Currently, the expression of HER2 in CRC is evaluated by using IHC and reflex ISH assays. IHC expression is based on the pattern and intensity of membranous reactivity and the percentage of immunoreactive cells. Both circumferential and basolateral/lateral patterns are considered, and the scoring ranges from 0 to 3+ similarly to breast and gastric cancer guidelines [44,45]. Score 3+ is considered positive (intense staining in >10% of tumor cells), and 2+ (weak to moderate staining in >10% of tumor cells) is determined to be equivocal similarly to other cancer types, while 1+ (faint staining in >10% of tumor cells) and 0 (no staining or staining in <10% of tumor cells) are considered negative. Similar to other tumor types, HER2 expression may not be homogenous in all CRC cells. Unlike breast or gastric cancers, HER2 in CRC, detected in the absence of membranous staining, in some reports is defined as the cytoplasmic expression and determined to be an adverse prognostic factor [4,6,14,20,46,47,48]. HER2 amplification is assessed by fluorescent in situ hybridization (FISH), silver in situ hybridization (SISH), or chromogenic in situ hybridization (CISH) counting the HER2/CEN17 or HER2/CEP17 signal ratio from 100 nuclei per case The HER2/CEN17 ratio ≥2.0 and HER2/CEP17 ratio >2.2 (CAP/ASCO guideline 2007) are considered amplified [10,14,48,49]. Comparing breast cancer and CRC, both tumor types exhibit a stronger correlation between HER2 protein expression and HER2 amplification by FISH and higher intratumor heterogeneity in the case of amplification, dissimilar to gastroesophageal cancer [47,49]. It has been proposed to mold the HER2 testing criteria and interpretation guidelines employed in breast and gastric cancer for HER2 status assessment in CRC [6,49]. However, the HER2 detection and scoring methods for CRC still lack standardization [4,6,14,20,49,50,51].
The HERACLES trial suggested both HER2 overexpression and amplification be accounted as positive predictive markers for anti-HER2 treatment response. Knowing that HER2 could also represent an important therapeutic target in KRAS wild-type metastatic colorectal cancer patients resistant to anti-EGFR treatment, the authors determined the prevalence rate for HER2 amplification in KRAS wild-type samples, which were almost identical (5.1% and 5.2%) [10,14,49,52,53]. Given the presence of RAS mutations in CRC, some researchers advocate for the use of next-generation sequencing (NGS) to detect HER2 amplifications and, thus, refine the selection of metastatic CRC patients, who may be candidates for anti-EGFR therapy [11,13,47,53,54].
4. Genetics and Actionable Mutations in HER2-Positive Colorectal Cancer
To achieve the most precise patient stratification and to choose the best therapeutic strategy, pathologists and oncologists are actively conducting remarkable work to deepen the clinical and molecular landscape of HER2-positive CRC [43]. From a molecular perspective, this is of major interest, considering that due to homo- or hetero-dimerization with other EGFR family members, HER2 causes transphosphorylation of the intracytoplasmic tyrosine kinase domain, which in turn activates several downstream signal transduction pathways, including RAS/RAF/ERK, PIK3K/AKT/mTOR, and JAK/STAT3 [17]. Thus, assessing co-occurring alterations in HER2 and these pathways may unravel the mechanisms of both primary and acquired resistance to HER2 inhibition. In breast cancer, among the most frequent alterations that can confer resistance to anti-HER2 drugs are PI3KCA mutations and/or PTEN loss [55,56]. Importantly, the study published by Loree et al. sought to determine the molecular landscape of HER2/ERBB3-mutated CRC in three different cohorts composed of a total of more than 2500 patients [57]. In terms of co-occurring alterations, the authors reported a strong association between PI3KCA and HER2 mutations, suggesting that concomitant genetic alterations in the former may represent a second hit to oncogenic signaling [57]. Moreover, they showed that microsatellite instability (MSI) can be correlated with HER2/ERBB3 mutations potentially explained by the hypermutator phenotype that characterizes these tumors [58,59,60,61]. These findings have been verified by an even larger study in which almost 9000 CRC cases were analyzed by comprehensive genomic profiling for alterations in 315 cancer-related genes, tumor mutational burden, and MSI [50]. Specifically, the authors demonstrated that HER2 short variant mutations had higher mutation frequencies in the PI3K, mismatch repair (MMR), and Wnt pathways. Regarding the data on co-occurring MSI/MMR-affected function and mutations in HER2/ERBB3, the recent study published by Qiu and colleagues reported that cases of HER2-mutated CRC are more likely to harbor an MSI-high status in comparison with the HER2 amplified ones [36]. On the other hand, there is still a debate related to the data on the presence of mutations in the RTK/RAS pathway in patients with HER2-positive CRC. While some studies demonstrated that most mutations in KRAS, NRAS, BRAF, HER2, and ERBB3 are mutually exclusive [62], others reported that KRAS alterations have a higher probability to cooccur with short variant mutations in HER2 than amplification [50]. Considering the clinical importance of co-occurring alterations in the setting of HER2-positive CRC, unraveling their prevalence and functional impact represents a crucial need to better establish the optimal targeting of this pathway. As shown in Figure 3, among HER2-altered CRCs (either HER2-mutated, amplified, or both), the most recurrently altered gene is adenomatous polyposis coli (APC), which is invariably targeted by truncating pathogenic mutations. Other highly recurrent mutations involve KRAS (44%); TP53 (44%); and members of the PIK3 family, such as PIK3CA and PIK3CG (25%).

