Asthma and Cacosmia Could Be Predictive Factors of Olfactory Dysfunction Persistence 9 Months after SARS-CoV-2 Infection: The ANOSVID Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Clinical and Paraclinical Data
2.3. Statistical Analysis
3. Results
3.1. OD Characteristics after SARS-CoV-2 Infection
3.2. Description of Patients with Persistent OD after SARS-CoV-2 Infection
3.3. Comparison of Two Groups
4. Discussion
4.1. OD Characteristics after SARS-CoV-2 Infection
4.2. Patients with Persistent OD after SARS-CoV-2 Infection
4.3. Comparison of Two Groups
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zu, Z.Y.; Di Jiang, M.; Xu, P.P.; Chen, W.; Ni, Q.Q.; Lu, G.M.; Zhang, L.J. Coronavirus Disease 2019 (COVID-19): A Perspective from China. Radiology 2020, 296, E15–E25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lechien, J.R.; Chiesa-Estomba, C.M.; De Siati, D.R.; Horoi, M.; Le Bon, S.D.; Rodriguez, A.; Dequanter, D.; Blecic, S.; El Afia, F.; Distinguin, L.; et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): A multicenter European study. Eur. Arch. Otorhinolaryngol. 2020, 277, 2251–2261. [Google Scholar] [CrossRef] [PubMed]
- Zayet, S.; Klopfenstein, T.; Mercier, J.; Kadiane-Oussou, N.J.; Wah, L.L.C.; Royer, P.-Y.; Toko, L.; Gendrin, V. Contribution of anosmia and dysgeusia for diagnostic of COVID-19 in outpatients. Infection 2020, 49, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Pang, K.W.; Chee, J.; Subramaniam, S.; Ng, C.L. Frequency and Clinical Utility of Olfactory Dysfunction in COVID-19: A System-atic Review and Meta-analysis. Curr. Allergy Asthma Rep. 2020, 20, 76. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Min, P.; Lee, S.; Kim, S.-W. Prevalence and Duration of Acute Loss of Smell or Taste in COVID-19 Patients. J. Korean Med. Sci. 2020, 35, e174. [Google Scholar] [CrossRef] [PubMed]
- Niazkar, H.R.; Zibaee, B.; Nasimi, A.; Bahri, N. The neurological manifestations of COVID-19: A review article. Neurol. Sci. 2020, 41, 1667–1671. [Google Scholar] [CrossRef] [PubMed]
- Vaira, L.A.; Salzano, G.; Fois, A.G.; Piombino, P.; De Riu, G. Potential pathogenesis of ageusia and anosmia in COVID-19 patients. Int. Forum Allergy Rhinol. 2020, 10, 1103–1104. [Google Scholar] [CrossRef]
- Villalba, N.L.; Maouche, Y.; Ortiz, M.B.A.; Sosa, Z.C.; Chahbazian, J.B.; Syrovatkova, A.; Pertoldi, P.; Andres, E.; Zulfiqar, A.A. Anosmia and Dysgeusia in the Absence of Other Respiratory Diseases: Should COVID-19 Infection Be Considered? Eur. J. Case Rep. Intern. Med. 2020, 7, 001641. Available online: https://www.ejcrim.com/index.php/EJCRIM/article/view/1641 (accessed on 1 May 2021).
