2.1. Example 1
In CKD, OS occurs early, progresses along with the deterioration of kidney function and is thought to promote ED and vascular calcification (VC), through oxidative modification of low-density lipoprotein (LDL) to oxidized LDL (oxLDL) within the vessel wall. The oxidation of LDL is the first, crucial step towards ED and atherosclerosis. However, the data regarding the association between oxLDL and VC in CKD patients remain limited. To evaluate whether oxLDL is an accurate biomarker for the diagnosis of VC in uremia, in a hypothetical cross-sectional clinical study, we enrolled a cohort of 120 predialysis CKD subjects (stages 2–5) and obtained measurements of circulating oxLDL for each patient. We aimed to estimate the classification accuracy by a ROC analysis as we had to determine the disease status for all subjects and classify our cohort into two groups, the diseased (those with VC) and the non-diseased group (those with no VC), by a gold standard method. To ensure that the classification was correct, we obtained autopsy data from the abdominal aorta of our patients, and VC was determined as the presence or absence of calcification within the artery. Since the definition of VC was based on an autopsy method, there was no overlapping between CKD patients with and without VC, thus indicating that aortic autopsy perfectly discriminated the diseased from the non-diseased (true positive rate = 100%, true negative rate = 100%, accuracy = 100%). To evaluate the discriminatory ability of a quantitative test (such as oxLDL for assessing VC) across a series of its values and determine the optimal threshold, we constructed a ROC curve. In this hypothetical cross-sectional study, 120 CKD patients were recruited to evaluate the overall accuracy of oxLDL to discriminate patients with and without VC as assessed by aortic autopsies. Hypothetically, the best cut-off value of oxLDL to identify patients with VC was 70 U/L. The diagnostic vale of the oxLDL threshold of 70 U/L to identify VC in CKD patients is presented in
Table 1.
Based on
Table 1, we can calculate the diagnostic value indices of oxLDL, as follows:
Sensitivity: 62/80 = 0.775 = 77.5%
Specificity: 32/40 = 0.80 = 80%
False positives (1-specificity):100 − 80 = 20%
Positive predictive value: 62/70 = 0.886 = 88.6%
Negative predictive value: 32/50 = 0.64 = 64%
Accuracy: 62 + 32/120 = 0.783 = 78.3%
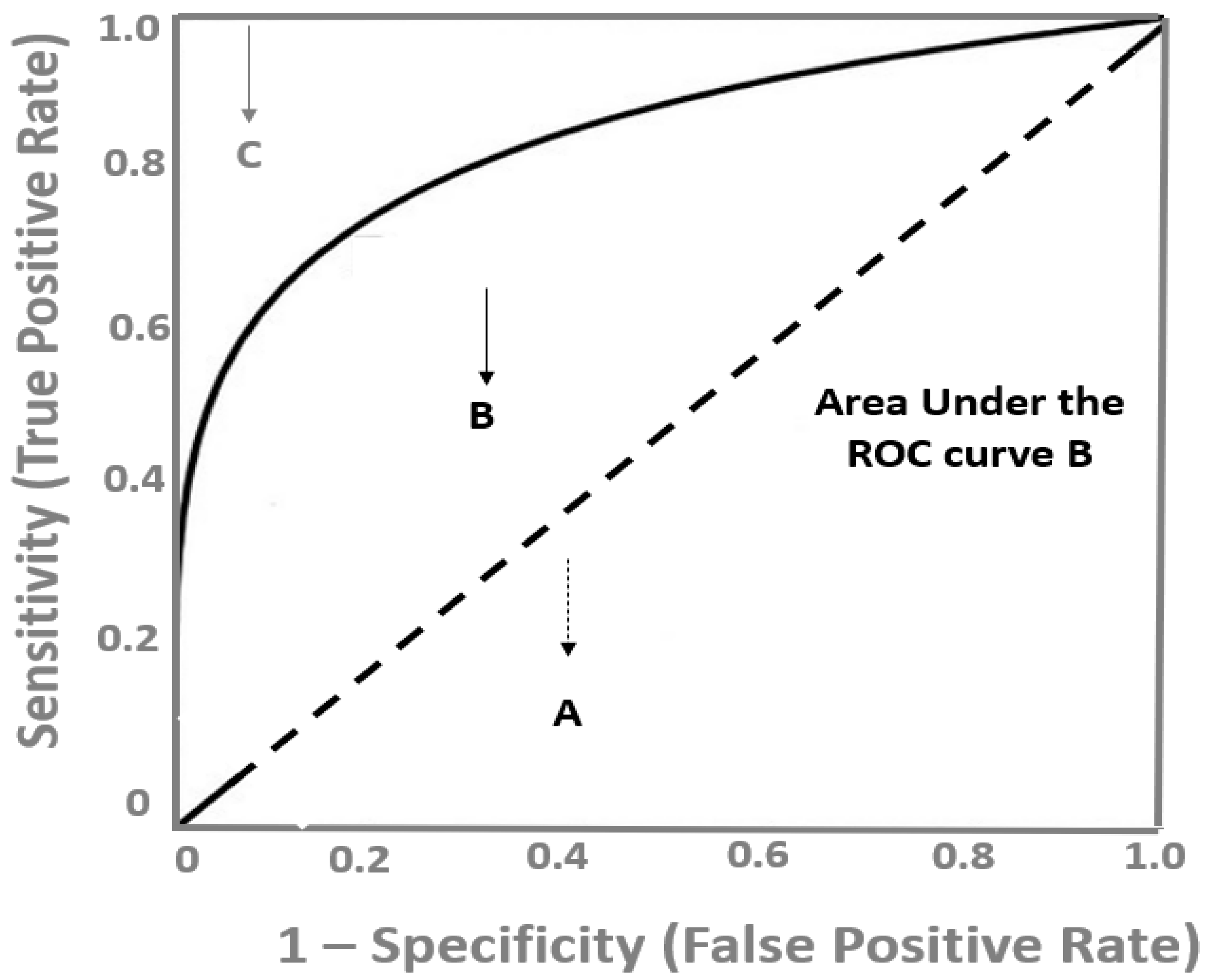
The ROC curve is a graphical plot that combines pairs of sensitivity (true positive rate) on the y axis and the complement of specificity (1—specificity, false positive rate) in the x axis, corresponding to several of the cut-off values covering the complete range of possible values that this test/marker might take. Since both specificity and sensitivity are not affected by the proportion of the diseased subjects, the ROC curve analysis is also unaffected by the prevalence of the disease.
As depicted in
Figure 1, a diagnostic test with a high discrimination ability for identifying the diseased subjects has a ROC curve close to the upper left corner of the graph, whereas the closer the ROC plot is to the diagonal reference line (also named as the chance line), the lower the diagnostic accuracy of the test. The overall discriminatory power of a test is assessed by the area under the ROC curve (AUC), which is widely recognized as a global estimate of diagnostic accuracy [
