Sublingual Vaccination with Live Influenza Virus Induces Better Protection Than Oral Immunization in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Cells and Viruses
2.3. Primary Administration of the Virus into Mice and Challenge Infection
2.4. Sample Collection and Preparation
2.5. Lung Viral Titer and Inflammatory Cytokine Responses
2.6. Antibody Responses and Hemagglutination Inhibition (HAI) Titer
2.7. Flow Cytometry
2.8. Statistical Analysis
3. Results
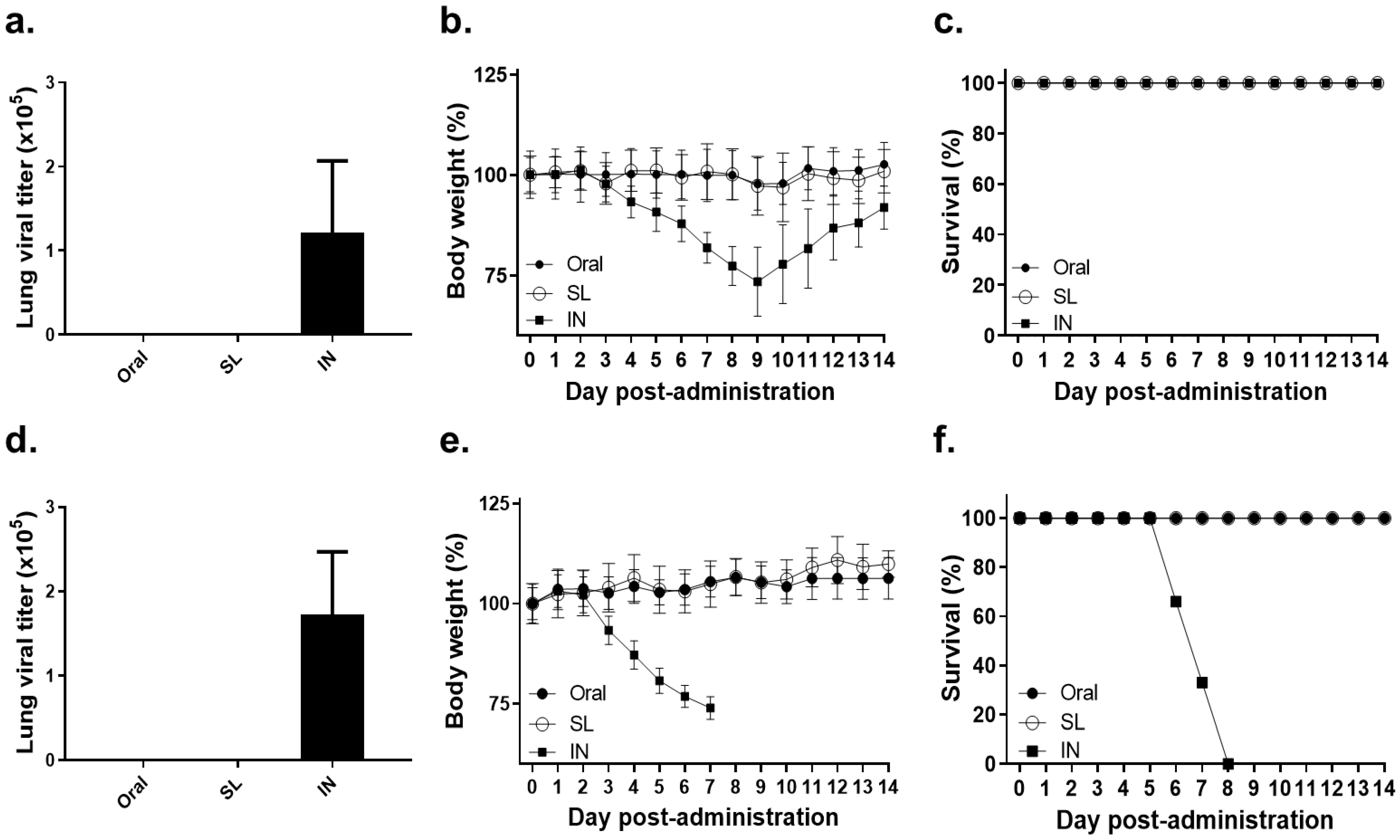
3.1. Lethal Dose of Influenza Virus Administration by SL or Oral Route Showed No Virus Replication and No Bodyweight Loss
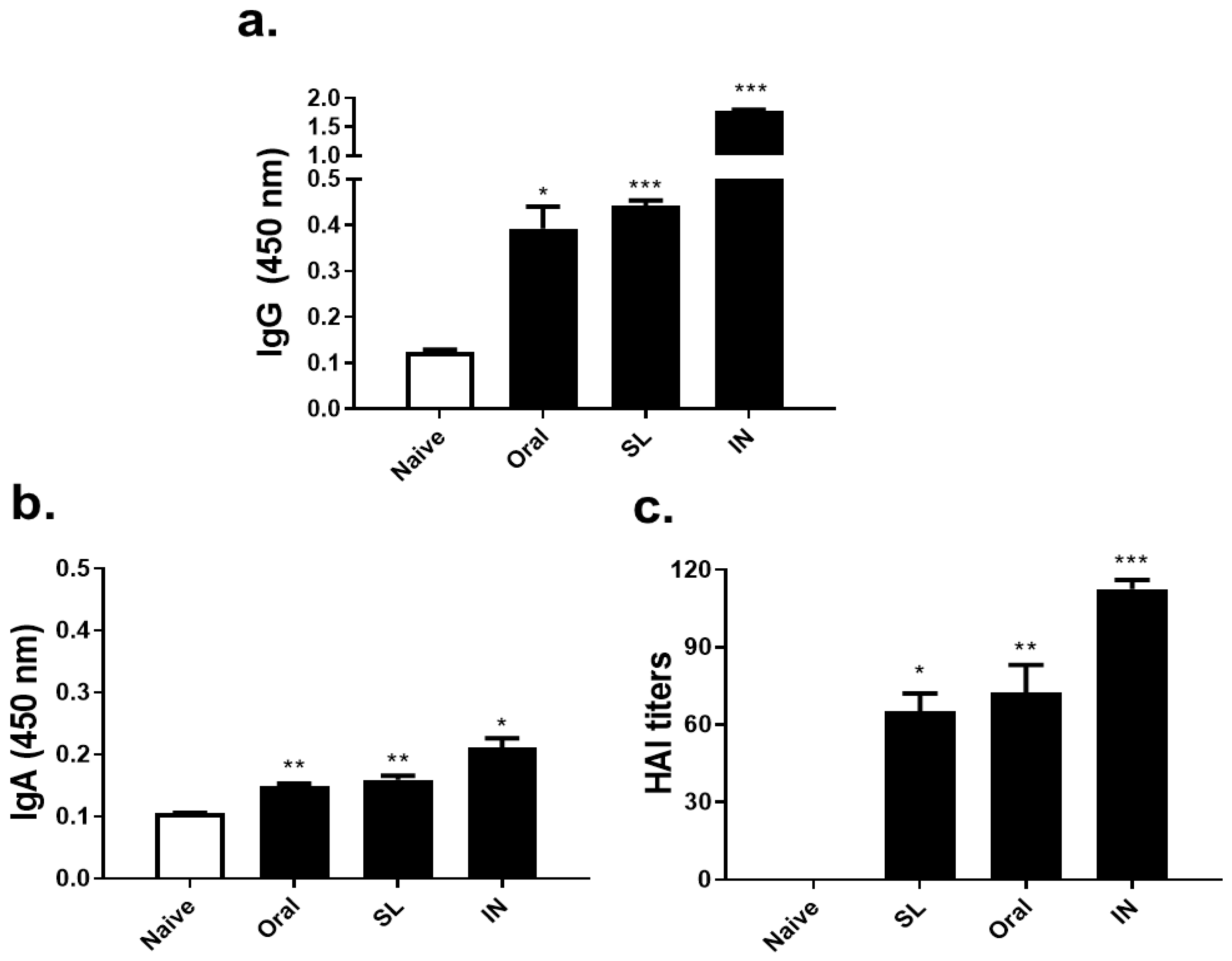
3.2. SL or Oral Administrations Elicited Virus-Specific Antibody Responses and HAI Titer
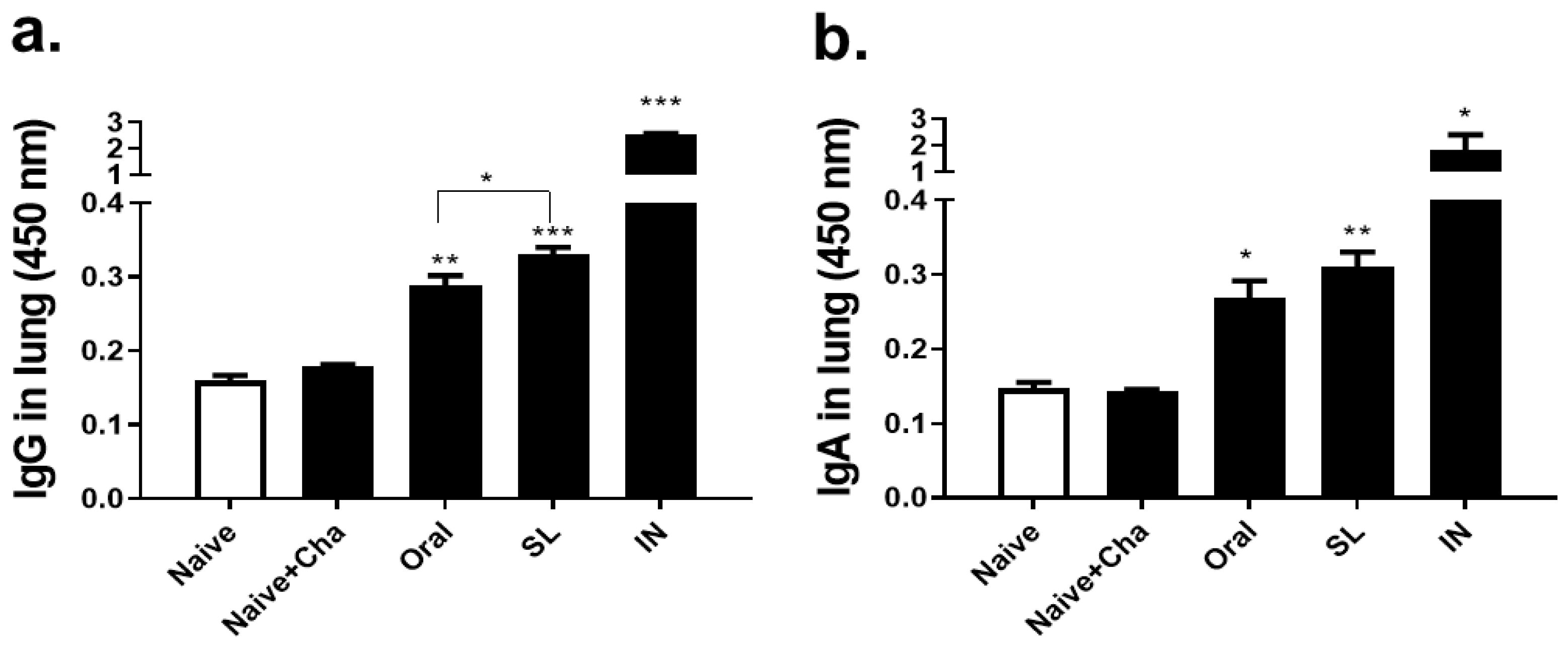
3.3. SL and Oral Virus Inoculation Induced Virus-Specific Antibody Responses in the Lungs
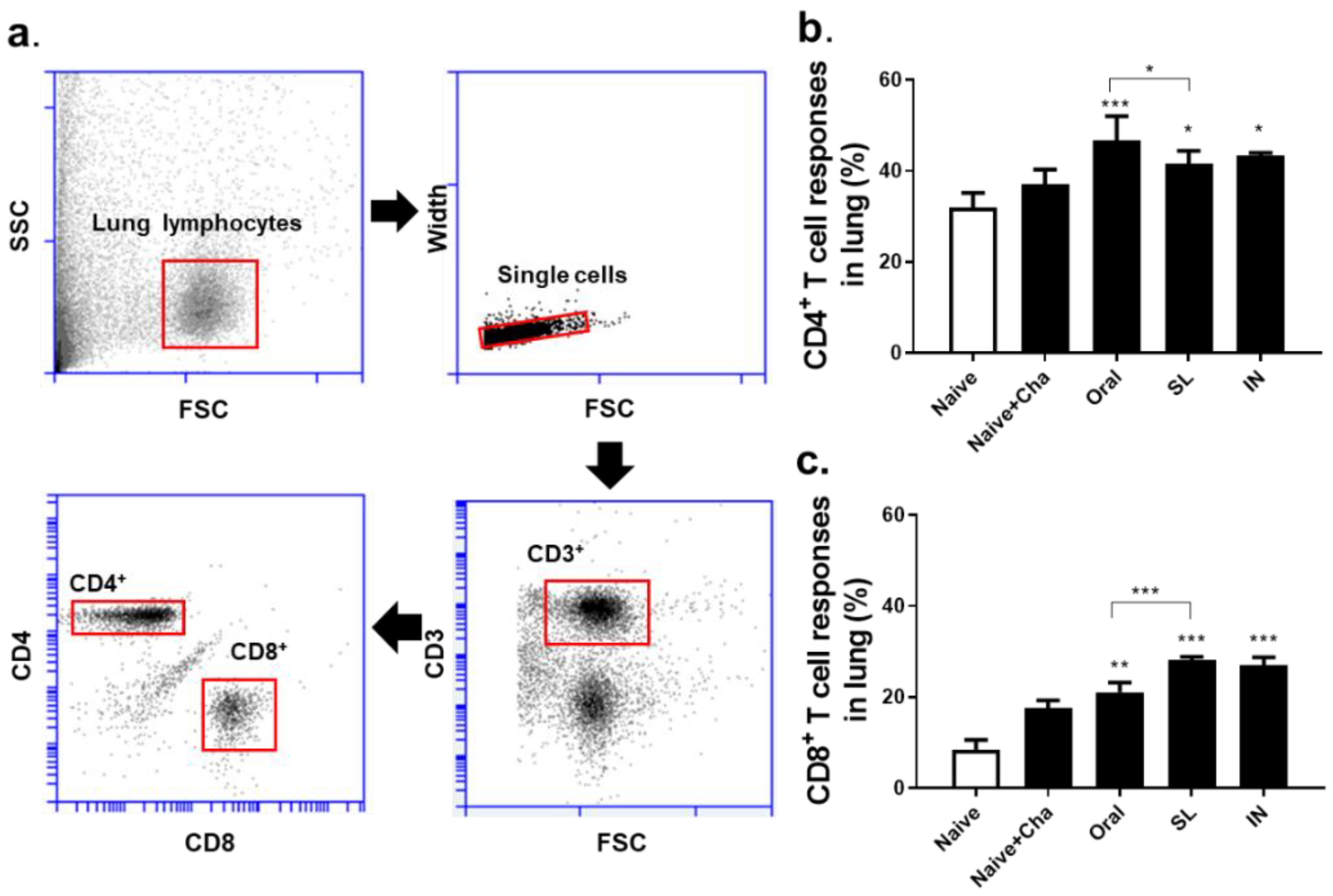
3.4. SL and Oral Administrations Induced Cellular Immune Responses in the Lungs
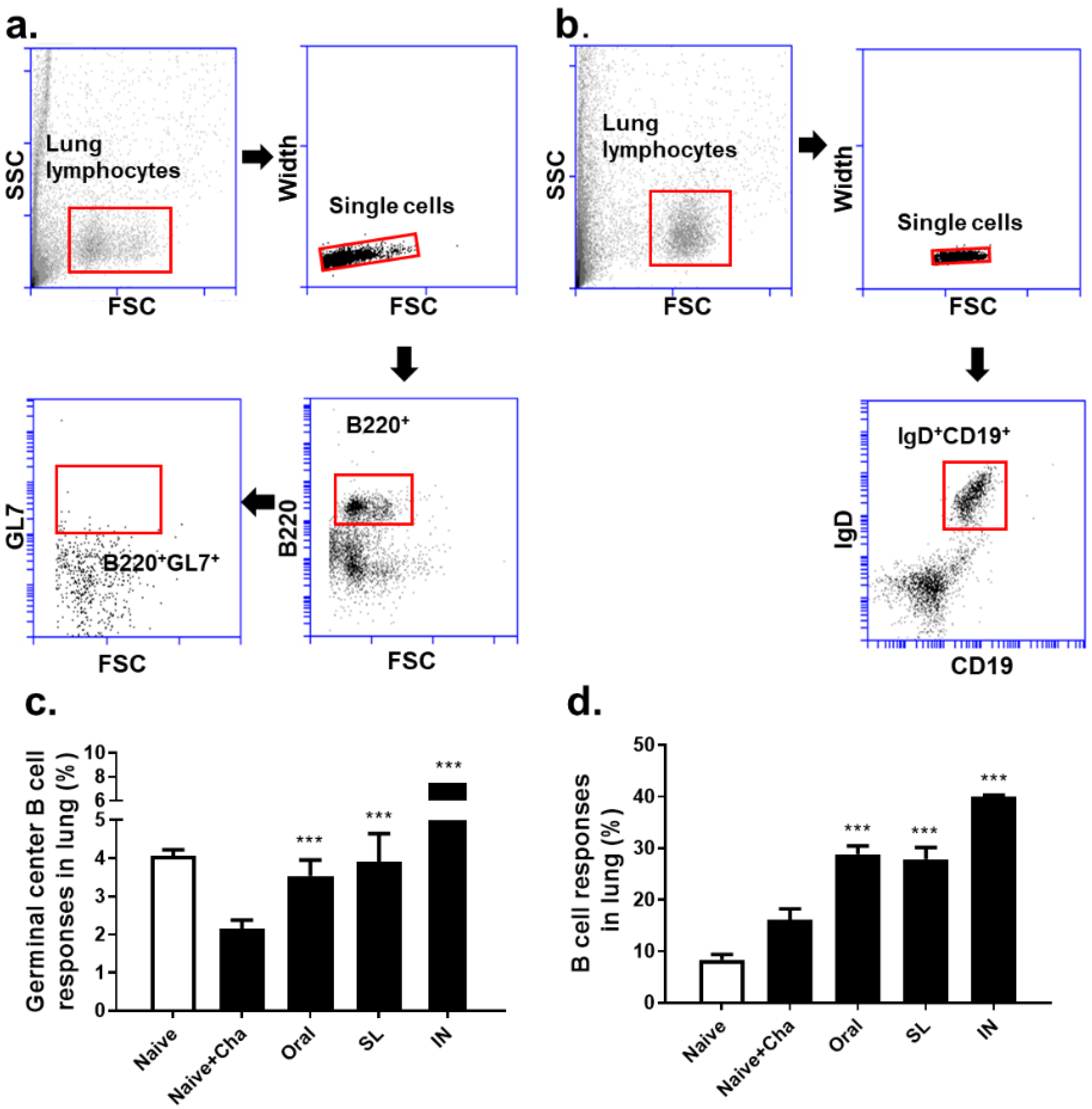
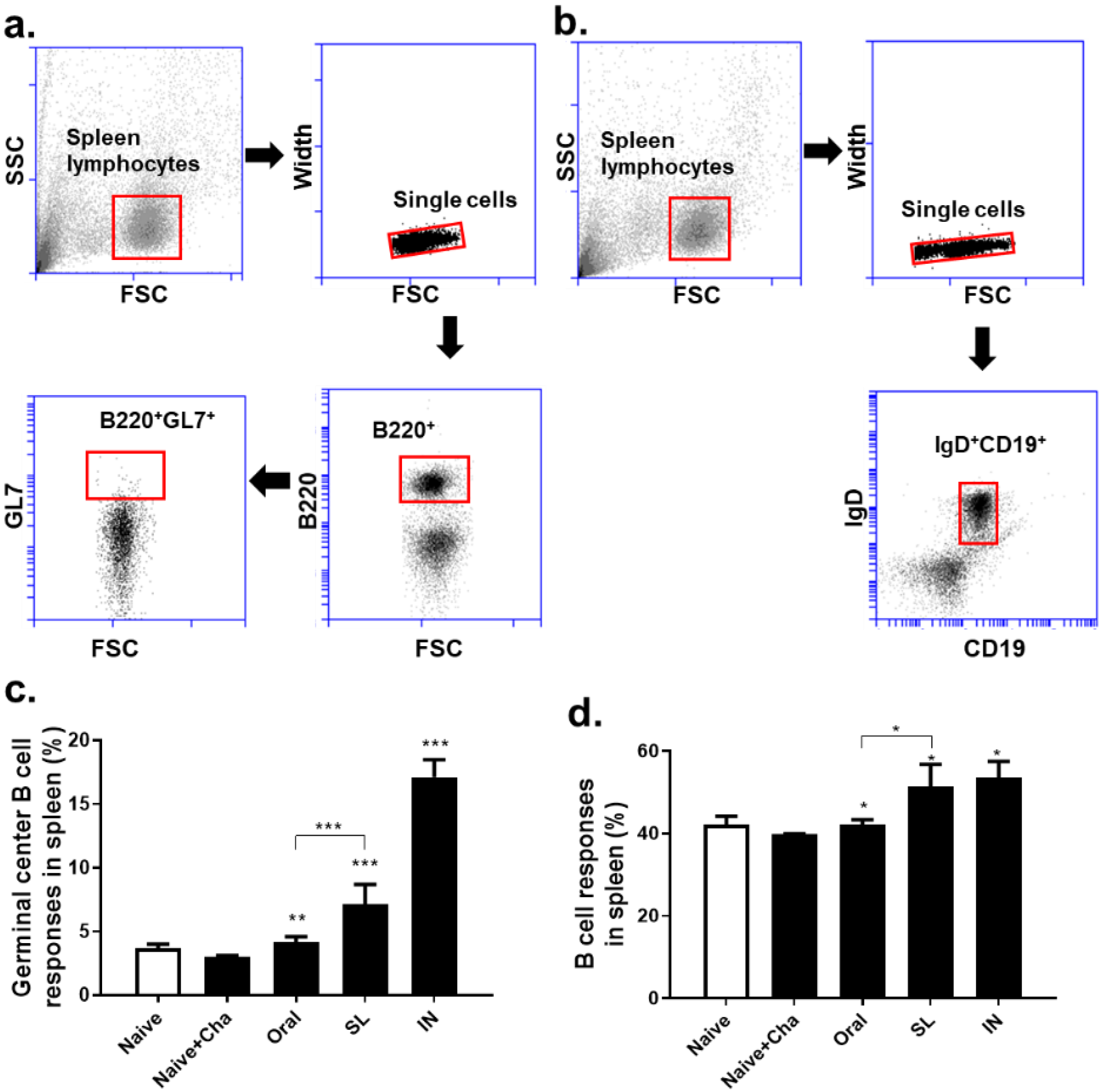
3.5. SL and Oral Administrations Evoked GC B Cell and B Cell Responses in Lungs and Spleens