- Printza, A.; Constantinidis, J. The role of self-reported smell and taste disorders in suspected COVID-19. Eur. Arch. Otorhinolaryngol. 2020, 277, 2625–2630. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, C.; Surda, P.; Vaira, L.; Lechien, J.; Safarian, M.; Saussez, S.; Kumar, N. Six month follow-up of self-reported loss of smell during the COVID-19 pandemic. Rhinol. J. 2021, 59, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Zayet, S.; Zahra, H.; Royer, P.Y.; Tipirdamaz, C.; Mercier, J.; Gendrin, V.; Lepiller, Q.; Marty-Quinternet, S.; Osman, M.; Belfeki, N.; et al. Post-COVID-19 Syndrome: Nine Months after SARS-CoV-2 Infection in a Cohort of 354 Patients: Data from the First Wave of COVID-19 in Nord Franche-Comté Hospital, France. Microorganisms 2021, 9, 1719. [Google Scholar] [CrossRef]
- El Sayed, S.; Shokry, D.; Gomaa, S.M. Post-COVID-19 fatigue and anhedonia: A cross-sectional study and their correlation to post-recovery period. Neuropsychopharmacol. Rep. 2020, 41, 50–55. [Google Scholar] [CrossRef]
- Tolba, M.; Abo Omirah, M.; Hussein, A.; Saeed, H. Assessment and Characterization of Post-COVID-19 manifestations. Int. J. Clin. Pract. 2020, 75, e13746. [Google Scholar]
- Mendelson, M.; Nel, J.; Blumberg, L.; Madhi, S.A.; Dryden, M.; Stevens, W.; Venter, F.W.D. Long-COVID: An evolving problem with an ex-tensive impact. S. Afr. Med. J. 2020, 111, 10–12. [Google Scholar] [CrossRef] [PubMed]
- Mahase, E. COVID-19: What do we know about “long covid”? BMJ 2020, 370, m2815. [Google Scholar] [CrossRef]
- Garg, P.; Arora, U.; Kumar, A.; Wig, N. The “post-COVID” syndrome: How deep is the damage? J. Med. Virol. 2021, 93, 673–674. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.W.T.W.; de Luca, R.D.; Mello Neto, H.O.; Barcellos, I. Post-COVID-19 Syndrome? New daily persistent headache in the af-termath of COVID-19. Arq. Neuropsiquiatr. 2020, 78, 753–754. [Google Scholar] [CrossRef]
- Klopfenstein, T.; Kadiane-Oussou, N.J.; Toko, L.; Royer, P.Y.; Lepiller, Q.; Gendrin, V.; Zayet, S. Features of anosmia in COVID-19. Med. Mal. Infect. 2020, 50, 436–439. [Google Scholar] [CrossRef]
- Mattos, J.L.; Ba, C.E.; Schlosser, R.J.; Hyer, M.; Mace, J.C.; Smith, T.L.; Soler, Z.M. A brief version of the questionnaire of olfactory disorders in patients with chronic rhinosinusitis. Int. Forum Allergy Rhinol. 2019, 9, 1144–1150. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, C.; Chiesa-Estomba, C.M.; Horoi, M.; Le Bon, S.D.; Hans, S.; Distinguin, L.; Chekkoury-Idrissi, Y.; Circiu, M.P.; Khalife, M.; Saussez, S.; et al. Validity and Reliability of the French Short Version of the Questionnaire of Olfactory Disorders-Negative Statements (sQOD-NS). Ear Nose Throat J. 2021, 1455613211032004. [Google Scholar] [CrossRef]