8]. In
Figure 1, the AUC of the B test is depicted as the grey area under this curve. The maximum value the AUC might take is 1.0, indicating a theoretical, ideal test with 100% specificity and 100% sensitivity (perfect discrimination) and the minimum value is 0.5, which corresponds to no discriminative power at all (50% specificity and 50% sensitivity), which is represented by the area under the diagonal, reference line (
Figure 1). There are various proposed interpretations for AUC values, but generally, ROC curves with an AUC above 0.75 might be considered as clinically useful and above 0.85 as having strong diagnostic accuracy, and therefore, potential clinical utility [
7].
The choice of the ideal cut-off value in a ROC curves analysis should ensure the highest sensitivity and specificity. However, it is usually a trade-off between specificity and sensitivity and the choice of a higher sensitivity is at the cost of lower specificity. Therefore, the best cut-off value is determined by clinical and probabilistic considerations. To determine the optimal cut-off point of ox-LDL to distinguish CKD patients with VC from those without VC, we determined the sensitivity and specificity over a range of different cut-off points (
Table 2).
Based on
Figure 2, which shows the ROC curve for oxLDL in the hypothetical cross-sectional study, a value of 70 U/L might be considered an optimal cut-off point to distinguish CKD patients with VC from those without VC. From a probabilistic point of view, we can use the co-ordinates of the ROC curve to determine the best oxLDL cut-off that provides the maximum discrimination or else the maximum sensitivity and specificity. In this example, this corresponds to the value of 70 U/L, which is the optimal probabilistic cut-off value and provides 80% specificity and 77.5% sensitivity. This could be interpreted as follows: among CKD patients, those with circulating oxLDL above 70 U/L probably have VC and those with oxLDL below 70 U/L more likely do not have VC. However, from a clinician point of view, it might be requested to minimize the false positive rate, and thus, set the specificity at 95% (only 5% will be diagnosed as false positives; that is, as diseased, although not having VC). In the ROC curve (
Figure 2), the oxLDL value that provides this specificity corresponds to a low sensitivity of 10%. On the other hand, if a clinician is interested in improving the identification of CKD subjects with VC at the maximum, an oxLDL cut-off value with high sensitivity is needed. If we set the sensitivity at 95%, the oxLDL value that provides this sensitivity corresponds to a modest specificity of 30% (
Figure 2). It should be noted that the ROC performance and the optimal cut-off value of a given diagnostic test might change when the test is applied to different patient populations; for example, in non-CKD or ESKD populations, the cut-off value of 70 U/L and the diagnostic indices of oxLDL for identifying VC cannot be applied.
