Anosmia Testing as Early Detection of SARS-CoV-2 Positivity; A Prospective Study under Screening Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Setting/Patient Recruitment
2.2. Olfactory Evaluation Card
2.3. Study Process, Demographic and Clinical Data Collection
2.4. Molecular Testing
2.5. External Conditions
2.6. Prediction of SARS-CoV-2 PCR Test Positivity Using Olfactory Evaluation as a Screening Tool
2.7. Scent Comparison
2.8. Statistical Analysis
2.9. Statistical Analysis—Performance of Feature Selection
3. Results
3.1. Study Population
3.2. External Conditions
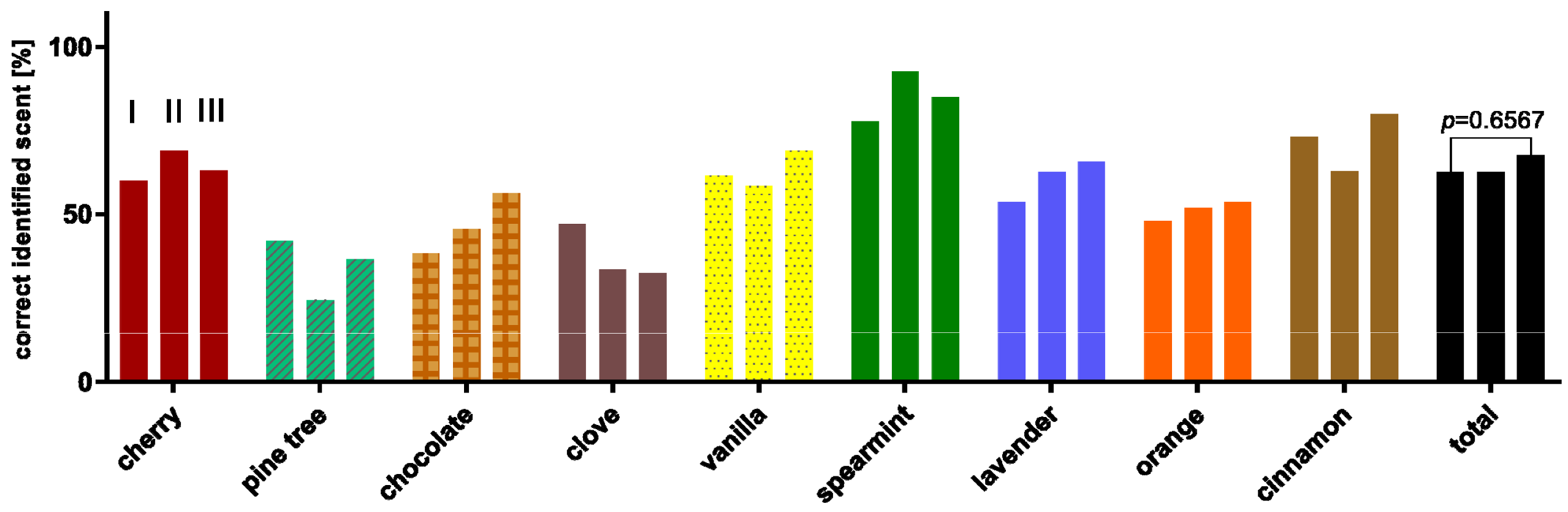
3.3. Evaluation of the Series Measurement
3.4. Evaluation of the Test Scenarios and Cut-Off Determination
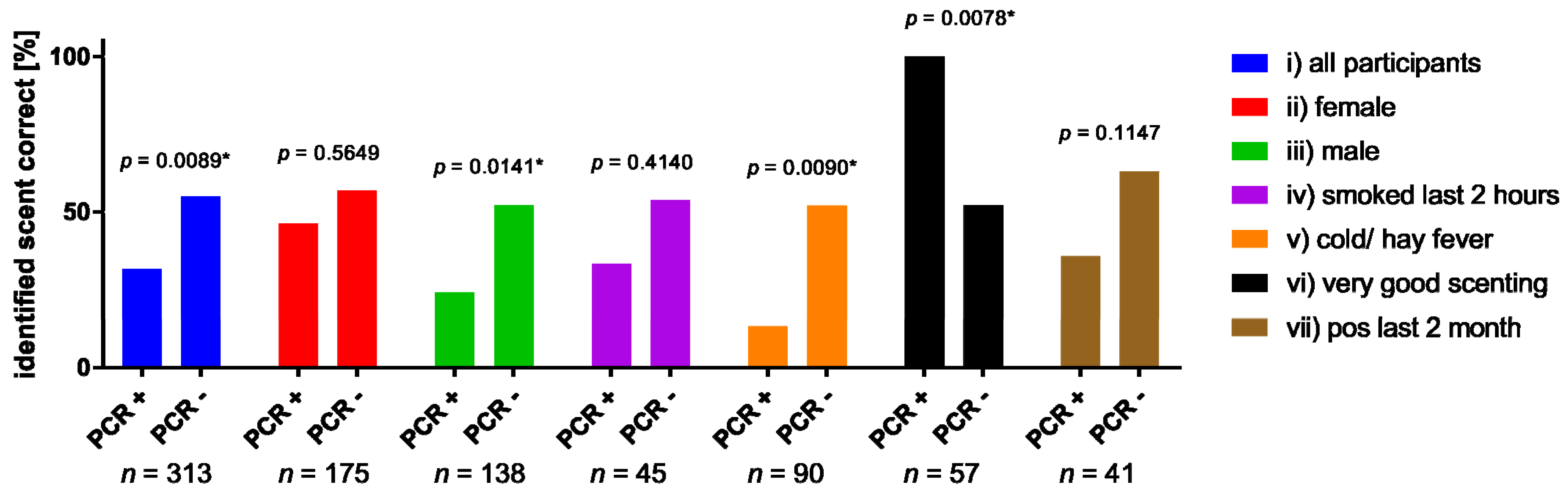
3.5. Subgroup Analysis
3.6. Scent Comparison
3.7. Supervised Machine Learning Based Feature Selection
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Xydakis, M.S.; Dehgani-Mobaraki, P.; Holbrook, E.H.; Geisthoff, U.W.; Bauer, C.; Hautefort, C.; Herman, P.; Manley, G.T.; Lyon, D.M.; Hopkins, C. Smell and taste dysfunction in patients with COVID-19. Lancet Infect. Dis. 2020, 20, 1015–1016. [Google Scholar] [CrossRef]
- Lechien, J.R.; Chiesa-Estomba, C.M.; De Siati, D.R.; Horoi, M.; Le Bon, S.D.; Rodriguez, A.; Dequanter, D.; Blecic, S.; El Afia, F.; Distinguin, L.; et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): A multicenter European study. Eur. Arch. Otorhinolaryngol. 2020, 277, 2251–2261. [Google Scholar] [CrossRef]