- Mercier, J.; Osman, M.; Bouiller, K.; Tipirdamaz, C.; Gendrin, V.; Chirouze, C.; Lepiller, Q.; Bouvier, E.; Royer, P.Y.; Pierron, A.; et al. Olfactory dysfunction in COVID-19, new in-sights from a cohort of 353 patients: The ANOSVID study. J. Med. Virol. 2022.
- Wu, D.; Wang, V.Y.; Chen, Y.H.; Ku, C.H.; Wang, P.C. The prevalence of olfactory and gustatory dysfunction in COVID-19—A systematic review. Auris Nasus Larynx 2021, 49, 165–175. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8279934/ (accessed on 10 August 2021). [CrossRef] [PubMed]
- Tong, J.Y.; Wong, A.; Zhu, D.; Fastenberg, J.H.; Tham, T. The Prevalence of Olfactory and Gustatory Dysfunction in COVID-19 Patients: A Systematic Review and Meta-analysis. Otolaryngol. Head Neck Surg. 2020, 163, 3–11. [Google Scholar] [CrossRef]
- Vaira, L.A.; Hopkins, C.; Petrocelli, M.; Lechien, J.R.; Chiesa-Estomba, C.M.; Salzano, G.; Cucurullo, M.; Salzano, F.A.; Saussez, S.; Boscolo-Rizzo, P.; et al. Smell and taste recovery in corona-virus disease 2019 patients: A 60-day objective and prospective study. J. Laryngol. Otol. 2020, 134, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Riestra-Ayora, J.; Yanes-Diaz, J.; Esteban-Sanchez, J.; Vaduva, C.; Molina-Quiros, C.; Larran-Jimenez, A.; Martin-Sanz, E. Long-term follow-up of olfactory and gustatory dysfunction in COVID-19: 6 months case–control study of health workers. Eur. Arch. Otorhinolaryngol. 2021, 278, 4831–4837. [Google Scholar] [CrossRef]
- Dell’Era, V.; Farri, F.; Garzaro, G.; Gatto, M.; Valletti, P.A.; Garzaro, M. Smell and taste disorders during COVID-19 outbreak: A Cross-sectional study on 355 patients. Head Neck 2020, 42, 1591–1596. [Google Scholar] [CrossRef]
- D’Ascanio, L.; Pandolfini, M.; Cingolani, C.; Latini, G.; Gradoni, P.; Capalbo, M.; Frausini, G.; Maranzano, M.; Brenner, M.J.; Di Stadio, A. Olfactory Dysfunction in COVID-19 Patients: Prevalence and Prognosis for Recovering Sense of Smell. Otolaryngol. Head Neck Surg. 2021, 164, 82–86. [Google Scholar] [CrossRef]
- Speth, M.M.; Singer-Cornelius, T.; Oberle, M.; Gengler, I.; Brockmeier, S.J.; Sedaghat, A.R. Olfactory Dysfunction and Sinonasal Symptomatology in COVID-19: Prevalence, Severity, Timing, and Associated Characteristics. Otolaryngol. Head Neck Surg. 2020, 163, 114–120. [Google Scholar] [CrossRef]
- Lovato, A.; de Filippis, C. Clinical Presentation of COVID-19: A Systematic Review Focusing on Upper Airway Symptoms. Ear Nose Throat J. 2020, 99, 569–576. [Google Scholar] [CrossRef] [Green Version]
- Leth, S.; Gunst, J.D.; Mathiasen, V.; Hansen, K.; Søgaard, O.; Østergaard, L.; Jensen-Fangel, S.; Storgaard, M.; Agergaard, J. Persistent Symptoms in Patients Recovering from COVID-19 in Denmark. Open Forum Infect. Dis. 2021, 8, ofab042. [Google Scholar] [CrossRef]
