Dl-3-n-Butylphthalide Reduced Neuroinflammation by Inhibiting Inflammasome in Microglia in Mice after Middle Cerebral Artery Occlusion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Establishment of the tMCAO Mouse Model
2.3. Neurobehavioral Tests Determination
2.4. Brain Infarct Volume Measurement
2.5. Immunohistochemical Staining
2.6. Western Blotting Analysis
2.7. Real-Time PCR
2.8. Cell Culture
2.9. Oxygen-Glucose Deprivation (OGD)
2.10. Cell Proliferation and Cytotoxicity Assay
2.11. Statistical Analysis
3. Results
3.1. NBP Reduced Infarct Volume and Neurological Behavior in tMCAO Mice
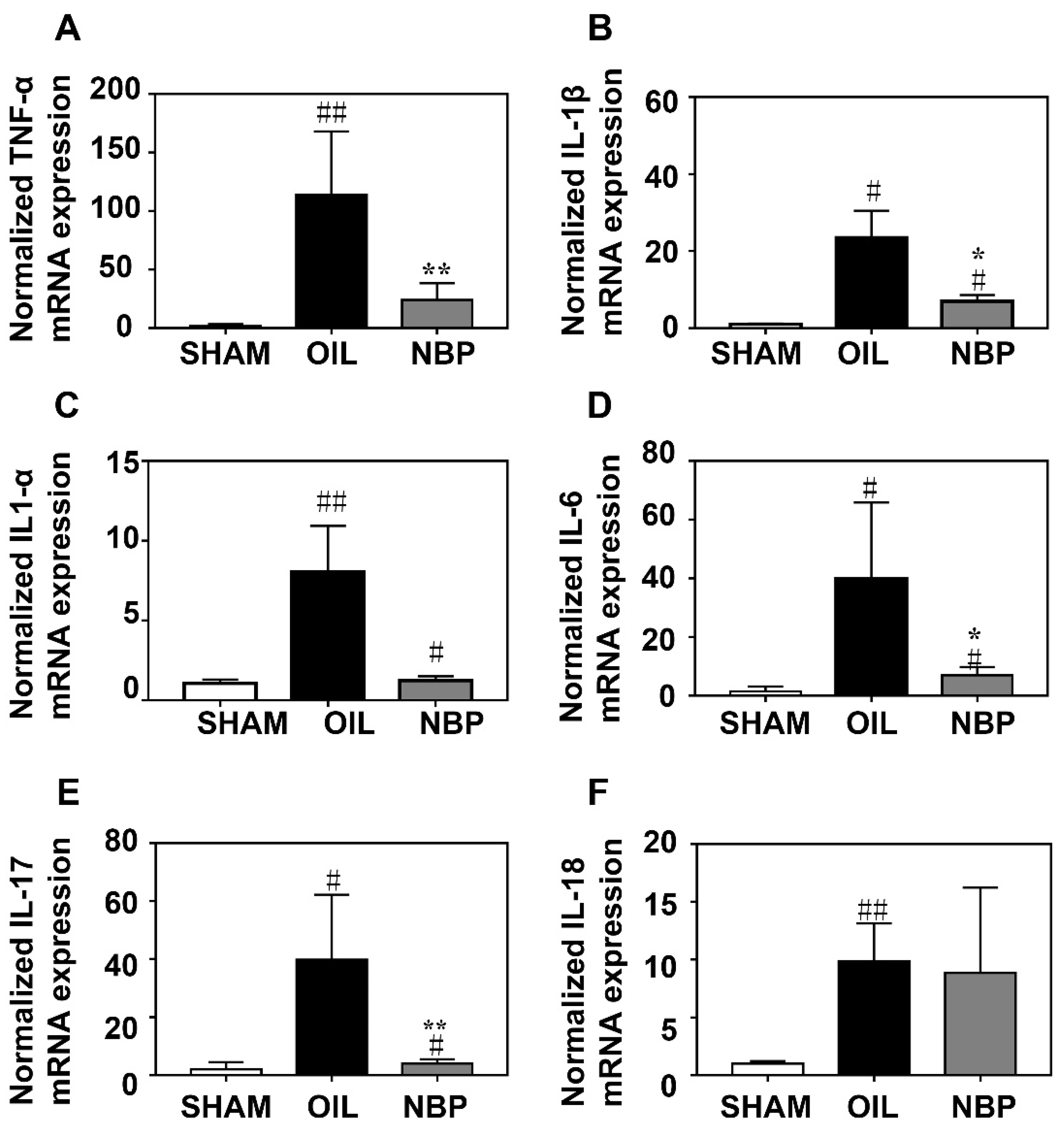
3.2. NBP Attenuated the Inflammatory Factor Expression after tMCAO
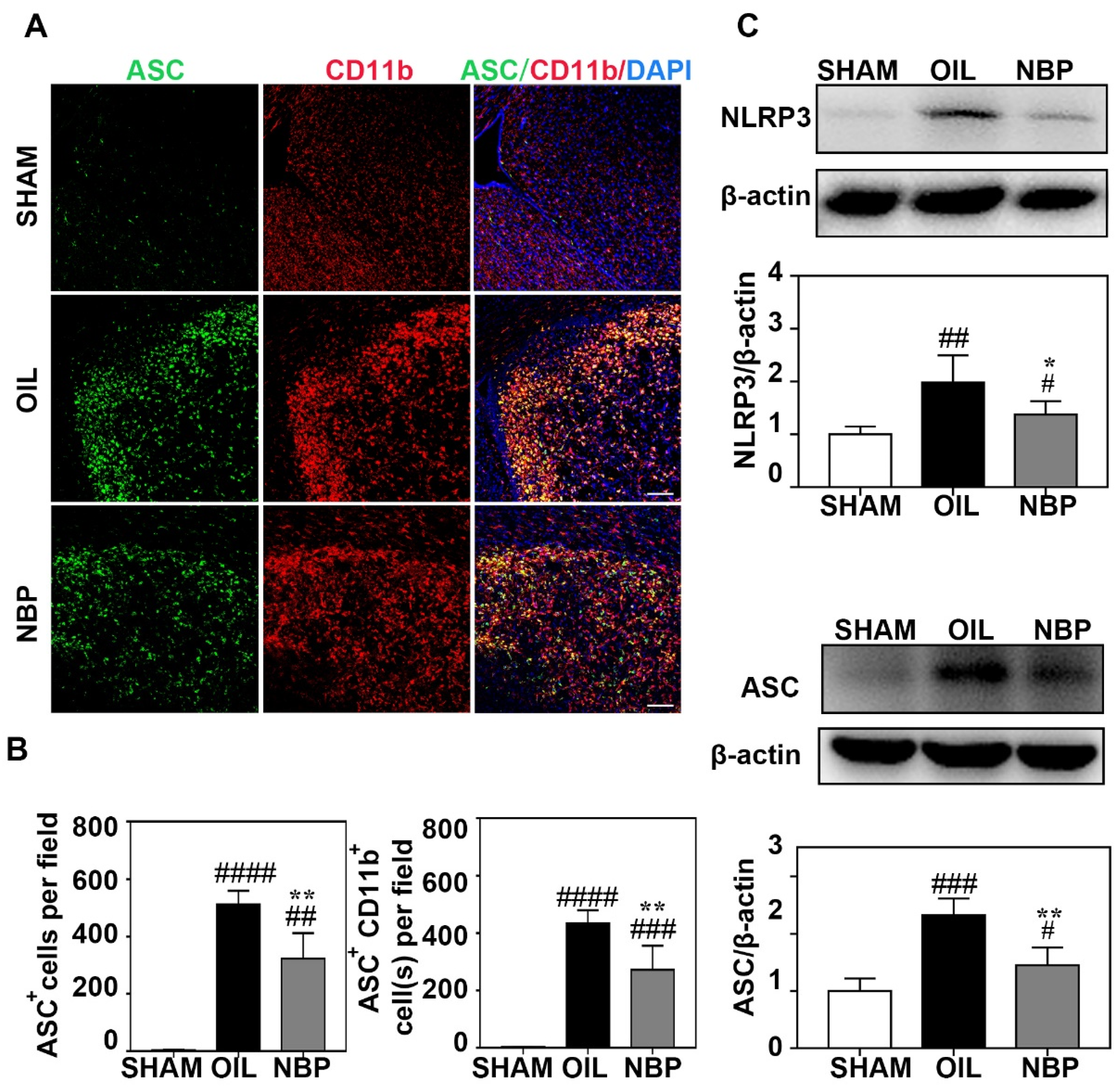
3.3. NBP Inhibited NLRP3 Inflammasome Expression in Microglia
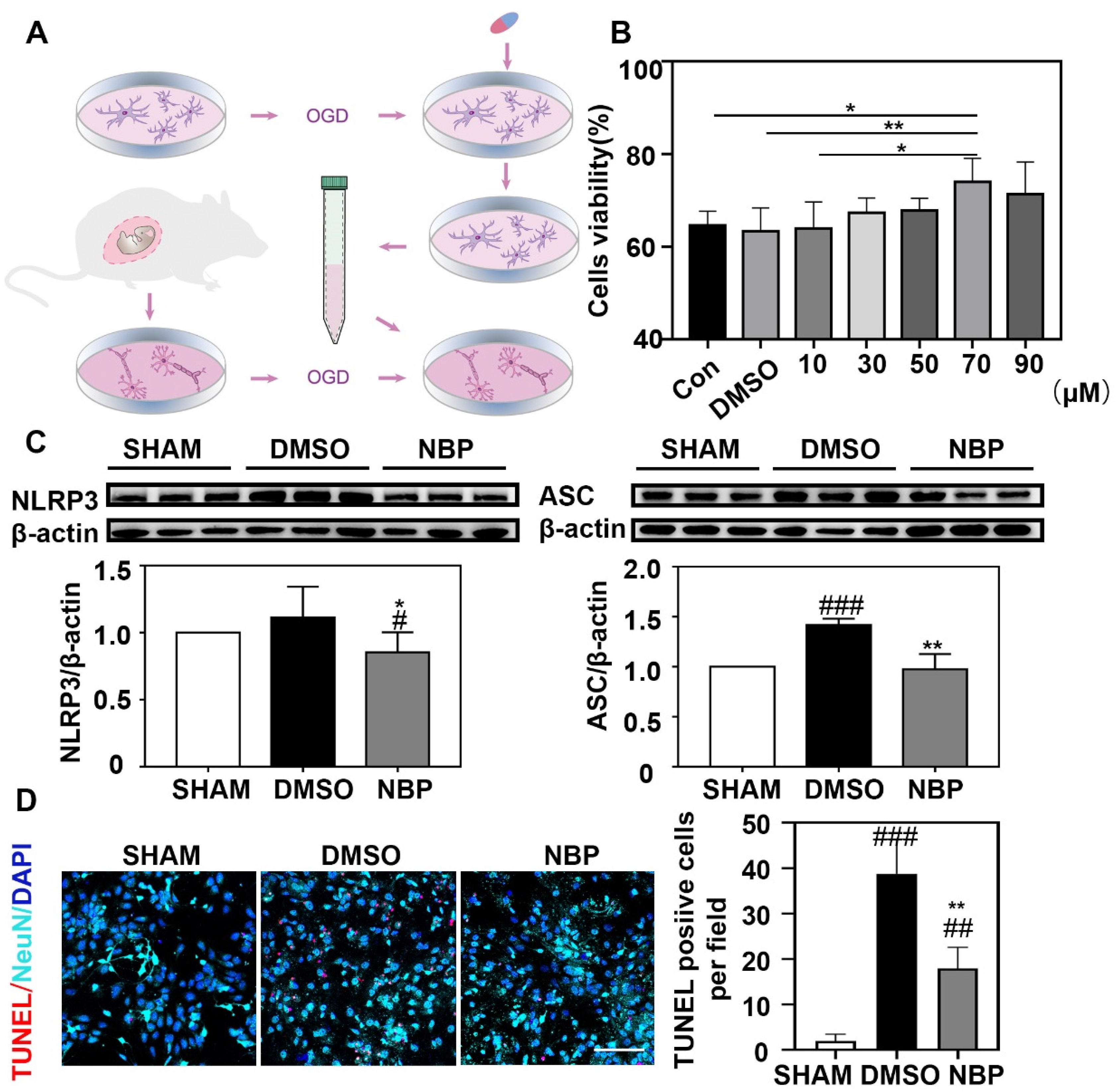
3.4. NBP Treatment Reduced CASP1 Expression in Neurons
3.5. NBP Treatment Indirectly Reduced the Apoptosis of Neurons In Vitro
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ma, Q.; Li, R.; Wang, L.; Yin, P.; Wang, Y.; Yan, C.; Ren, Y.; Qian, Z.; Vaughn, M.; McMillin, S.; et al. Temporal trend and attributable risk factors of stroke burden in China, 1990–2019: An analysis for the Global Burden of Disease Study 2019. Lancet Public Health 2021, 6, e897–e906. [Google Scholar] [CrossRef]
- Naghavi, M.; Abajobir, A.A.; Abbafati, C.; Abbas, K.M.; Abd-Allah, F.; Abera, S.F.; Aboyans, V.; Adetokunboh, O.; Afshin, A.; Agrawal, A.; et al. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1151–1210. [Google Scholar] [CrossRef]
- Khoshnam, S.E.; Winlow, W.; Farzaneh, M.; Farbood, Y.; Moghaddam, H.F. Pathogenic mechanisms following ischemic stroke. Neurol. Sci. 2017, 38, 1167–1186. [Google Scholar] [CrossRef] [PubMed]