Figure 3.
Recurrent genomic alterations in colorectal carcinomas with HER2 alterations. Oncoprint visualization of the most frequently mutated cancer genes in colorectal carcinomas harboring HER2 amplification, mutations, or both types of alterations. TGCA Firehose Legacy series (16 samples) from https://www.cbioportal.org/, accessed on 11 August 2022.
5. Perspectives for Individualized Therapeutic Schemes
An open issue in the treatment of HER2-positive mCRC is the continuation of HER2 blockade in patients developing resistance to anti-HER2 therapy. The HERACLES RESCUE trial aims to investigate the activity of TDM-1 in patients already treated with lapatinib + trastuzumab in the HERACLES-A trial. In preclinical models of xenograft derived from these patients with acquired resistance to trastuzumab + lapatinib and subsequent exposure to TDM-1, substantial tumor regression was detected [63]. Multiple studies are evaluating the combination of checkpoint inhibitors with anti-HER2 agents. BDC-1001 is a novel immune-stimulating antibody conjugate (ISAC), consisting of a trastuzumab biosimilar chemically conjugated to a toll-like receptor 7/8 agonist. This compound combines the precision of a tumor-targeting antibody with the therapeutic effect of an immune-modulator and activates at the same time both the innate and adaptive immune responses. A phase I-II study is evaluating BDC-1001 alone and in combination with pembrolizumab in HER2-positive tumors. Preliminary results showed a clinical benefit in three out of five mCRC patients treated with one PR and two SD in patients with microsatellite-stable tumors [41,64]. Another ongoing study is evaluating BDC-1001 as a single agent or in combination with nivolumab (NCT04278144). Another ISAC, SBT6050, is being tested alone and in combination with PD-1 inhibitors pembrolizumab and cemiplimab in the same subset of patients (NCT04460456). Zanidatamab (ZW25) is a HER2-bispecific antibody targeting simultaneously the juxtamembrane domain (ECD4) and the dimerization domain (ECD2) of HER2 [65]. A phase II, open-label, two-part, first-line study is testing the combination of chemotherapy and ZW25 in different primary tumors including mCRC (NCT03929666). Different cancer vaccines including HER2 peptides recognized by T-lymphocytes have been developed so far, such as the TAEK-VAC-HerBy vaccine that is being tested in a phase I study including HER2-positive tumors (NCT04246671). Moreover, different trials are testing chimeric antigen receptor (CAR) T-cells targeting HER2, such as HER2-specific CAR T-cells in combination with an intra-tumor injection of CAdVEC, an oncolytic adenovirus that is designed to help the immune system, including HER2-specific CAR T-cells, react to the tumor (NCT03740256) [66,67]. Natural killer (NK) cells and chimeric antigen receptor macrophages have also been developed [17]. For instance, ACE 1702 is an NK cell product targeting HER2 expressing solid tumors. A phase I study is ongoing with patients receiving treatment with cyclophosphamide and fludarabine followed by ACE1702 (NCT04319757). In addition, CT-0508 adenoviral transduced autologous macrophages engineered to contain an anti-HER2 CAR are also under testing in a phase I study of subjects with HER2 overexpressing solid tumors (NCT04660929).
6. Conclusions
HER2 is an established therapeutic target with the continuous evolution of specific anti-HER2 therapeutic agents (monoclonal antibodies, antibody-drug conjugates, and bispecific antibodies targeting) [4,5,6]. Recent data indicate that HER2 mutations may be successfully targeted by anti-HER2 therapies in various cancer types [4,5]. Clinical trials already report impressive results in overall breast and gastric cancer survival improvement. The trials on HER2-targeted therapies’ application in other solid tumor types are still ongoing [4,68]. HER2 gene amplification and protein overexpression are identified in about 6% of CRC patients and may be successfully evaluated both by IHC and ISH with high concordance rates, giving these patients new treatment opportunities by making them potential candidates for anti-HER2 therapy [6,10,12,14,50]. Available data in HER2-positive metastatic CRC provide future directions for biomarker-driven research and clinical trials, where the main strategies focus on targeting HER2 as the primary carcinogenesis driver and attempt to overcome other target therapies’ resistance, mediated by HER2 alterations [6,10,12,14,50].