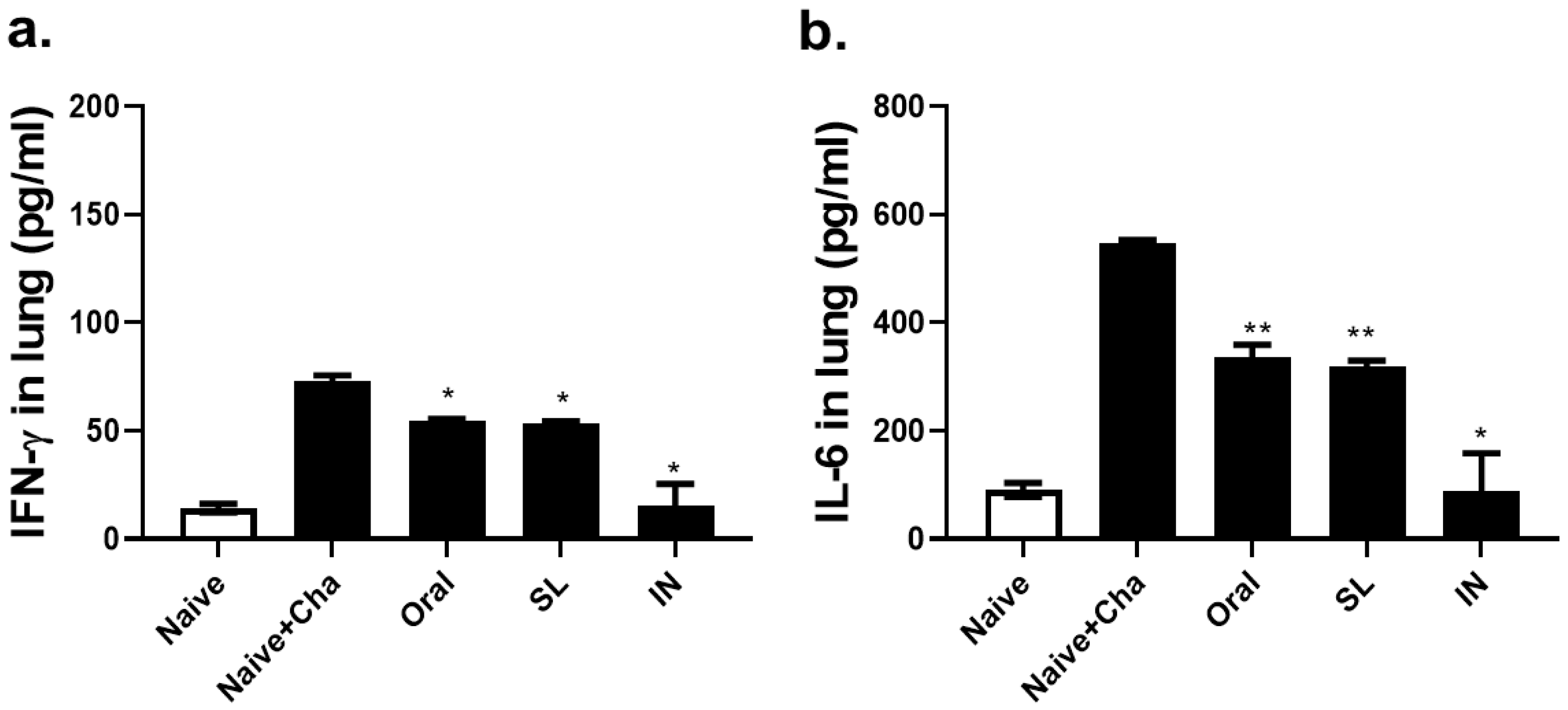
3.6. SL and Oral Administrations Lessened the Lung Inflammatory Responses
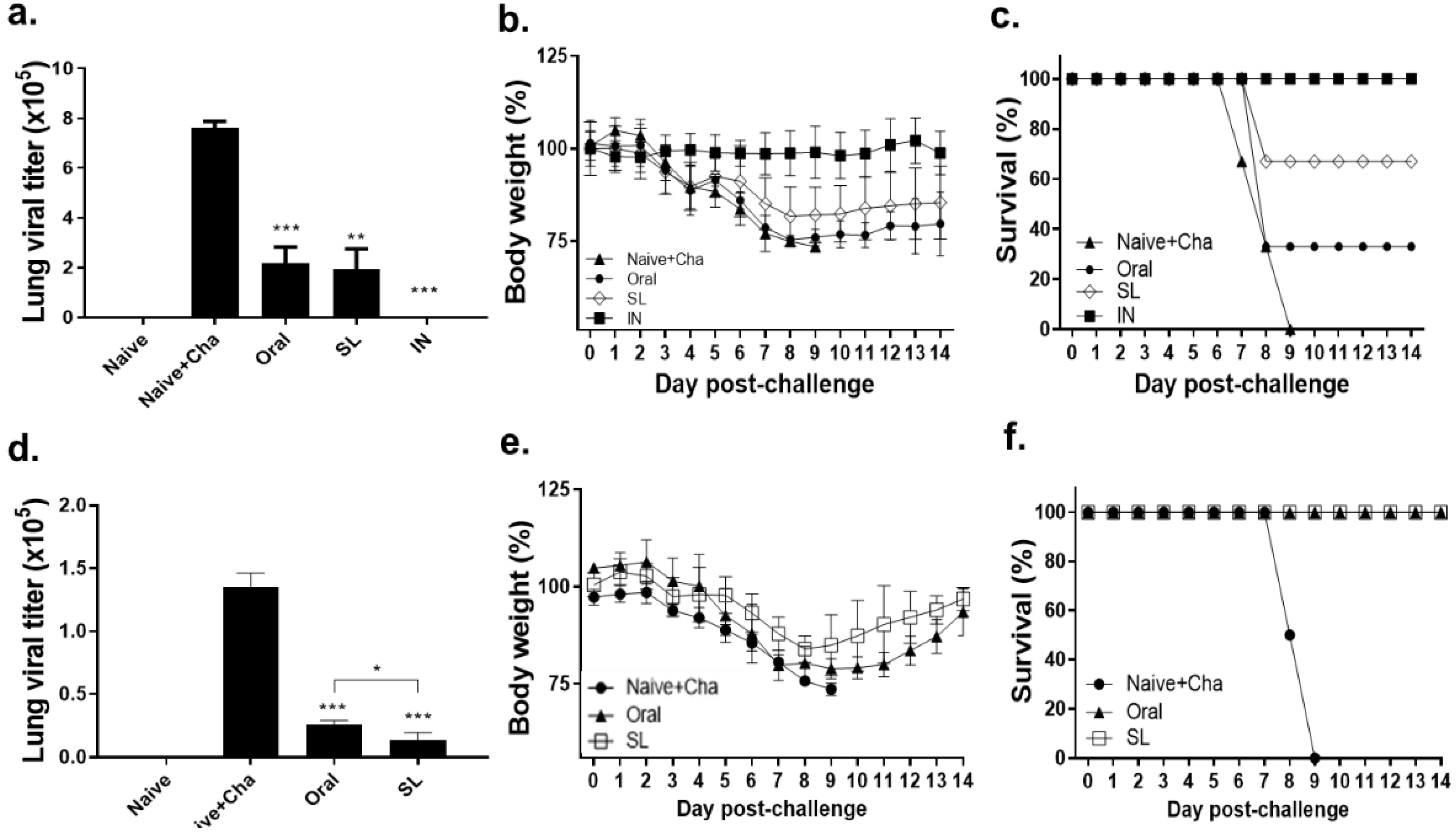
3.7. SL and Oral Immunization Diminished Lung Viral Load and Fully Protected Mice against a Low Dose of Infection but Conferred Partial Protection against a High Dose of Infection
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kang, S.M.; Yoo, D.G.; Lipatov, A.S.; Song, J.M.; Davis, C.T.; Quan, F.S.; Chen, L.M.; Donis, R.O.; Compans, R.W. Induction of long-term protective immune responses by influenza H5N1 virus-like particles. PLoS ONE 2009, 4, e4667. [Google Scholar] [CrossRef] [PubMed]
- Krammer, F. The human antibody response to influenza A virus infection and vaccination. Nat. Rev. Immunol. 2019, 19, 383–397. [Google Scholar] [CrossRef] [PubMed]
- Durando, P.; Iudici, R.; Alicino, C.; Alberti, M.; de Florentis, D.; Ansaldi, F.; Icardi, G. Adjuvants and alternative routes of administration towards the development of the ideal influenza vaccine. Hum. Vaccin. 2011, 7, 29–40. [Google Scholar] [CrossRef] [Green Version]