- Hura, N.; Xie, D.X.; Choby, G.W.; Schlosser, R.J.; Orlov, C.P.; Seal, S.M.; Rowan, N.R. Treatment of post-viral olfactory dysfunction: An evidence-based review with recommendations. Int. Forum Allergy Rhinol. 2020, 10, 1065–1086. [Google Scholar] [CrossRef]
- Vaira, L.A.; Salzano, G.; Fois, A.G.; Piombino, P.; De Riu, G. Potential pathogenesis of ageusia and anosmia in COVID-19 patients. Int. Forum Allergy Rhinol. 2020, 10, 1103–1104. [Google Scholar] [CrossRef]
- Fotuhi, M.; Mian, A.; Meysami, S.; Raji, C.A. Neurobiology of COVID-19. J. Alzheimer’s Dis. 2020, 76, 3–19. [Google Scholar] [CrossRef]
- Lee, Y.; Min, P.; Lee, S.; Kim, S.W. Prevalence and Duration of Acute Loss of Smell or Taste in COVID-19 Patients. J. Korean Med. Sci. 2020, 35, e174. [Google Scholar] [CrossRef]
- Scohy, A.; Anantharajah, A.; Bodeus, M.; Kabamba-Mukadi, B.; Verroken, A.; Rodriguez-Villalobos, H. Low performance of rapid antigen detection test as frontline testing for COVID-19 diagnosis. J. Clin. Virol. 2020, 129, 104455. [Google Scholar] [CrossRef]
- Hummel, T.; Sekinger, B.; Wolf, S.R.; Pauli, E.; Kobal, G. Sniffin sticks: Olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem. Senses 1997, 22, 39–52. [Google Scholar] [CrossRef]
- Baden-Württemberg, M. SARS-CoV-2 Teststrategie in Baden-Württemberg. Available online: Sozialministerium.baden-wuerttemberg.de%2Ffileadmin%2Fredaktion%2Fm-sm%2Fintern%2Fdownloads%2FDownloads_Gesundheitsschutz%2FCorona_Uebersicht_Teststrategie-BW_20201009.pdf (accessed on 13 April 2022).