- Albers, G.W.; Bates, V.E.; Clark, W.M.; Bell, R.; Verro, P.S.A. Intravenous tissue-type plasminogen activator for treatment of acute stroke: The Standard Treatment with Alteplase to Reverse Stroke (STARS) study. JAMA 2000, 283, 1145–1150. [Google Scholar] [CrossRef] [PubMed]
- Goyal, M.; Menon, B.K.; van Zwam, W.H.; Dippel, D.W.; Mitchell, P.J.; Demchuk, A.M.; Dávalos, A.; Majoie, C.B.; van der Lugt, A.; de Miquel, M.A.; et al. Endovascular thrombectomy after large-vessel ischaemic stroke: A meta-analysis of individual patient data from five randomised trials. Lancet 2016, 387, 1723–1731. [Google Scholar] [CrossRef]
- Qin, C.; Zhou, L.Q.; Ma, X.T.; Hu, Z.W.; Yang, S.; Chen, M.; Bosco, D.B.; Wu, L.J.; Tian, D.S. Dual Functions of Microglia in Ischemic Stroke. Neurosci. Bull. 2019, 35, 921–933. [Google Scholar] [CrossRef] [PubMed]
- Amantea, D.; Nappi, G.; Bernardi, G.; Bagetta, G.; Corasaniti, M.T. Post-ischemic brain damage: Pathophysiology and role of inflammatory mediators. FEBS J. 2009, 276, 13–26. [Google Scholar] [CrossRef]
- Arumugam, T.V.; Selvaraj, P.K.; Woodruff, T.M.; Mattson, M.P. Targeting ischemic brain injury with intravenous immunoglobulin. Expert Opin. Ther. Targets 2008, 12, 19–29. [Google Scholar] [CrossRef]
- Abulafia, D.P.; Vaccari, J.P.d.; Lozano, J.D.; Lotocki, G.; Keane, R.W.; Dietrich, W.D. Inhibition of the inflammasome complex reduces the inflammatory response after thromboembolic stroke in mice. J. Cereb. Blood Flow Metab. 2009, 29, 534–544. [Google Scholar] [CrossRef]
- Wu, A.G.; Zhou, X.G.; Qiao, G.; Yu, L.; Tang, Y.; Yan, L.; Qiu, W.Q.; Pan, R.; Yu, C.L.; Law, B.Y.; et al. Targeting microglial autophagic degradation in NLRP3 inflammasome-mediated neurodegenerative diseases. Ageing Res. Rev. 2021, 65, 101202. [Google Scholar] [CrossRef]