- Mutsch, M.; Zhou, W.G.; Rhodes, P.; Bopp, M.; Chen, R.T.; Linder, T.; Spyr, C.; Steffen, R. Use of the Inactivated Intranasal Influenza Vaccine and the Risk of Bell’s Palsy in Switzerland. N. Engl. J. Med. 2004, 350, 896–903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shinya, K.; Shimada, A.; Ito, T.; Otsuki, K.; Morita, T.; Tanaka, H.; Takada, A.; Kida, H.; Umemura, T. Avian influenza virus intranasally inoculated infects the central nervous system of mice through the general visceral afferent nerve. Arch. Virol. 2000, 145, 187–195. [Google Scholar] [CrossRef]
- Holmgren, J.; Czerkinsky, C. Mucosal immunity and vaccines. Nat. Med. 2005, 11, S45–S53. [Google Scholar] [CrossRef]
- Trincado, V.; Gala, R.P.; Morales, J.O. Buccal and Sublingual Vaccines: A Review on Oral Mucosal Immunization and Delivery Systems. Vaccines 2021, 9, 1177. [Google Scholar] [CrossRef]
- Czerkinsky, C.; Cuburu, N.; Kweon, M.N.; Anjuere, F.; Holmgren, J. Sublingual vaccination. Hum. Vaccin. 2011, 7, 110–114. [Google Scholar] [CrossRef] [Green Version]
- Song, J.H.; Nguyen, H.H.; Cuburu, N.; Horimoto, T.; Ko, S.Y.; Park, S.H.; Czerkinsky, C.; Kweon, M.N. Sublingual vaccination with influenza virus protects mice against lethal viral infection. Proc. Natl. Acad. Sci. USA 2008, 105, 1644–1649. [Google Scholar] [CrossRef] [Green Version]