- Meije, Y.; Duarte-Borges, A.; Sanz, X.; Clemente, M.; Ribera, A.; Ortega, L.; González-Pérez, R.; Cid, R.; Pareja, J.; Cantero, I.; et al. Long-term outcomes of patients following hospi-talization for coronavirus disease 2019: A prospective observational study. Clin. Microbiol. Infect. 2021, 27, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Bénézit, F.; Le Turnier, P.; Declerck, C.; Paillé, C.; Revest, M.; Dubée, V.; Tattevin, P.; Arvieux, C.; Baldeyrou, M.; Chapplain, J.M.; et al. Utility of hyposmia and hypogeusia for the diagnosis of COVID-19. Lancet Infect. Dis. 2020, 20, 1014–1015. [Google Scholar] [CrossRef]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef]
- Sun, B.; Tang, N.; Peluso, M.J.; Iyer, N.S.; Torres, L.; Donatelli, J.L.; Munter, S.E.; Nixon, C.C.; Rutishauser, R.L.; Rodriguez-Barraquer, I.; et al. Characterization and Biomarker Analyses of Post-COVID-19 Complications and Neurological Manifestations. Cells 2021, 10, 386. [Google Scholar] [CrossRef] [PubMed]
- Afshari, J.T.; Hosseini, R.F.; Farahabadi, S.H.; Heydarian, F.; Boskabady, M.H.; Khoshnavaz, R.; Razavi, A.; Karimiani, E.G.; Ghasemi, G. Association of the expression of IL-4 and IL-13 genes, IL-4 and IgE serum levels with allergic asthma. Iran. J. Allergy Asthma Immunol. 2007, 6, 67–72. [Google Scholar]
- Lee, Y.C.; Lee, K.H.; Lee, H.B.; Rhee, Y.K. Serum levels of interleukins (IL)-4, IL-5, IL-13, and interferon-gamma in acute asthma. J. Asthma 2001, 38, 665–671. [Google Scholar] [CrossRef]
- Koitka, A.; Cooper, M.; Thomas, M.; Tikellis, C. Angiotensin converting enzyme 2 in the kidney. Clin. Exp. Pharmacol. Physiol. 2008, 35, 420–425. [Google Scholar] [CrossRef]
- Altin, F.; Cingi, C.; Uzun, T.; Bal, C. Olfactory and gustatory abnormalities in COVID-19 cases. Eur. Arch. Otorhinolaryngol. 2020, 277, 2775–2781. [Google Scholar] [CrossRef]
- Cooper, K.; Brann, D.H.; Farruggia, M.C.; Bhutani, S.; Pellegrino, R.; Tsukahara, T.; Weinreb, C.; Joseph, P.V.; Larson, E.D.; Parma, V.; et al. COVID-19 and the Chemical Senses: Supporting Players Take Center Stage. Neuron 2020, 107, 219–233. [Google Scholar] [CrossRef]
- Las Casas Lima MH de Cavalcante, A.L.B.; Leão, S.C. Pathophysiological relationship between COVID-19 and olfactory dys-function: A systematic review. Braz. J. Otorhinolaryngol. 2021; in press. [Google Scholar] [CrossRef]
- Chen, M.; Shen, W.; Rowan, N.R.; Kulaga, H.; Hillel, A.; Ramanathan, M.; Lane, A.P. Elevated ACE-2 expression in the olfactory neu-roepithelium: Implications for anosmia and upper respiratory SARS-CoV-2 entry and replication. Eur. Respir. J. 2020, 56, 2001948. [Google Scholar] [CrossRef] [PubMed]