- Herman, F.J.; Pasinetti, G.M. Principles of inflammasome priming and inhibition: Implications for psychiatric disorders. Brain Behav. Immun. 2018, 73, 66–84. [Google Scholar] [CrossRef] [PubMed]
- Shichita, T.; Ito, M.; Yoshimura, A. Post-ischemic inflammation regulates neural damage and protection. Front. Cell Neurosci. 2014, 8, 319. [Google Scholar] [CrossRef]
- Frank, M.G.; Weber, M.D.; Fonken, L.K.; Hershman, S.A.; Watkins, L.R.; Maier, S.F. The redox state of the alarmin HMGB1 is a pivotal factor in neuroinflammatory and microglial priming: A role for the NLRP3 inflammasome. Brain Behav. Immun. 2016, 55, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yamashita, T.; Kurata, T.; Kono, S.; Hishikawa, N.; Deguchi, K.; Zhai, Y.; Abe, K. Protective effect of telmisartan on neurovascular unit and inflammasome in stroke-resistant spontaneously hypertensive rats. Neurol. Res. 2015, 37, 491–501. [Google Scholar] [CrossRef]
- Bergsbaken, T.; Fink, S.L.; Cookson, B.T. Pyroptosis: Host cell death and inflammation. Nat. Rev. Microbiol. 2009, 7, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Tan, Z.; Wang, M.; Xing, Y.; Dong, F.; Zhang, F. Inhibition of NLRP3 Inflammasome: A Prospective Target for the Treatment of Ischemic Stroke. Front. Cell. Neurosci. 2020, 14, 155. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Sun, M.; Li, R.; Tao, Y.; Luo, X.; Xu, J.; Wu, X.; Xie, Z. Activation of RKIP Binding ASC Attenuates Neuronal Pyroptosis and Brain Injury via Caspase-1/GSDMD Signaling Pathway after Intracerebral Hemorrhage in Mice. Transl. Stroke Res. 2022; Online ahead of print. [Google Scholar] [CrossRef]