- Verma, H.; Verma, S.; Prasad, S.B.; Singh, H. Sublingual Delivery of Frovatriptan: An Indication of Potential Alternative Route. Int. Sch. Res. Not. 2014, 2014, 675868. [Google Scholar] [CrossRef] [Green Version]
- Basak, S.; Kang, H.J.; Lee, S.H.; Chu, K.B.; Moon, E.K.; Quan, F.S. Influenza vaccine efficacy induced by orally administered recombinant baculoviruses. PLoS ONE 2020, 15, e0233520. [Google Scholar] [CrossRef] [PubMed]
- Quan, F.S.; Compans, R.W.; Kang, S.M. Oral vaccination with inactivated influenza vaccine induces cross-protective immunity. Vaccine 2012, 30, 180–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, H.J.; Chu, K.B.; Yoon, K.W.; Eom, G.D.; Mao, J.; Kim, M.J.; Lee, S.H.; Moon, E.K.; Quan, F.S. Multiple Neuraminidase Containing Influenza Virus-like Particle Vaccines Protect Mice from Avian and Human Influenza Virus Infection. Viruses 2022, 14, 429. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.J.; Chu, K.B.; Lee, D.H.; Lee, S.H.; Park, B.R.; Kim, M.C.; Kang, S.M.; Quan, F.S. Influenza M2 virus-like particle vaccination enhances protection in combination with avian influenza HA VLPs. PLoS ONE 