The prognostic role of HER2 in metastatic CRC remains unclear, although personalized treatment strategies allow for the overall survival of patients to improve and significantly decrease drug-related toxicity. Common widespread next-generation sequencing may provide additional helpful insights [69,70]. The results of novel clinical trials will likely allow HER2 to become a validated therapeutic target in metastatic CRC [4,5,11,47,71].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/life12091403/s1, Figure S1: HER2 pathways schematic overview. L, ligand, binds to the of human epidermal growth factor receptor (HeR1/3/4) extracellular domain, stabilizing the active HER2 heterodimers formation; PI3K, phosphoinositide 3-kinase; AKT, protein kinase B; mTOR, mammalian target of rapamycin; SOS, Son of Sevenless protein; RAS, member of signal cell transduction proteins; RAF, proto-oncogene serine/threonine-protein kinase; MEK, mitogen-activated protein kinase kinase enzyme; MAPK, mitogen-activated protein kinase.
Author Contributions
Conceptualization, N.F. and M.G.; methodology, N.F.; software, N.F.; validation, E.G.-R., L.B. and M.G.M.; formal analysis, M.I, K.V., and N.F.; resources, N.F.; data curation, N.F.; writing—original draft preparation, M.I., K.V. and M.G.; writing—review and editing, E.G.-R., L.B. and M.G.M.; visualization, M.I. and N.F.; supervision, N.F. and O.G.; project administration, N.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded by Grant Ricerca Corrente 2021, Italian Ministry of Health.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gaye, E.; Penel, N.; Lebellec, L. Novel treatment approaches for HER2 positive solid tumors (excluding breast cancer). Curr. Opin. Oncol. 2022, 34, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Roy-Chowdhuri, S.; Davies, K.D.; Ritterhouse, L.L.; Snow, A.N. ERBB2 (HER2) Alterations in Colorectal Cancer. J. Mol. Diagn. 2022. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Miao, J.; Wen, Y.; Xia, X.; Chen, Y.; Huang, M.; Chen, S.; Zhao, Z.; Zhang, Y.; Chen, C.; et al. Molecular Landscape of ERBB2 Alterations in 14,956 Solid Tumors. Pathol. Oncol. Res. POR 2022, 28, 1610360. [Google Scholar] [CrossRef]
- Oh, D.-Y.; Bang, Y.-J. HER2-targeted therapies—A role beyond breast cancer. Nat. Rev. Clin. Oncol. 2020, 17, 33–48. [Google Scholar] [CrossRef]
- Vranić, S.; Bešlija, S.; Gatalica, Z. Targeting HER2 expression in cancer: New drugs and new indications. Bosn. J. Basic Med. Sci. 2021, 21, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Seo, A.N.; Kwak, Y.; Kim, D.W.; Kang, S.B.; Choe, G.; Kim, W.H.; Lee, H.S. HER2 status in colorectal cancer: Its clinical significance and the relationship between HER2 gene amplification and expression. PLoS ONE 2014, 9, e98528. [Google Scholar] [CrossRef]
- Fusco, N.; Bosari, S. HER2 aberrations and heterogeneity in cancers of the digestive system: Implications for pathologists and gastroenterologists. World J. Gastroenterol. 2016, 22, 7926–7937. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Kavuri, S.M.; Jain, N.; Galimi, F.; Cottino, F.; Leto, S.M.; Migliardi, G.; Searleman, A.C.; Shen, W.; Monsey, J.; Trusolino, L.; et al. HER2 activating mutations are targets for colorectal cancer treatment. Cancer Discov. 2015, 5, 832–841. [Google Scholar] [CrossRef] [PubMed]
- El-Deiry, W.S.; Vijayvergia, N.; Xiu, J.; Scicchitano, A.; Lim, B.; Yee, N.S.; Harvey, H.A.; Gatalica, Z.; Reddy, S. Molecular profiling of 6892 colorectal cancer samples suggests different possible treatment options specific to metastatic sites. Cancer Biol. Ther. 2015, 16, 1726–1737. [Google Scholar] [CrossRef] [Green Version]