- Xu, Z.Q.; Zhou, Y.; Shao, B.Z.; Zhang, J.J.; Liu, C. A Systematic Review of Neuroprotective Efficacy and Safety of DL-3-N-Butylphthalide in Ischemic Stroke. Am. J. Chin. Med. 2019, 47, 507–525. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Yun, W.; Zhang, Q.; Cai, X.; Li, X.; Hui, G.; Zhou, X. Mobilization of Circulating Endothelial Progenitor Cells by dl-3-n-Butylphthalide in Acute Ischemic Stroke Patients. J. Stroke Cerebrovasc. Dis. 2016, 25, 752–760. [Google Scholar] [CrossRef]
- Wang, S.; Ma, F.; Huang, L.; Zhang, Y.; Peng, Y.; Xing, C.; Feng, Y.; Wang, X.; Peng, Y. Dl-3-n-Butylphthalide (NBP): A Promising Therapeutic Agent for Ischemic Stroke. CNS Neurol. Disord. Drug Targets 2018, 17, 338–347. [Google Scholar] [CrossRef]
- Mamtilahun, M.; Wei, Z.; Qin, C.; Wang, Y.; Tang, Y.; Shen, F.; Tian, H.; Zhang, Z.; Yang, G. DL-3n-Butylphthalide Improves Blood-Brain Barrier Integrity in Rat After Middle Cerebral Artery Occlusion. Front. Cell. Neurosci. 2020, 14, 610714. [Google Scholar] [CrossRef]
- Yang, G.Y. Advancement in stroke research. Stroke Vasc. Neurol. 2019, 4, 61–62. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Xu, Y.; Wang, X.; Guo, C. Dl-3-n-Butylphthalide Inhibits NLRP3 Inflammasome and Mitigates Alzheimer’s-Like Pathology via Nrf2-TXNIP-TrX Axis. Antioxid. Redox Signal. 2019, 30, 1411–1431. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, R.; Fu, D.; Wu, H.; Zhao, X.; Sun, Y.; Wang, M.; Pu, X. Dl-3-n-butylphthalide inhibits neuroinflammation by stimulating foxp3 and Ki-67 in an ischemic stroke model. Aging 2021, 13, 3763–3778. [Google Scholar] [CrossRef]
- Zhang, L.; Pan, J.; Mamtilahun, M.; Zhu, Y.; Wang, L.; Venkatesh, A.; Shi, R.; Tu, X.; Jin, K.; Wang, Y.; et al. Microglia exacerbate white matter injury via complement C3/C3aR pathway after hypoperfusion. Theranostics 2020, 10, 74–90. [Google Scholar] [CrossRef] [PubMed]
- Mamtilahun, M.; Jiang, L.; Song, Y.; Shi, X.; Liu, C.; Jiang, Y.; Deng, L.; Zheng, H.; Shen, H.; Li, Y.; et al. Plasma from healthy donors protects blood-brain barrier integrity via FGF21 and improves the recovery in a mouse model of cerebral ischaemia. Stroke Vasc. Neurol. 2021, 6, 561–571. [Google Scholar] [CrossRef]