- Robert Koch, I. Informationen zur Ausweisung internationaler Risikogebiete. Available online: https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Transport/Archiv_Risikogebiete/Risikogebiete_2021-07-23.pdf?__blob=publicationFile (accessed on 2 March 2022).
- Time and Date AS. Wetter-Rückblick Mannheim, Baden-Württemberg, Deutschland—Gestern, Letzte Wochen & Monate. Available online: https://www.timeanddate.de/wetter/deutschland/mannheim/rueckblick (accessed on 14 May 2011).
- Degenhardt, F.; Seifert, S.; Szymczak, S. Evaluation of variable selection methods for random forests and omics data sets. Brief. Bioinform. 2019, 20, 492–503. [Google Scholar] [CrossRef] [Green Version]
- Acharjee, A.; Larkman, J.; Xu, Y.; Cardoso, V.R.; Gkoutos, G.V. A random forest based biomarker discovery and power analysis framework for diagnostics research. BMC Med. Genom. 2020, 13, 178. [Google Scholar] [CrossRef]
- Stadt, M. Fallzahlen COVID-19 Mannheim Stadtbezirke. 2021. Available online: https://www.mannheim.de/de/informationen-zu-corona/aktuelle-situation-in-mannheim/inzidenzzahl (accessed on 15 April 2022).
- Cohen, J. Statistical power analysis. Curr. Dir. Psychol. Sci. 1992, 1, 98–101. [Google Scholar] [CrossRef]
- Michael, F. Corrgrams: Exploratory displays for correlation matrices. Am. Stat. 2002, 56, 316–324. [Google Scholar]
- Wender, I. Intensität und Qualität in der Geruchswahrnehmung. Psychol. Forsch. 1968, 32, 244–276. [Google Scholar] [CrossRef]
- Yan, C.H.; Faraji, F.; Prajapati, D.P.; Boone, C.E.; DeConde, A.S. Association of chemosensory dysfunction and COVID-19 in patients presenting with influenza-like symptoms. Int. Forum. Allergy Rhinol. 2020, 10, 806–813. [Google Scholar] [CrossRef] [Green Version]
- Jungbauer, F.; Huber, L.; Lammert, A.; Ludwig, S.; Rotter, N.; Zaubitzer, L.; Schell, A. Prevalence of subjective impairments of the sense of smell and taste in employees of retirement and nursing homes during SARS-CoV-2 pandemic. Nurs. Open 2022, 9, 175–180. [Google Scholar] [CrossRef]
- Rocke, J.; Hopkins, C.; Philpott, C.; Kumar, N. Is loss of sense of smell a diagnostic marker in COVID-19: A systematic review and meta-analysis. Clin. Otolaryngol. 2020, 45, 914–922. [Google Scholar] [CrossRef]
- Weiss, J.J.; Attuquayefio, T.N.; White, E.B.; Li, F.; Herz, R.S.; White, T.L.; Campbell, M.; Geng, B.; Datta, R.; Wyllie, A.L. Tracking Smell Loss to Identify Healthcare Workers with SARS-CoV-2 Infection. PLoS ONE 2021, 16, e0248025. [Google Scholar] [CrossRef]
- Basu, B.; Riya, P.A.; Issac, J.; Parvathy, S.; Nair, B.S.; Tokdar, P.; Kumar, D.S.; Kulkarni, P.R.; Hanumanram, G.; Jagadeesan, M. COVID-Anosmia Checker: A rapid and low-cost alternative tool for mass screening of COVID-19. medRxiv 2020. [Google Scholar] [CrossRef]
- Albert Mudry Md, P. Corowell: New Simple App-Combined Smell Test to Screen Patients for Suspicion of COVID-19 Infection. Available online: https://corowell.com/de/clinical/ (accessed on 2 June 2022).