- Netland, J.; Meyerholz, D.K.; Moore, S.; Cassell, M.; Perlman, S. Severe Acute Respiratory Syndrome Coronavirus Infection Causes Neuronal Death in the Absence of Encephalitis in Mice Transgenic for Human ACE2. J. Virol. 2008, 82, 7264–7275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Douaud, G.; Lee, S.; Alfaro-Almagro, F.; Arthofer, C.; Wang, C.; McCarthy, P.; Lange, F.; Andersson, J.L.; Griffanti, L.; Duff, E.; et al. SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature 2022, 604, 697–707. [Google Scholar] [CrossRef]
- Lauring, A.S.; Malani, P.N. Variants of SARS-CoV-2. JAMA 2021, 326, 880. [Google Scholar] [CrossRef] [PubMed]
- Twohig, K.A.; Nyberg, T.; Zaidi, A.; Thelwall, S.; Sinnathamby, M.A.; Aliabadi, S.; Seaman, S.R.; Harris, R.J.; Hope, R.; Lopez-Bernal, J.; et al. Hospital admission and emergency care attendance risk for SARS-CoV-2 delta (B.1.617.2) compared with alpha (B.1.1.7) variants of concern: A cohort study. Lancet Infect. Dis. 2021, 22, 35–42. Available online: https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(21)00475-8/abstract (accessed on 29 August 2021). [CrossRef]
- Butowt, R.; Von Bartheld, C.S. Anosmia in COVID-19: Underlying Mechanisms and Assessment of an Olfactory Route to Brain Infection. Neuroscientist 2020, 27, 1073858420956905. [Google Scholar] [CrossRef]

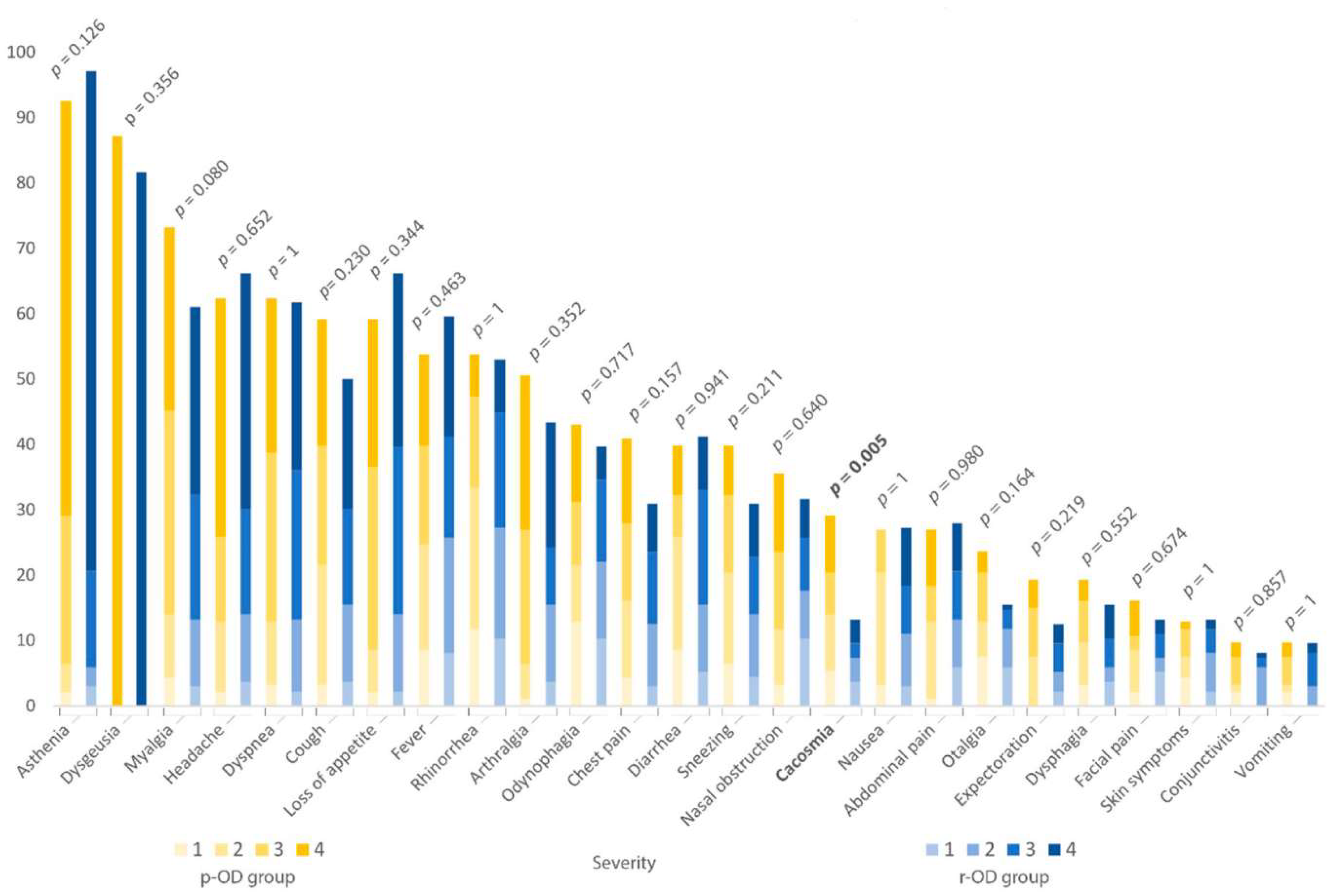
| Variables | Proportion of Patients | p-Value | ||
|---|---|---|---|---|
| Resolved Olfactory Dysfunction (n = 136) (59.4%) | Persistent Olfactory Dysfunction (n = 93) (40.6%) | Total (n = 229) (100%) | ||
| Time between questionnaire answer and symptoms onset, day (mean, extremes, SD) | 285.0 [211–335] ± 23.9 | 282.2 [281–366] ± 29.2 | 283.9 [211–366] ± 26.1 | 0.435 |
| Demographic and baseline characteristics | ||||
| Age, year (mean, extremes, SD) | 49.6 [20–94] ± 18.1 | 51.4 [19–98] ± 20.2 | 50.4 [19–98] ± 18.9 | 0.505 |
| Sex, % (no.) | 0.094 | |||
| Male | 41.9 (57) | 30.1 (28) | 37.1 (85) | |
| Female | 58.1 (79) | 69.9 (65) | 62.9 (144) | |
| HCW, % (no.) | 49.3 (67) | 49.5 (46) | 49.3 (113) | 1 |
| BMI (kg/m2) (mean, extremes, SD) | 26.8 [17.5–43.2] ± 5.6 | 26.3 [15.9–47] ± 5.7 | 26.6 [15.9–47] ± 5.7 | 0.594 |
| <18.5 | 3.0 (4) | 8.6 (8) | 5.2 (12) | 0.194 |
| [18.5–25] | 40.4 (55) | 35.5 (33) | 38.4 (88) | 0.548 |
| [25–30] | 29.4 (40) | 32.3 (30) | 30.6 (70) | 0.741 |
| ≥30 | 25.7 (35) | 22.6 (21) | 24.5 (56) | 0.708 |
| Pregnancy, % (no.) | 0.7 (1) | 2.2 (2) | 1.3 (3) | 0.567 |
| Current smoking, % (no.) | 6.6 (9) | 8.6 (8) | 7.4 (17) | 1 |
| Comorbidities | ||||
| Comorbidities, % (no.) | ||||
| Yes | 58.8 (80) | 60.2 (56) | 59.4 (136) | 1 |
| No | 41.2 (56) | 39.8 (37) | 40.6 (93) | 1 |