2019, 14, e0216871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quan, F.S.; Huang, C.; Compans, R.W.; Kang, S.M. Virus-like particle vaccine induces protective immunity against homologous and heterologous strains of influenza virus. J. Virol. 2007, 81, 3514–3524. [Google Scholar] [CrossRef] [Green Version]
- Moldoveanu, Z.; Clementst, M.L.; Prince, S.J.; Murphy, B.R.; Mestecky, J. Human immune responses to influenza virus vaccines administered by systemic or mucosal routes. Vaccine 1995, 13, 1006–1012. [Google Scholar] [CrossRef]
- Kawashima, K.; Ishihara, S.; Masuhara, M.; Mikami, H.; Okimoto, E.; Oshima, N.; Ishimura, N.; Araki, A.; Maruyama, R.; Kinoshita, Y. Development of eosinophilic esophagitis following sublingual immunotherapy with cedar pollen extract: A case report. Allergol. Int. 2018, 67, 515–517. [Google Scholar] [CrossRef]
- Paris, A.L.; Colomb, E.; Verrier, B.; Anjuere, F.; Monge, C. Sublingual vaccination and delivery systems. J. Control Release 2021, 332, 553–562. [Google Scholar] [CrossRef]
- Narang, N.; Sharma, J. Sublingual Mucosa as a route for systemic drug delivery. Int. J. Pharm. Pharm. Sci. 2011, 3, 18–22. [Google Scholar]