- Wang, J.; Li, G.; Deng, L.; Mamtilahun, M.; Jiang, L.; Qiu, W.; Zheng, H.; Sun, J.; Xie, Q.; Yang, G.Y. Transcranial Focused Ultrasound Stimulation Improves Neurorehabilitation after Middle Cerebral Artery Occlusion in Mice. Aging Dis. 2021, 12, 50–60. [Google Scholar] [CrossRef]
- Li, W.; He, T.; Shi, R.; Song, Y.; Wang, L.; Zhang, Z.; Tang, Y.; Yang, G.Y.; Wang, Y. Oligodendrocyte Precursor Cells Transplantation Improves Stroke Recovery via Oligodendrogenesis, Neurite Growth and Synaptogenesis. Aging Dis. 2021, 12, 2096–2112. [Google Scholar] [CrossRef]
- Singer, C.A.; Figueroa-Masot, X.A.; Batchelor, R.H.; Dorsa, D.M. The mitogen-activated protein kinase pathway mediates estrogen neuroprotection after glutamate toxicity in primary cortical neurons. J. Neurosci. 1999, 19, 2455–2463. [Google Scholar] [CrossRef]
- Luo, R.; Wangqin, R.; Zhu, L.; Bi, W. Neuroprotective mechanisms of 3-n-butylphthalide in neurodegenerative diseases. Biomed. Rep. 2019, 11, 235–240. [Google Scholar] [CrossRef]
- Swanson, K.V.; Deng, M.; Ting, J.P. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef]
- Alishahi, M.; Farzaneh, M.; Ghaedrahmati, F.; Nejabatdoust, A.; Sarkaki, A.; Khoshnam, S. NLRP3 inflammasome in ischemic stroke: As possible therapeutic target. Int. J. Stroke Off. J. Int. Stroke Soc. 2019, 14, 574–591. [Google Scholar] [CrossRef] [PubMed]
- Fricker, M.; Tolkovsky, A.M.; Borutaite, V.; Coleman, M.; Brown, G.C. Neuronal Cell Death. Physiol. Rev. 2018, 98, 813–880. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Dong, Q.; Song, Z.; Shen, F.; Shi, J.; Li, Y. Advances in nanocarriers enabled brain targeted drug delivery across blood brain barrier. Int. J. Pharm. 2019, 559, 360–372. [Google Scholar]