| Arterial hypertension, % (no.) | 22.8 (31) | 16.1 (15) | 20.1 (46) | 0.290 |
| Cardio-vascular diseases, % (no.) | 30.1 (41) | 23.7 (22) | 27.5 (63) | 0.674 |
| Cardiac arrhythmia | 4.4 (6) | 7.5 (7) | 5.7 (13) | 0.465 |
| Heart failure | 3.7 (5) | 3.2 (3) | 3.5 (8) | 1 |
| Coronary heart disease | 2.9 (4) | 1.1 (1) | 2.2 (5) | 0.651 |
| Diabetes mellitus, % (no.) | 4.4 (6) | 10.8 (10) | 7.0 (16) | 0.111 |
| Chronic kidney failure, % (no.) | 3.7 (5) | 1.1 (1) | 2.6 (6) | 0.405 |
| Neurologic diseases 1, % (no.) | 4.4 (6) | 9.7 (9) | 6.6 (15) | 0.188 |
| ORL diseases, % (no.) | 18.4 (25) | 25.8 (24) | 21.4 (49) | 0.221 |
| Rhinosinusitis nasal polyps | 0.0 (0) | 3.2 (3) | 1.3 (3) | 0.064 |
| Surgical rhinoplasty | 2.2 (3) | 3.2 (3) | 2.6 (6) | 0.687 |
| Allergic rhinitis | 14.0 (19) | 19.4 (18) | 16.2 (37) | 0.347 |
| Chronic rhinosinusitis | 3.7 (5) | 5.4 (5) | 4.4 (10) | 0.530 |
| Respiratory diseases, % (no.) | 10.3 (14) | 23.7 (22) | 15.7 (36) | 0.010 |
| COPD | 2.2 (3) | 1.1 (1) | 1.7 (4) | 0.650 |
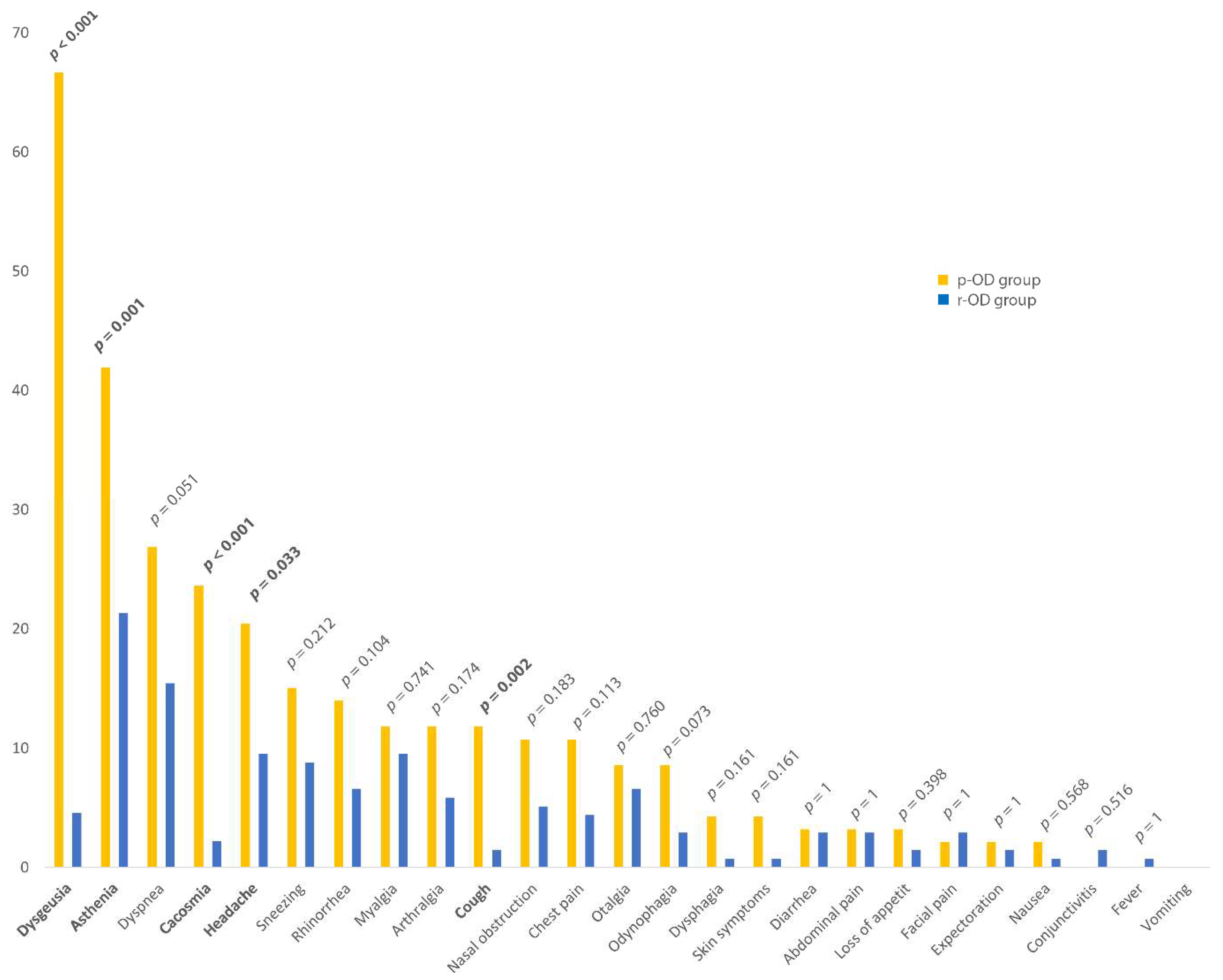
| Asthma | 7.4 (10) | 20.4 (19) | 12.7 (29) | 0.006 |
| Others 2 | 0.7 (1) | 2.2 (2) | 1.3 (3) | 0.567 |
| Past history of malignancy, % (no.) | 6.6 (9) | 7.5 (7) | 7.0 (16) | 0.982 |
| Actually treated | 0.0 (0) | 4.3 (4) | 1.7 (4) | 0.025 |
| Use of immunosuppressants,% (no.) | 0.0 (0) | 2.2 (2) | 2.2 (2) | 0.162 |
| Psychiatric disorders, % (no.) | 5.1 (7) | 7.5 (7) | 6.1 (14) | 0.643 |
| Depressive disorder | 4.4 (6) | 6.5 (6) | 5.2 (12) | 0.553 |
| Others 3 | 0.7 (1) | 1.1 (1) | 0.9 (2) | 1 |
| Hospitalization | ||||
| Hospitalization, % (no.) | 37.5 (51) | 37.6 (35) | 37.8 (86) | 1 |
| Duration of hospitalization, days (mean, extremes, SD) | 12.2 [1–55] ± 12.1 | 12.4 [1–73] ± 14.3 | 12.3 [1–73] ± 13.0 | 0.938 |
| Treatments received, % (no.) | Over 51 hospitalized patients | Over 35 hospitalized patients | Over 86 hospitalized patients | |
| Antibiotics | 82.4 (42) | 82.3 (29) | 82.6 (71) | 1 |
| Hydroxychloroquine | 66.7 (34) | 71.4 (25) | 68.6 (59) | 0.817 |
| Lopinavir/Ritonavir | 3.9 (2) | 5.7 (2) | 4.7 (4) | 1 |
| Steroids (Dexamethasone) | 19.6 (10) | 14.3 (5) | 17.4 (15) | 0.727 |
| Anti-IL-6 (Tocilizumab) | 5.9 (3) | 2.9 (1) | 4.7 (4) | 0.643 |
| Laboratory data | Over 49 analyzed patients | Over 35 analyzed patients | Over 84 analyzed patients | |
| Laboratory data on admission (mean, extremes, DS) | ||||
| White-cell count/mm3 (4.00–10.00/mm3) | 7.45 [2.66–23.95] ± 3.75 | 7.55 [3.!98–17.51] ± 3.37 | 7.49 [2.66–23.95] ± 3.58 | 0.895 |
| Lymphocytes/mm3 (1.50–4.00/mm3) | 0.95 [0.15–2.70] ± 0.50 | 0.95 [0.33–1.83] ± 0.42 | 0.95 [0.15–2.70] ± 0.46 | 0.991 |