- Pedersen, G.; Cox, R. The mucosal vaccine quandary: Intranasal vs. sublingual immunization against influenza. Hum. Vaccin. Immunother. 2012, 8, 689–693. [Google Scholar] [CrossRef]
- Shim, B.S.; Choi, Y.; Cheon, I.S.; Song, M.K. Sublingual delivery of vaccines for the induction of mucosal immunity. Immune Netw. 2013, 13, 81–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergmann, K.C.; Waldman, R.H. Oral Immunization with Influenza Virus: Experimental and Clinical Studies. Curr. Top. Microbiol. Immunol. 1986, 146, 83–89. [Google Scholar]
- Vela Ramirez, J.E.; Sharpe, L.A.; Peppas, N.A. Current state and challenges in developing oral vaccines. Adv. Drug Deliv. Rev. 2017, 114, 116–131. [Google Scholar] [CrossRef]
- Beverley, P.C.; Sridhar, S.; Lalvani, A.; Tchilian, E.Z. Harnessing local and systemic immunity for vaccines against tuberculosis. Mucosal. Immunol. 2014, 7, 20–26. [Google Scholar] [CrossRef] [PubMed]
- La Gruta, N.L.; Turner, S.J. T cell mediated immunity to influenza: Mechanisms of viral control. Trends Immunol. 