- Klopfenstein, T.; Kadiane-Oussou, N.J.; Toko, L.; Royer, P.Y.; Lepiller, Q.; Gendrin, V.; Zayet, S. Features of anosmia in COVID-19. Med. Mal. Infect. 2020, 50, 436–439. [Google Scholar] [CrossRef]
- Oliva, A.D.; Gupta, R.; Issa, K.; Abi Hachem, R.; Jang, D.W.; Wellford, S.A.; Moseman, E.A.; Matsunami, H.; Goldstein, B.J. Aging-related olfactory loss is associated with olfactory stem cell transcriptional alterations in humans. J. Clin. Investig. 2022, 132, 1. [Google Scholar] [CrossRef] [PubMed]
- Da Re, A.F.; Gurgel, L.G.; Buffon, G.; Moura, W.E.R.; Marques Vidor, D.C.G.; Maahs, M.A.P. Tobacco Influence on Taste and Smell: Systematic Review of the Literature. Int. Arch. Otorhinolaryngol. 2018, 22, 81–87. [Google Scholar] [CrossRef] [Green Version]
- Drews, T.; Nehring, M.; Werner, A.; Hummel, T. The sense of smell is not strongly affected by ambient temperature and humidity: A prospective study in a controlled environment. Eur. Arch. Otorhinolaryngol. 2021, 278, 1465–1469. [Google Scholar] [CrossRef] [PubMed]
- Kuehn, M.; Welsch, H.; Zahnert, T.; Hummel, T. Changes of pressure and humidity affect olfactory function. Eur. Arch. Otorhinolaryngol. 2008, 265, 299–302. [Google Scholar] [CrossRef] [PubMed]
- Mohrhardt, J.; Nagel, M.; Fleck, D.; Ben-Shaul, Y.; Spehr, M. Signal Detection and Coding in the Accessory Olfactory System. Chem. Senses 2018, 43, 667–695. [Google Scholar] [CrossRef] [Green Version]
- Sai-Guan, L.; Husain, S.; Zahedi, F.D.; Ahmad, N.; Gendeh, B.S. Cultural Adaptation of Sniffin’ Sticks Smell Identification Test: The Malaysian Version. Iran J. Otorhinolaryngol. 2020, 32, 213–222. [Google Scholar] [CrossRef]
- Balungwe, P.; Huart, C.; Matanda, R.; Bisimwa, G.; Mouraux, A.; Rombaux, P. Adaptation of the Sniffin’ Sticks Test in South-Kivu. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2020, 137, 467–471. [Google Scholar] [CrossRef]
- Kamrava, S.K.; Jalessi, M.; Ghalehbaghi, S.; Amini, E.; Alizadeh, R.; Rafiei, F.; Moosa, S.; Farhadi, M. Validity and Reliability of Persian Smell Identification Test. Iran J. Otorhinolaryngol. 2020, 32, 65–71. [Google Scholar] [CrossRef]
- Bundesamt, D.-S. Ausländische Bevölkerung nach Bundesländern. Available online: https://www.destatis.de/DE/Themen/Gesellschaft-Umwelt/Bevoelkerung/Migration-Integration/Tabellen/auslaendische-bevoelkerung-bundeslaender.html (accessed on 12 March 2022).
- Pellegrino, R.; Sinding, C.; de Wijk, R.A.; Hummel, T. Habituation and adaptation to odors in humans. Physiol. Behav. 2017, 177, 13–19. [Google Scholar] [CrossRef]
- Alonso, C.C.G.; Silva, F.G.; Costa, L.O.P.; Freitas, S. Smell tests to distinguish Parkinson’s disease from other neurological disorders: A systematic review and meta-analysis. Expert Rev. Neurother. 2021, 21, 365–379. [Google Scholar] [CrossRef]
- Nepal, G.; Rehrig, J.H.; Shrestha, G.S.; Shing, Y.K.; Yadav, J.K.; Ojha, R.; Pokhrel, G.; Tu, Z.L.; Huang, D.Y. Neurological manifestations of COVID-19: A systematic review. Crit. Care 2020, 24, 421. [Google Scholar] [CrossRef] [PubMed]
- Goh, Y.; Beh, D.L.L.; Makmur, A.; Somani, J.; Chan, A.C.Y. Pearls & Oy-sters: Facial nerve palsy in COVID-19 infection. Neurology 2020, 95, 364–367. [Google Scholar] [CrossRef] [PubMed]
- Jungbauer, F.; Hulse, R.; Lu, F.; Ludwig, S.; Held, V.; Rotter, N.; Schell, A. Case Report: Bilateral Palsy of the Vocal Cords After COVID-19 Infection. Front. Neurol. 2021, 12, 619545. [Google Scholar] [CrossRef]