- Afrăsânie, V.A.; Marinca, M.V.; Alexa-Stratulat, T.; Gafton, B.; Păduraru, M.; Adavidoaiei, A.M.; Miron, L.; Rusu, C. KRAS, NRAS, BRAF, HER2 and microsatellite instability in metastatic colorectal cancer—Practical implications for the clinician. Radiol. Oncol. 2019, 53, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Siena, S.; Di Bartolomeo, M.; Raghav, K.; Masuishi, T.; Loupakis, F.; Kawakami, H.; Yamaguchi, K.; Nishina, T.; Fakih, M.; Elez, E.; et al. Trastuzumab deruxtecan (DS-8201) in patients with HER2-expressing metastatic colorectal cancer (DESTINY-CRC01): A multicentre, open-label, phase 2 trial. Lancet Oncol. 2021, 22, 779–789. [Google Scholar] [CrossRef]
- Dienstmann, R.; Ciner, A.; Hochster, H.S. Should next-generation sequencing testing be routinely used in metastatic colorectal cancer? Lancet Oncol. 2018, 19, 1434–1435. [Google Scholar] [CrossRef]
- Sartore-Bianchi, A.; Trusolino, L.; Martino, C.; Bencardino, K.; Lonardi, S.; Bergamo, F.; Zagonel, V.; Leone, F.; Depetris, I.; Martinelli, E.; et al. Dual-targeted therapy with trastuzumab and lapatinib in treatment-refractory, KRAS codon 12/13 wild-type, HER2-positive metastatic colorectal cancer (HERACLES): A proof-of-concept, multicentre, open-label, phase 2 trial. Lancet Oncol. 2016, 17, 738–746. [Google Scholar] [CrossRef]
- Henry, N.L.; Somerfield, M.R.; Dayao, Z.; Elias, A.; Kalinsky, K.; McShane, L.M.; Moy, B.; Park, B.H.; Shanahan, K.M.; Sharma, P.; et al. Biomarkers for Systemic Therapy in Metastatic Breast Cancer: ASCO Guideline Update. J. Clin. Oncol. 2022, JCO2201063. [Google Scholar] [CrossRef]
- Bartley, A.N.; Washington, M.K.; Colasacco, C.; Ventura, C.B.; Ismaila, N.; Benson, A.B.; Carrato, A.; Gulley, M.L.; Jain, D.; Kakar, S.; et al. HER2 Testing and Clinical Decision Making in Gastroesophageal Adenocarcinoma: Guideline from the College of American Pathologists, American Society for Clinical Pathology, and the American Society of Clinical Oncology. J. Clin. Oncol. 2017, 35, 446–464. [Google Scholar] [CrossRef]
- Djaballah, S.A.; Daniel, F.; Milani, A.; Ricagno, G.; Lonardi, S. HER2 in colorectal cancer: The long and winding road from negative predictive factor to positive actionable target. Am. Soc. Clin. Oncol. Educ. Book 2022, 42, 219–232. [Google Scholar] [CrossRef]
- Modi, S.; Jacot, W.; Yamashita, T.; Sohn, J.; Vidal, M.; Tokunaga, E.; Tsurutani, J.; Ueno, N.T.; Chae, Y.S.; Lee, K.S.; et al. Trastuzumab deruxtecan (T-DXd) versus treatment of physician’s choice (TPC) in patients (pts) with HER2-low unresectable and/or metastatic breast cancer (mBC): Results of DESTINY-Breast04, a randomized, phase 3 study. J. Clin. Oncol. 2022, 40, LBA3. [Google Scholar] [CrossRef]
- Clark, J.W.; Niedzwiecki, D.; Hollis, D.M.R. Phase-II trial of 5-fluororuacil (5-FU), leucovorin (LV), oxaliplatin (Ox), and trastuzamab (T) for patients with metastatic colorectal cancer (CRC) refractory to initial therapy. Onkologie 2003, 26, 13–46. [Google Scholar]
- Ramanathan, R.K.; Hwang, J.J.; Zamboni, W.C.; Sinicrope, F.A.; Safran, H.; Wong, M.K.; Earle, M.; Brufsky, A.; Evans, T.; Troetschel, M.; et al. Low overexpression of HER-2/neu in advanced colorectal cancer limits the usefulness of trastuzumab (Herceptin) and irinotecan as therapy. A phase II trial. Cancer Investig. 2004, 22, 858–865. [Google Scholar] [CrossRef]