| Hemoglobin, g/dL (13.5–17.5 g/dL) | 13.7 [10.4–18.2] ± 1.4 | 13.6 [10.5–17.0] ± 1.7 | 13.6 [10.4–18.2] ± 1.5 | 0.695 |
| Creatinine, μmol/L (65–120 μmol/L) | 88.2 [46–403] ± 57.0 | 70.7 [43–139] ± 21.6 | 80.9 [43–403] ± 44.3 | 0.055 |
| Creatinine clearance CKD-EPI, mL/min/1.73 m2 (69–119 mL/min/1.73 m2) | 83.3 [8–122] ± 22.4 | 92.1 [29–132] ± 22.2 | 87.0 [8–132] ± 46.3 | 0.078 |
| Alanine aminotransferase, U/L (8–45 U/L) | 49.6 [13–188] ± 39.2 | 54.0 [12–175] ± 42.5 | 51.4 [12–188] ± 40.1 | 0.660 |
| Aspartate aminotransferase, U/L (10–40 U/L) | 52.2 [11–159] ± 34.0 | 53.9 [17–193] ± 42.8 | 52.9 [11–193] ± 37.3 | 0.862 |
| C-reactive protein, mg/L (<5 mg/L) | 117.5 [10.1–478.7] ± 91.7 | 117.3 [0.1–377.6] ± 86.8 | 117.4 [0.1–478.7] ± 89.1 | 0.992 |
| C-reactive protein >100 mg/L, % (no.) | 44.9 (22) | 51.4 (18) | 47.6 (40) | 0.712 |
| RT-PCR SARS-CoV-2 CT E (mean, extremes, SD) | 27.0 [16.4–37.6] ± 5.81 | 26.3 [19.6–32.5] ± 5.06 | 26.8 [16.4–37.6] ± 5.53 | 0.661 |
| Radiological data | Over 38 CT-scanned patients | Over 27 CT-scanned patients | Over 65 CT-scanned patients | |
| Thoracic imaging features, % (no.) | ||||
| GGO | 89.5 (34) | 100 (27) | 93.4 (61) | 0.135 |
| Consolidation opacities | 73.7 (28) | 81.5 (22) | 76.9 (50) | 0.662 |
| Crazy-paving sign | 44.7 (17) | 44.4 (12) | 44.6 (29) | 1 |
| <25% extension | 55.3 (21) | 59.3 (16) | 56.9 (37) | 0.947 |
| >50% extension | 10.5 (4) | 14.9 (4) | 12.3 (8) | 0.709 |
| Complications % (no.) | Over 51 hospitalized patients | Over 35 hospitalized patients | Over 86 hospitalized patients | |
| SARS | 11.8 (6) | 14.3 (5) | 12.8 (11) | 0.752 |
| Transferred to ICU | 15.7 (8) | 14.3 (5) | 15.1 (13) | 1 |
| Mechanical ventilation | 15.7 (8) | 14.3 (5) | 15.1 (13) | 1 |
| Pleural Effusion | 11.8 (6) | 5.7 (2) | 9.3 (8) | 0.464 |
| Hepatitis | 15.7 (8) | 14.3 (5) | 15.1 (13) | 1 |
| Variables | Proportion of Patients | p-Value | ||
|---|---|---|---|---|
| Resolved Olfactory Dysfunction (n = 136) (59.4%) | Persistent Olfactory Dysfunction (n = 93) (40.6%) | Total (n = 229) (100%) | ||
| Gustatory dysfunction rate % (no.) | ||||
| Total | 81.6 (111) | 87.1 (81) | 83.4 (192) | 0.356 |
| Hypogeusia | 21.3 (29) | 30.1 (28) | 24.9 (57) | 0.176 |
| Ageusia | 60.3 (82) | 57.0 (53) | 59.0 (135) | 0.717 |
| Duration | ||||
| Recovery time of gustatory dysfunction, days (mean, extremes, SD) | 26.0 [0–252] ± 34.0 | 25.8 [1–113] ± 29.3 | 25.9 [0–252] ± 33.1 | 0.985 |
| Persistent gustatory dysfunction | ||||
| Over 111 patients | Over 81 patients | Over 192 patients | ||
| Gustatory dysfunction persistence rate % (no.) | ||||
| Total | 4.6 (5) | 66.7 (54) | 30.7 (59) | <0.001 |
| Persistent hypogeusia | 4.50 (5) | 64.2 (52) | 29.7 (57) | <0.001 |