2014, 35, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Yewdell, W.T.; Smolkin, R.M.; Belcheva, K.T.; Mendoza, A.; Michaels, A.J.; Cols, M.; Angeletti, D.; Yewdell, J.W.; Chaudhuri, J. Temporal dynamics of persistent germinal centers and memory B cell differentiation following respiratory virus infection. Cell Rep. 2021, 37, 109961. [Google Scholar] [CrossRef] [PubMed]
- Boyden, A.W.; Legge, K.L.; Waldschmidt, T.J. Pulmonary infection with influenza A virus induces site-specific germinal center and T follicular helper cell responses. PLoS ONE 2012, 7, e40733. [Google Scholar] [CrossRef] [Green Version]
- Legge, K.L.; Braciale, T.J. Accelerated Migration of Respiratory Dendritic Cells to the Regional Lymph Nodes Is Limited to the Early Phase of Pulmonary Infection. Immunity 2003, 18, 165–277. [Google Scholar] [CrossRef] [Green Version]
- Chiu, C.; Ellebedy, A.H.; Wrammert, J.; Ahmed, R. B cell responses to influenza infection and vaccination. Curr. Top Microbiol. Immunol. 2015, 386, 381–398. [Google Scholar] [CrossRef]
- Chiu, C.; Openshaw, P.J. Antiviral B cell and T cell immunity in the lungs. Nat. Immunol. 2015, 16, 18–26. [Google Scholar] [CrossRef]
- Takahashi, Y.; Onodera, T.; Adachi, Y.; Ato, M. Adaptive B Cell Responses to Influenza Virus Infection in the Lung. Viral Immunol. 2017, 30, 431–437. [Google Scholar] [CrossRef] [PubMed]