2.2. Example 2
Since subclinical overhydration is associated with increased OS, hypertension and CV events in ESKD patients, it is crucial to be recognized early and managed in these patients. Alexiadis et al. [
9] aimed to investigate and compare the accuracy of several available techniques for evaluating the hydration status in 53 ESKD patients undergoing maintenance hemodialysis (HD), thrice weekly. In all patients, before and after a HD session, the hydration status was assessed by four different techniques: indexed inferior vena cava diameter (IVCDi), continuous blood volume monitoring (Crit-line), bioelectrical impedance analysis (BIA) and lung comets score with lung ultrasonography. In this setting, to determine the “true disease status”—that is the overhydrated patients—the authors chose IVCDi as the gold standard reference technique and all patients were categorized to overhydrated or underhydrated, based on the results of this test. When determining the gold standard, two major pitfalls should be avoided: first, the measurement error that occurs when there is no true gold standard or when the one used might be flawed. Second, verification bias, which might occur when the gold standard test is accurate only when evaluated in subjects with a known disease status. In the study by Alexiadis et al. patients were characterized as overhydrated when IVCMi was above 11.5 mm/m
2 and patients were classified accordingly, and based on the results of Crit-Line, BIA and lung comets score, three different thresholds were set for each test. The ROC analysis, besides evaluating the discriminatory ability of a certain test, can also compare more than two methods at once, using a reference method.
Figure 3 shows the ROC curves evaluating the performance of lung comet score, BIA and Crit-Line test in predicting overhydration, as determined using IVCDi as the reference method, in HD patients. The lung comet score showed more promising results predicting overhydration, as assessed by the AUCs (0.81 for lung comet score, 0.71 for BIA and 0.61 for Crit-Line). Moreover, the difference between AUCs was statistically significant (lung comet score vs. BIA: 0.100,
p = 0.032), (lung comet score vs. Crit-Line: 0.20,
p = 0.001). Since 0.50 is the lowest AUC value, a given test has discriminatory power if the 95% confidence interval (CI) of the AUC does not include this value. In
Figure 3, the 95%CI for the lung comet score was 0.74–0.87, for BIA 0.63–0.78 and for Crit-Line 0.53–0.68. Of note, the ROC curve for Crit-Line was very close to the diagonal reference line and the AUC was very close to 0.5, indicating that this test had very low diagnostic power. However, the AUC for the lung comet score was 0.81, indicating that if we theoretically selected random pairs of overhydrated and not overhydrated HD patients, the test result (lung comet score) would be higher 81% of the times in the overhydrated patients. In
Figure 3, it also shown that at the optimal threshold of ≥11, the lung comet score displayed a sensitivity of 77% and a specificity of 74%, whereas BIA at the cut-off of ≥0.45 presented a sensitivity of 90% and a specificity of 45% and Crit-Line at the cut-off of ≥10.5 presented a sensitivity of 82% and a specificity of 39%.
2.3. Example 3
A logistic regression analysis might be used to describe the association between an independent variable and a dependent one. In multiple regression models, we can calculate the regression coefficients, statistical significance and the 95%CIs of independent variables (that can be continuous or not) for predicting the dependent variable; that is, a dichotomous variable that can only take the values of 0 = non-diseased or 1 = diseased. In a recent study [
10], aiming to examine the possible association between biomarkers of OS and inflammation and cardiac status, 100 patients presenting with non-ST-elevation myocardial infraction (NSTEMI) at the emergency department were enrolled. Ejection fraction (EF), troponin, interleukin 6 and 10 (IL-6, IL-10), myeloperoxidase (MPO), high-sensitive C-reactive protein (CRP) and fibrinogen were assessed at admission, along with other markers of cardiac (alanine transaminase, aspartate transaminase, brain natriuretic peptide, creatinine phosphokinase, creatinine phosphokinase myocardial band) and kidney function (eGFR and albuminuria). A multiple logistic regression analysis was used to determine independent predictors (independent variables) of patients with the worst cardiac status after the NSTEMI, determined as those with high troponin (dependent variable) and low EF (dependent variable) with two separate models. MPO, IL-10 and AST were independent predictors of high troponin (β = −1.69, 95% CI −3.1 to −0.24,
p = 0.02, β = 0.15, 95% CI 0.04–0.25,
p = 0.006, β = 0.04, 95% CI 0.02–0.06,
p = 0.001, respectively), whereas Hs-CRP, BNP and eGFR were independent predictors of low EF (β = −0.11, 95% CI −0.18 to −0.05,
p = 0.001, β = −0.011, 95% CI −0.018 to −0.003,
p = 0.004, β = 0.12, 95% CI 0.012–0.22,
p = 0.029, respectively).