- Siena, S.; Sartore-Bianchi, A.; Marsoni, S.; Hurwitz, H.I.; McCall, S.J.; Penault-Llorca, F.; Srock, S.; Bardelli, A.; Trusolino, L. Targeting the human epidermal growth factor receptor 2 (HER2) oncogene in colorectal cancer. Ann. Oncol. 2018, 29, 1108–1119. [Google Scholar] [CrossRef] [PubMed]
- Tosi, F.; Sartore-Bianchi, A.; Lonardi, S.; Amatu, A.; Leone, F.; Ghezzi, S.; Martino, C.; Bencardino, K.; Bonazzina, E.; Bergamo, F.; et al. Long-term Clinical Outcome of Trastuzumab and Lapatinib for HER2-positive Metastatic Colorectal Cancer. Clin. Colorectal Cancer 2020, 19, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Sartore-Bianchi, A.; Lonardi, S.; Martino, C.; Fenocchio, E.; Tosi, F.; Ghezzi, S.; Leone, F.; Bergamo, F.; Zagonel, V.; Ciardiello, F.; et al. Pertuzumab and trastuzumab emtansine in patients with HER2-amplified metastatic colorectal cancer: The phase II HERACLES-B trial. ESMO Open 2020, 5, e000911. [Google Scholar] [CrossRef] [PubMed]
- Meric-Bernstam, F.; Hurwitz, H.; Raghav, K.P.S.; McWilliams, R.R.; Fakih, M.; VanderWalde, A.; Swanton, C.; Kurzrock, R.; Burris, H.; Sweeney, C.; et al. Pertuzumab plus trastuzumab for HER2-amplified metastatic colorectal cancer (MyPathway): An updated report from a multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol. 2019, 20, 518–530. [Google Scholar] [CrossRef]
- Nakamura, Y.; Okamoto, W.; Kato, T.; Esaki, T.; Kato, K.; Komatsu, Y.; Yuki, S.; Masuishi, T.; Nishina, T.; Ebi, H.; et al. Circulating tumor DNA-guided treatment with pertuzumab plus trastuzumab for HER2-amplified metastatic colorectal cancer: A phase 2 trial. Nat. Med. 2021, 27, 1899–1903. [Google Scholar] [CrossRef]
- Gupta, M.; Wang, B.; Carrothers, T.J.; LoRusso, P.M.; Chu, Y.W.; Shih, T.; Loecke, D.; Joshi, A.; Saad, O.; Yi, J.H.; et al. Effects of Trastuzumab Emtansine (T-DM1) on QT Interval and Safety of Pertuzumab Plus T-DM1 in Patients with Previously Treated Human Epidermal Growth Factor Receptor 2-Positive Metastatic Breast Cancer. Clin. Pharmacol. Drug Dev. 2013, 2, 11–24. [Google Scholar] [CrossRef]
- Yoshino, T.; Di Bartolomeo, M.; Raghav, K.; Masuishi, T.; Loupakis, F.; Kawakami, H.; Yamaguchi, K.; Nishina, T.; Wainberg, Z.; Elez, E.; et al. Trastuzumab deruxtecan (T-DXd; DS-8201) in patients (pts) with HER2-expressing metastatic colorectal cancer (mCRC): Final results from a phase 2, multicenter, open-label study (DESTINY-CRC01). In Proceedings of the 2021 ASCO Annual Meeting, Virtual, 4–8 June 2021. [Google Scholar]
- Strickler, J.H.; Zemla, T.; Ou, F.S.; Cercek, A.; Wu, C.; Sanchez, F.A.; Hubbard, J.; Jaszeeski, B.; Bandel, L.; Schweitzer, B.; et al. Trastuzumab and tucatinib for the treatment of HER2 amplified metastatic colorectal cancer (mCRC): Initial results from the MOUNTAINEER trial. In Proceedings of the ESMO Annual Meeting, Barcelona, Spain, 27 September–1 October 2019. [Google Scholar]
- Li, X.; Yang, C.; Wan, H.; Zhang, G.; Feng, J.; Zhang, L.; Chen, X.; Zhong, D.; Lou, L.; Tao, W.; et al. Discovery and development of pyrotinib: A novel irreversible EGFR/HER2 dual tyrosine kinase inhibitor with favorable safety profiles for the treatment of breast cancer. Eur. J. Pharm. Sci. 2017, 110, 51–61. [Google Scholar] [CrossRef]
- Li, W.; Chang, J.; Xu, M.; Zhu, X. Anti-HER2 therapy with pyrotinib and trastuzumab in refractory HER2-positive metastatic colorectal cancer: A preliminary report from HER2-FUSCC-G study. In Proceedings of the 2022 ASCO Gastrointestinal Cancers Symposium, San Francisco, CA, USA, 20–22 January 2022. [Google Scholar]