- CAIV-T. Influenza virus vaccine live intranasal—MedImmune vaccines: CAIV-T, influenza vaccine live intranasal. Drugs R D 2003, 4, 312–319. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, J.; Eom, G.-D.; Yoon, K.-W.; Kang, H.-J.; Chu, K.-B.; Quan, F.-S. Sublingual Vaccination with Live Influenza Virus Induces Better Protection Than Oral Immunization in Mice. Life 2022, 12, 975. https://doi.org/10.3390/life12070975
Mao J, Eom G-D, Yoon K-W, Kang H-J, Chu K-B, Quan F-S. Sublingual Vaccination with Live Influenza Virus Induces Better Protection Than Oral Immunization in Mice. Life. 2022; 12(7):975. https://doi.org/10.3390/life12070975
Chicago/Turabian StyleMao, Jie, Gi-Deok Eom, Keon-Woong Yoon, Hae-Ji Kang, Ki-Back Chu, and Fu-Shi Quan. 2022. "Sublingual Vaccination with Live Influenza Virus Induces Better Protection Than Oral Immunization in Mice" Life 12, no. 7: 975. https://doi.org/10.3390/life12070975
APA StyleMao, J., Eom, G.-D., Yoon, K.-W., Kang, H.-J., Chu, K.-B., & Quan, F.-S. (2022). Sublingual Vaccination with Live Influenza Virus Induces Better Protection Than Oral Immunization in Mice. Life, 12(7), 975. https://doi.org/10.3390/life12070975