- Ludwig, S.; Schell, A.; Berkemann, M.; Jungbauer, F.; Zaubitzer, L.; Huber, L.; Warken, C.; Held, V.; Kusnik, A.; Teufel, A.; et al. Post-COVID-19 Impairment of the Senses of Smell, Taste, Hearing, and Balance. Viruses 2022, 14, 849. [Google Scholar] [CrossRef]
- Joshi, A.; Thaploo, D.; Yan, X.; Zang, Y.; Warr, J.; Hummel, T. Habitual Exposure to Trigeminal Stimuli and Its Effects on the Processing of Chemosensory Stimuli. Neuroscience 2021, 470, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Callaway, E. Heavily mutated Omicron variant puts scientists on alert. Nature 2021, 600, 21. [Google Scholar] [CrossRef]
- Suzuki, R.; Yamasoba, D.; Kimura, I.; Wang, L.; Kishimoto, M.; Ito, J.; Morioka, Y.; Nao, N.; Nasser, H.; Uriu, K.; et al. Attenuated fusogenicity and pathogenicity of SARS-CoV-2 Omicron variant. Nature 2022, 603, 700–705. [Google Scholar] [CrossRef]
- Brandal, L.T.; MacDonald, E.; Veneti, L.; Ravlo, T.; Lange, H.; Naseer, U.; Feruglio, S.; Bragstad, K.; Hungnes, O.; Odeskaug, L.E.; et al. Outbreak caused by the SARS-CoV-2 Omicron variant in Norway, November to December 2021. Eurosurveillance 2021, 26, 2101147. [Google Scholar] [CrossRef] [PubMed]
- Butowt, R.; Bilińska, K.; von Bartheld, C. Why does the Omicron Variant Largely Spare Olfactory Function? Implications for the Pathogenesis of Anosmia in COVID-19. J. Infect. Dis. 2022, jiac113. [Google Scholar] [CrossRef]
- Lippi, G.; Nocini, R.; Henry, B.M. Analysis of online search trends suggests that SARS-CoV-2 Omicron (B.1.1.529) variant causes different symptoms. J. Infect. 2022, 84, e76–e77. [Google Scholar] [CrossRef]
- Armando, F.; Beythien, G.; Kaiser, F.; Allnoch, L.; Heydemann, L.; Rosiak, M.; Becker, S.; Gonzalez-Hernandez, M.; Lamers, M.; Haagmans, B. SARS-CoV-2 Omicron Variant Causes Mild Pathology in the Upper and Lower Respiratory Tract of Syrian Golden Hamsters (Mesocricetus auratus). 2022. Available online: https://assets.researchsquare.com/files/rs-1315280/v1/f4b6924c-c9bd-41ed-a712-81d875e8ef92.pdf?c=1655795514b (accessed on 15 May 2022).
- Rodriguez-Sevilla, J.J.; Güerri-Fernádez, R.; Bertran Recasens, B. Is There Less Alteration of Smell Sensation in Patients With Omicron SARS-CoV-2 Variant Infection? Front. Med. 2022, 9, 852998. [Google Scholar] [CrossRef] [PubMed]
- Haehner, A.; Marquardt, B.; Kardashi, R.; de With, K.; Rößler, S.; Landis, B.N.; Welge-Luessen, A.; Hummel, T. SARS-CoV-2 Leads to Significantly More Severe Olfactory Loss than Other Seasonal Cold Viruses. Life 2022, 12, 461. [Google Scholar] [CrossRef] [PubMed]
- Hummel, T.; Rissom, K.; Reden, J.; Hahner, A.; Weidenbecher, M.; Huttenbrink, K.B. Effects of olfactory training in patients with olfactory loss. Laryngoscope 2009, 119, 496–499. [Google Scholar] [CrossRef] [PubMed]



| Overall (n = 313) | |
|---|---|
| PCRRes | |
| SARS-CoV-2 PCR positive | 38 (12.1%) |
| SARS-CoV-2 PCR negative | 275 (87.9%) |
| Sex | |
| male | 138 (44.1%) |
| female | 175 (55.9%) |
| Age | |
| Mean (SD) | 31.9 (14.3) |
| Range | 8.0–77.0 |
| hay fever/cold | |
| No | 223 (71.2%) |
| Yes | 90 (28.8%) |
| tested positive for SARS-CoV-2 last 2 month | |
| No | 272 (86.9%) |
| Yes | 41 (13.1%) |
| smoked last 2 h | |
| No | 268 (85.6%) |
| Yes | 45 (14.4%) |
| scent problems since longer time | |