- Gao, L.; Dong, Q.; Song, Z.; Shen, F.; Shi, J. NLRP3 inflammasome: A promising target in ischemic stroke. Inflamm. Res. 2017, 66, 17–24. [Google Scholar] [CrossRef]
- Shao, B.Z.; Xu, Z.Q.; Han, B.Z.; Su, D.F.; Liu, C. NLRP3 inflammasome and its inhibitors: A review. Front. Pharmacol. 2015, 6, 262. [Google Scholar] [CrossRef]
- Que, R.; Zheng, J.; Chang, Z.; Zhang, W.; Li, H.; Xie, Z.; Huang, Z.; Wang, H.; Xu, J.; Jin, D.; et al. Dl-3-n-Butylphthalide Rescues Dopaminergic Neurons in Parkinson’s Disease Models by Inhibiting the NLRP3 Inflammasome and Ameliorating Mitochondrial Impairment. Front. Immunol. 2021, 12, 794770. [Google Scholar] [CrossRef]
- Bai, J. Clinical efficacy and safety of urinary kallindinogenase combined with butylphthalide in the treatment of progressive cerebral infarction. Am. J. Transl. Res. 2021, 13, 13909–13915. [Google Scholar]
- Broderick, L.; de Nardo, D.; Franklin, B.S.; Hoffman, H.M.; Latz, E. The inflammasomes and autoinflammatory syndromes. Annu. Rev. Pathol. 2015, 10, 395–424. [Google Scholar] [CrossRef]
- Datta, A.; Sarmah, D.; Mounica, L.; Kaur, H.; Kesharwani, R.; Verma, G.; Veeresh, P.; Kotian, V.; Kalia, K.; Borah, A.; et al. Cell Death Pathways in Ischemic Stroke and Targeted Pharmacotherapy. Transl. Stroke Res. 2020, 11, 1185–1202. [Google Scholar] [CrossRef]
- Franklin, B.S.; Bossaller, L.; de Nardo, D.; Ratter, J.M.; Stutz, A.; Engels, G.; Brenker, C.; Nordhoff, M.; Mirandola, S.R.; Al-Amoudi, A.; et al. The adaptor ASC has extracellular and ‘prionoid’ activities that propagate inflammation. Nat. Immunol. 2014, 15, 727–737. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, H.; Kouadir, M.; Song, H.; Shi, F. Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell Death Dis. 2019, 10, 128. [Google Scholar] [CrossRef] [PubMed]