- Jacobs, S.A.; Lee, J.J.; George, T.J.; Wade, J.L., III; Stella, P.J.; Wang, D.; Sama, A.R.; Piette, F.; Pogue-Geile, K.L.; Kim, R.S.; et al. Neratinib-Plus-Cetuximab in Quadruple-WT (KRAS, NRAS, BRAF, PIK3CA) Metastatic Colorectal Cancer Resistant to Cetuximab or Panitumumab: NSABP FC-7, A Phase Ib Study. Clin. Cancer Res. 2021, 27, 1612–1622. [Google Scholar] [CrossRef]
- Bitar, L.; Zouein, J.; Haddad, F.G.; Eid, R.; Kourie, H.R. HER2 in metastatic colorectal cancer: A new to target to remember. Biomark Med. 2021, 15, 133–136. [Google Scholar] [CrossRef]
- Nazemalhosseini Mojarad, E.; Kuppen, P.J. HER2 and immunotherapy using monoclonal antibodies in colorectal cancer. Immunotherapy 2013, 5, 1267–1269. [Google Scholar] [CrossRef]
- Shi, Y.; Fan, X.; Meng, W.; Deng, H.; Zhang, N.; An, Z. Engagement of immune effector cells by trastuzumab induces HER2/ERBB2 downregulation in cancer cells through STAT1 activation. Breast Cancer Res. 2014, 16, R33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, X.; He, Z.; Qin, C.; Zhang, P.; Sui, C.; Deng, X.; Fang, Y.; Li, G.; Shi, J. Inhibition of PFKFB3 in HER2-positive gastric cancer improves sensitivity to trastuzumab by inducing tumour vessel normalisation. Br. J. Cancer 2022, 127, 811–823. [Google Scholar] [CrossRef] [PubMed]
- Qiu, M.Z.; He, C.Y.; Yang, X.H.; Yang, L.Q.; Lin, J.Z.; Zhou, D.L.; Long, Y.K.; Guan, W.L.; Jin, Y.; Li, Y.H.; et al. Relationship of HER2 Alteration and Microsatellite Instability Status in Colorectal Adenocarcinoma. Oncologist 2021, 26, e1161–e1170. [Google Scholar] [CrossRef] [PubMed]
- Andre, T.; Shiu, K.K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer. N. Engl. J. Med. 2020, 383, 2207–2218. [Google Scholar] [CrossRef] [PubMed]
- Passardi, A.; Canale, M.; Valgiusti, M.; Ulivi, P. Immune Checkpoints as a Target for Colorectal Cancer Treatment. Int. J. Mol. Sci. 2017, 18, 1324. [Google Scholar] [CrossRef]
- Xie, Y.H.; Chen, Y.X.; Fang, J.Y. Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct. Target Ther. 2020, 5, 22. [Google Scholar] [CrossRef]
- Sahin, I.H.; Akce, M.; Alese, O.; Shaib, W.; Lesinski, G.B.; El-Rayes, B.; Wu, C. Immune checkpoint inhibitors for the treatment of MSI-H/MMR-D colorectal cancer and a perspective on resistance mechanisms. Br. J. Cancer 2019, 121, 809–818. [Google Scholar] [CrossRef]
- Sharma, M.; Carvajal, R.D.; Hanna, G.J.; Li, B.T.; Moore, K.N.; Pegram, M.D.; Rasco, D.W.; Spira, A.I.; Alonso, M.; Fang, L.; et al. Preliminary results from a phase 1/2 study of BDC-1001, a novel HER2 targeting TLR7/8 immune-stimulating antibody conjugate (ISAC), in patients (pts) with advanced HER2-expressing solid tumors. In Proceedings of the ASCO Annual Meeting, Virtual, 4–8 June 2021. [Google Scholar]
- Lefler, D.S.; Snook, A.E.; Bashir, B. Immune checkpoint inhibitors in luminal gastrointestinal malignancies: Going beyond MSI-H/dMMR, TMB and PD-L1. Immunotherapy 2022, 14, 885–902. [Google Scholar] [CrossRef]
- Guarini, C.; Grassi, T.; Pezzicoli, G.; Porta, C. Beyond RAS and BRAF: HER2, a New Actionable Oncotarget in Advanced Colorectal Cancer. Int. J. Mol. Sci. 2021, 22, 6813. [Google Scholar] [CrossRef]
- Sajjadi, E.; Venetis, K.; Ivanova, M.; Fusco, N. Improving HER2 testing reproducibility in HER2-low breast cancer. Cancer Drug Resist. 2022, 5, 882–888. [Google Scholar] [CrossRef]