| Persistent ageusia | 0.0 (0) | 2.50 (2) | 1.04 (2) | 0.164 |
| Short Version QOD-NS Items | Resolved OD Group (n = 136) | Persistent OD Group (n = 93) | Total (n = 229) | p-Value |
|---|---|---|---|---|
| Changes in my sense of smell isolate me socially. | 2.18 ± 1.15 | 2.10 ± 1.15 | 2.14 ± 1.15 | 0.812 |
| The problems with my sense of smell have a negative impact on my daily social activities. | 2.03 ± 1.16 | 1.78 ± 1.15 | 1.93 ± 1.16 | 0.319 |
| The problems with my sense of smell make me more irritable. | 1.97 ± 1.14 | 1.75 ± 1.14 | 1.88 ± 1.14 | 0.116 |
| Because of the problems with my sense of smell, I eat out less. | 1.46 ± 1.36 | 1.37 ± 1.36 | 1.42 ± 1.36 | 0.091 |
| Because of the problems with my sense of smell, I eat less than before (loss of appetite). | 0.99 ± 1.13 | 0.94 ± 1.12 | 0.97 ± 1.13 | 0.567 |
| Because of problems with my sense of smell, I must make more effort to relax. | 1.92 ± 1.17 | 1.83 ± 1.16 | 1.88 ± 1.17 | 0.949 |
| I am afraid I will never be able to get used to the problems with my sense of smell. | 1.36 ± 1.22 | 0.97 ± 1.21 | 1.21 ± 1.21 | 0.102 |
| Short version QOD-NS total score | 12.0 ± 6.03 | 10.7 ± 5.89 | 11.0 ± 5.94 | 0.137 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tipirdamaz, C.; Zayet, S.; Osman, M.; Mercier, J.; Bouvier, E.; Gendrin, V.; Bouiller, K.; Lepiller, Q.; Toko, L.; Pierron, A.; et al. Asthma and Cacosmia Could Be Predictive Factors of Olfactory Dysfunction Persistence 9 Months after SARS-CoV-2 Infection: The ANOSVID Study. Life 2022, 12, 929. https://doi.org/10.3390/life12070929
Tipirdamaz C, Zayet S, Osman M, Mercier J, Bouvier E, Gendrin V, Bouiller K, Lepiller Q, Toko L, Pierron A, et al. Asthma and Cacosmia Could Be Predictive Factors of Olfactory Dysfunction Persistence 9 Months after SARS-CoV-2 Infection: The ANOSVID Study. Life. 2022; 12(7):929. https://doi.org/10.3390/life12070929
Chicago/Turabian StyleTipirdamaz, Can, Souheil Zayet, Molka Osman, Julien Mercier, Elodie Bouvier, Vincent Gendrin, Kévin Bouiller, Quentin Lepiller, Lynda Toko, Alix Pierron, and et al. 2022. "Asthma and Cacosmia Could Be Predictive Factors of Olfactory Dysfunction Persistence 9 Months after SARS-CoV-2 Infection: The ANOSVID Study" Life 12, no. 7: 929. https://doi.org/10.3390/life12070929
APA StyleTipirdamaz, C., Zayet, S., Osman, M., Mercier, J., Bouvier, E., Gendrin, V., Bouiller, K., Lepiller, Q., Toko, L., Pierron, A., Royer, P.-Y., Garnier, P., Kadiane-Oussou, N.-J., Chirouze, C., & Klopfenstein, T. (2022). Asthma and Cacosmia Could Be Predictive Factors of Olfactory Dysfunction Persistence 9 Months after SARS-CoV-2 Infection: The ANOSVID Study. Life, 12(7), 929. https://doi.org/10.3390/life12070929