| Primer | Sequence (5′-3′) |
|---|---|
| TNF-α-F | TAGCCAGGAGGGAGAACAGA |
| TNF-α-R | CCAGTGAGTGAAAGGGACAGA |
| IL-1α-F | CGCTTGAGTCGGCAAAGAAAT |
| IL-1α-R | CTTCCCGTTGCTTGACGTTG |
| IL-1β-F | TACATCAGCACCTCACAAGC |
| IL-1β-R | AGAAACAGTCCAGCCCATACT |
| GAPDH-F | AAATGGTGAAGGTCGGTGTG |
| GAPDH-R | AGGTCAATGAAGGGGTCGTT |
| IL-6-F | ACCAAGACCATCCAATTCATC |
| IL-6-R | CTGACCACAGTGAGGAATGTC |
| IL-17-F | GTTCGTGCTATTGATTTTCAGC |
| IL-17-R | GGACCCCTTTACACCTTCTTT |
| IL-18-F | AGGACACTTTCTTGCTTGCCA |
| IL-18-R | CACAAACCCTCCCCACCTAAC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; Zheng, H.; Liu, Z.; Guo, Y.; Wang, S.; Tang, Y.; Tian, H.; Zhang, Z.; Yang, G. Dl-3-n-Butylphthalide Reduced Neuroinflammation by Inhibiting Inflammasome in Microglia in Mice after Middle Cerebral Artery Occlusion. Life 2022, 12, 1244. https://doi.org/10.3390/life12081244
Liu M, Zheng H, Liu Z, Guo Y, Wang S, Tang Y, Tian H, Zhang Z, Yang G. Dl-3-n-Butylphthalide Reduced Neuroinflammation by Inhibiting Inflammasome in Microglia in Mice after Middle Cerebral Artery Occlusion. Life. 2022; 12(8):1244. https://doi.org/10.3390/life12081244
Chicago/Turabian StyleLiu, Mengdi, Haoran Zheng, Ze Liu, Yiyan Guo, Shuhong Wang, Yaohui Tang, Hengli Tian, Zhijun Zhang, and Guoyuan Yang. 2022. "Dl-3-n-Butylphthalide Reduced Neuroinflammation by Inhibiting Inflammasome in Microglia in Mice after Middle Cerebral Artery Occlusion" Life 12, no. 8: 1244. https://doi.org/10.3390/life12081244
APA StyleLiu, M., Zheng, H., Liu, Z., Guo, Y., Wang, S., Tang, Y., Tian, H., Zhang, Z., & Yang, G. (2022). Dl-3-n-Butylphthalide Reduced Neuroinflammation by Inhibiting Inflammasome in Microglia in Mice after Middle Cerebral Artery Occlusion. Life, 12(8), 1244. https://doi.org/10.3390/life12081244