- Fusco, N.; Rocco, E.G.; Del Conte, C.; Pellegrini, C.; Bulfamante, G.; Di Nuovo, F.; Romagnoli, S.; Bosari, S. HER2 in gastric cancer: A digital image analysis in pre-neoplastic, primary and metastatic lesions. Mod. Pathol. 2013, 26, 816–824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ocaña, A.; Amir, E.; Pandiella, A. HER2 heterogeneity and resistance to anti-HER2 antibody-drug conjugates. Breast Cancer Res. 2020, 22, 15. [Google Scholar] [CrossRef] [PubMed]
- Nowak, J.A. HER2 in Colorectal Carcinoma: Are We There yet? Surg. Pathol. Clin. 2020, 13, 485–502. [Google Scholar] [CrossRef] [PubMed]
- Sawada, K.; Nakamura, Y.; Yamanaka, T.; Kuboki, Y.; Yamaguchi, D.; Yuki, S.; Yoshino, T.; Komatsu, Y.; Sakamoto, N.; Okamoto, W.; et al. Prognostic and Predictive Value of HER2 Amplification in Patients with Metastatic Colorectal Cancer. Clin. Colorectal Cancer 2018, 17, 198–205. [Google Scholar] [CrossRef]
- Valtorta, E.; Martino, C.; Sartore-Bianchi, A.; Penaullt-Llorca, F.; Viale, G.; Risio, M.; Rugge, M.; Grigioni, W.; Bencardino, K.; Lonardi, S.; et al. Assessment of a HER2 scoring system for colorectal cancer: Results from a validation study. Mod. Pathol. 2015, 28, 1481–1491. [Google Scholar] [CrossRef]
- Ross, J.S.; Fakih, M.; Ali, S.M.; Elvin, J.A.; Schrock, A.B.; Suh, J.; Vergilio, J.A.; Ramkissoon, S.; Severson, E.; Daniel, S.; et al. Targeting HER2 in colorectal cancer: The landscape of amplification and short variant mutations in ERBB2 and ERBB3. Cancer 2018, 124, 1358–1373. [Google Scholar] [CrossRef]
- Owen, D.R.; Wong, H.-L.; Bonakdar, M.; Jones, M.; Hughes, C.S.; Morin, G.B.; Jones, S.J.M.; Renouf, D.J.; Lim, H.; Laskin, J.; et al. Molecular characterization of ERBB2-amplified colorectal cancer identifies potential mechanisms of resistance to targeted therapies: A report of two instructive cases. Cold Spring Harb. Mol. Case Stud. 2018, 4, a002535. [Google Scholar] [CrossRef]
- Schmoll, H.-J. Targeting HER2: Precision oncology for colorectal cancer. Lancet Oncol. 2016, 17, 685–686. [Google Scholar] [CrossRef]
- Mosele, F.; Remon, J.; Mateo, J.; Westphalen, C.B.; Barlesi, F.; Lolkema, M.P.; Normanno, N.; Scarpa, A.; Robson, M.; Meric-Bernstam, F.; et al. Recommendations for the use of next-generation sequencing (NGS) for patients with metastatic cancers: A report from the ESMO Precision Medicine Working Group. Ann. Oncol. 2020, 31, 1491–1505. [Google Scholar] [CrossRef]
- Cenaj, O.; Ligon, A.H.; Hornick, J.L.; Sholl, L.M. Detection of ERBB2 Amplification by Next-Generation Sequencing Predicts HER2 Expression in Colorectal Carcinoma. Am. J. Clin. Pathol. 2019, 152, 97–108. [Google Scholar] [CrossRef]
- Fusco, N.; Malapelle, U.; Fassan, M.; Marchiò, C.; Buglioni, S.; Zupo, S.; Criscitiello, C.; Vigneri, P.; Dei Tos, A.P.; Maiorano, E.; et al. PIK3CA Mutations as a Molecular Target for Hormone Receptor-Positive, HER2-Negative Metastatic Breast Cancer. Front Oncol. 2021, 11, 644737. [Google Scholar] [CrossRef] [PubMed]
- Fusco, N.; Sajjadi, E.; Venetis, K.; Gaudioso, G.; Lopez, G.; Corti, C.; Rocco, E.G.; Criscitiello, C.; Malapelle, U.; Invernizzi, M. PTEN Alterations and Their Role in Cancer Management: Are We Making Headway on Precision Medicine? Genes 2020, 11, 719. [Google Scholar] [CrossRef]
- Loree, J.M.; Bailey, A.M.; Johnson, A.M.; Yu, Y.; Wu, W.; Bristow, C.A.; Davis, J.S.; Shaw, K.R.; Broaddus, R.; Banks, K.C.; et al. Molecular Landscape of ERBB2/ERBB3 Mutated Colorectal Cancer. J. Natl. Cancer Inst. 2018, 110, 1409–1417. [Google Scholar] [CrossRef] [PubMed]