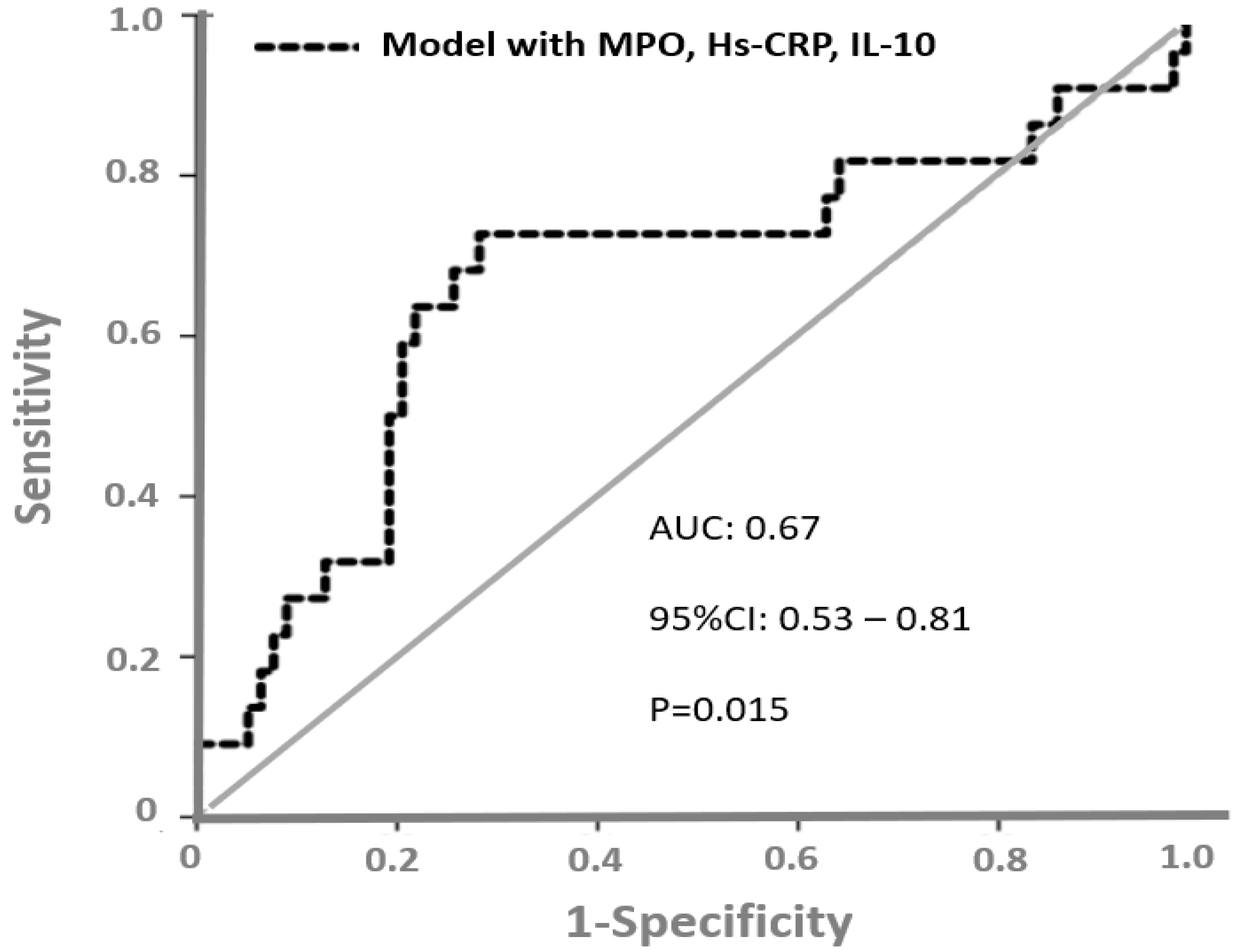
The authors found that MPO and IL-10 were independent predictors of high troponin and Hs-CRP predicted low EF. To determine patients with the worst cardiac outcome (assessed by low EF and high troponin), the authors constructed a predictive risk model including MPO, IL-10 and Hs-CRP. A ROC curve analysis (
Figure 4) showed that the AUC of this risk predictive model to discriminate patients with the worst cardiac outcome was 0.67 (95%CI = 0.53–0.81,
p = 0.015).
In another paper [
11], the authors investigated the relationship between cardiac natriuretic peptides (atrial natriuretic peptide [ANP] and brain natriuretic peptide [BNP]) and norepinephrine (NE) with LAV changes over time in 199 dialysis patients. The ROC curve area for predicting the left atrial volume (LAV) changes (>3 mL/per year) of a model based on standard risk factors was 0.72. Plasma BNP (+12%;
p = 0.004), ANP (+8%;
p = 0.03), NE (+8%;
p = 0.05) and midwall fraction shortening (+8%;
p = 0.05) increased the area under the ROC curve to a significant extent. The authors concluded that BNP and ANP predict LAV changes over time in dialysis patients and that the measurement of the plasma concentration of these compounds might be useful for guiding treatment in this patient population.