| No | 247 (78.9%) |
| Yes (daily) | 52 (16.6%) |
| Yes (occasionally) | 14 (4.5%) |
| subjective scent ability | |
| very good | 69 (22.0%) |
| good | 143 (45.7%) |
| normal/ average | 82 (26.2%) |
| bad | 16 (5.1%) |
| very bad | 1 (0.3%) |
| not specified | 2 (0.6%) |
| ability to inspire through nose | |
| very good | 36 (11.5%) |
| good | 194 (62.0%) |
| normal/ average | 34 (10.9%) |
| bad | 45 (14.4%) |
| very bad | 4 (1.3%) |
| 1 Out of 3 | 2 Out of 3 | 3 Out of 3 | First Ticket Correct | |
|---|---|---|---|---|
| Negative Tests | 265 | 177 | 76 | 163 |
| True Test | 249 | 187 | 102 | 177 |
| Wrong Test | 64 | 125 | 210 | 136 |
| Sensitivity | 28.9 % | 65.8 % | 86.8 % | 68.4 % |
| Specificity | 86.5 % | 59.6 % | 25.8 % | 54.9 % |
| Accuracy | 79.6 % | 60.4 % | 33.2 % | 56.5 % |
| Prevalence | 4.8 % | 4.8 % | 4.8 % | 4.8 % |
| Positive Predictive Value | 22.9 % | 18.4 % | 13.9 % | 17.3 % |
| Negative Predictive Value | 89.8 % | 92.7 % | 93.4 % | 92.6 % |
| Post-test Disease Probability | 9.9 % | 7.7 % | 5.6 % | 7.2 % |
| Post-test Health Probability | 96.0 % | 97.2 % | 97.5 % | 97.2 % |
| Positive Likelihood Ratio | 2.15 | 1.63 | 1.17 | 1.52 |
| Negative Likelihood Ratio | 0.821 | 0.574 | 0.510 | 0.575 |
| Correct Scenting | Incorrect Scenting | Two Tailed Fisher’s Exact Test | ||
|---|---|---|---|---|
| all participants | PCR + | 31.6% (12) | 68.4% (26) | p= 0.0089 * |
| PCR - | 54.9% (151) | 45.1 (124) | ||
| Female | PCR + | 46.2% (6) | 53.8% (7) | p = 0.5649 |
| PCR - | 56.8% (92) | 43.2% (70) | ||
| Male | PCR + | 24% (6) | 76% (19) | p= 0.0141 * |
| PCR - | 52.2% (59) | 47.8% (54) | ||
| smoked last 2 h | PCR + | 33.3% (2) | 66.7% (4) | p = 0.4140 |
| PCR - | 53.8% (21) | 46.2% (18) | ||
| cold/ hay fever | PCR + | 13.3% (2) | 86.7% (13) | p= 0.0090 * |
| PCR - | 52% (39) | 48% (36) | ||
| Scenting sense: Good and very good | PCR + | 100% (9) | 0% (0) | p= 0.0078 * |
| PCR - | 52.1% (25) | 47.9% (23) | ||
| tested positive for SARS-CoV-2 during the last 2 month | PCR + | 35.7% (5) | 64.3% (9) | p = 0.1147 |
| PCR - | 63% (17) | 37% (10) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jungbauer, F.; Gerhards, C.; Thiaucourt, M.; Behnes, M.; Rotter, N.; Schell, A.; Haselmann, V.; Neumaier, M.; Kittel, M. Anosmia Testing as Early Detection of SARS-CoV-2 Positivity; A Prospective Study under Screening Conditions. Life 2022, 12, 968. https://doi.org/10.3390/life12070968
Jungbauer F, Gerhards C, Thiaucourt M, Behnes M, Rotter N, Schell A, Haselmann V, Neumaier M, Kittel M. Anosmia Testing as Early Detection of SARS-CoV-2 Positivity; A Prospective Study under Screening Conditions. Life. 2022; 12(7):968. https://doi.org/10.3390/life12070968
Chicago/Turabian StyleJungbauer, Frederic, Catharina Gerhards, Margot Thiaucourt, Michael Behnes, Nicole Rotter, Angela Schell, Verena Haselmann, Michael Neumaier, and Maximilian Kittel. 2022. "Anosmia Testing as Early Detection of SARS-CoV-2 Positivity; A Prospective Study under Screening Conditions" Life 12, no. 7: 968. https://doi.org/10.3390/life12070968
APA StyleJungbauer, F., Gerhards, C., Thiaucourt, M., Behnes, M., Rotter, N., Schell, A., Haselmann, V., Neumaier, M., & Kittel, M. (2022). Anosmia Testing as Early Detection of SARS-CoV-2 Positivity; A Prospective Study under Screening Conditions. Life, 12(7), 968. https://doi.org/10.3390/life12070968