- Sajjadi, E.; Venetis, K.; Piciotti, R.; Invernizzi, M.; Guerini-Rocco, E.; Haricharan, S.; Fusco, N. Mismatch repair-deficient hormone receptor-positive breast cancers: Biology and pathological characterization. Cancer Cell Int. 2021, 21, 266. [Google Scholar] [CrossRef]
- Piciotti, R.; Venetis, K.; Sajjadi, E.; Fusco, N. Mismatch Repair Status Characterization in Oncologic Pathology: Taking Stock of the Real-World Possibilities. J. Mol. Pathol. 2021, 2, 93–100. [Google Scholar] [CrossRef]
- Lopez, G.; Venetis, K.; Sajjadi, E.; Fusco, N. Mismatch Repair System Genomic Scars in Gastroesophageal Cancers: Biology and Clinical Testing. Gastrointest. Disord. 2020, 2, 341–352. [Google Scholar] [CrossRef]
- Corti, C.; Sajjadi, E.; Fusco, N. Determination of Mismatch Repair Status in Human Cancer and Its Clinical Significance: Does One Size Fit All? Adv. Anat. Pathol. 2019, 26, 270–279. [Google Scholar] [CrossRef]
- Zaidi, S.H.; Harrison, T.A.; Phipps, A.I.; Steinfelder, R.; Trinh, Q.M.; Qu, C.; Banbury, B.L.; Georgeson, P.; Grasso, C.S.; Giannakis, M.; et al. Landscape of somatic single nucleotide variants and indels in colorectal cancer and impact on survival. Nat. Commun. 2020, 11, 3644. [Google Scholar] [CrossRef]
- Siena, S.; Bardelli, A.; Sartore-Bianchi, A.; Martino, C.; Siravegna, G.; Magrì, A.; Leone, F.; Zagonel, V.; Lonardi, S.; Amatu, A.; et al. HER2 amplification as a ‘molecular bait’ for trastuzumab-emtansine (T-DM1) precision chemotherapy to overcome anti-HER2 resistance in HER2 positive metastatic colorectal cancer: The HERACLES-RESCUE trial. In Proceedings of the 2016 Gastrointestinal Cancers Symposium, San Francisco, CA, USA, 21–23 June 2016. [Google Scholar]
- Saude-Conde, R.; Rasschaert, G.; Bregni, G.; Hendlisz, A.; Sclafani, F. Mind the target: Human epidermal growth factor receptor 2 in colorectal cancer. Curr. Opin. Oncol. 2022, 34, 382–388. [Google Scholar] [CrossRef]
- Antonarelli, G.; Giugliano, F.; Corti, C.; Repetto, M.; Tarantino, P.; Curigliano, G. Research and Clinical Landscape of Bispecific Antibodies for the Treatment of Solid Malignancies. Pharmaceuticals 2021, 14, 884. [Google Scholar] [CrossRef]
- Corti, C.; Venetis, K.; Sajjadi, E.; Zattoni, L.; Curigliano, G.; Fusco, N. CAR-T cell therapy for triple-negative breast cancer and other solid tumors: Preclinical and clinical progress. Expert Opin. Investig. Drugs 2022, 31, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Venetis, K.; Invernizzi, M.; Sajjadi, E.; Curigliano, G.; Fusco, N. Cellular immunotherapy in breast cancer: The quest for consistent biomarkers. Cancer Treat. Rev. 2020, 90, 102089. [Google Scholar] [CrossRef] [PubMed]
- Venetis, K.; Crimini, E.; Sajjadi, E.; Corti, C.; Guerini-Rocco, E.; Viale, G.; Curigliano, G.; Criscitiello, C.; Fusco, N. HER2 low, ultra-low, and novel complementary biomarkers: Expanding the spectrum of HER2 positivity in breast cancer. Front. Mol. Biosci. 2022, 9, 834651. [Google Scholar] [CrossRef] [PubMed]
- Angerilli, V.; Galuppini, F.; Pagni, F.; Fusco, N.; Malapelle, U.; Fassan, M. The Role of the Pathologist in the Next-Generation Era of Tumor Molecular Characterization. Diagnostics 2021, 11, 339. [Google Scholar] [CrossRef]
- Pisapia, P.; L’Imperio, V.; Galuppini, F.; Sajjadi, E.; Russo, A.; Cerbelli, B.; Fraggetta, F.; d’Amati, G.; Troncone, G.; Fassan, M.; et al. The evolving landscape of anatomic pathology. Crit. Rev. Oncol./Hematol. 2022, 178, 103776. [Google Scholar] [CrossRef]
- Greally, M.; Kelly, C.M.; Cercek, A. HER2: An emerging target in colorectal cancer. Curr. Probl. Cancer 2018, 42, 560–571. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).