Carbon Pool in Mexican Wetland Soils: Importance of the Environmental Service
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Importance of Natural Wetlands to Ecosystem Services
3.2. Wetland Types
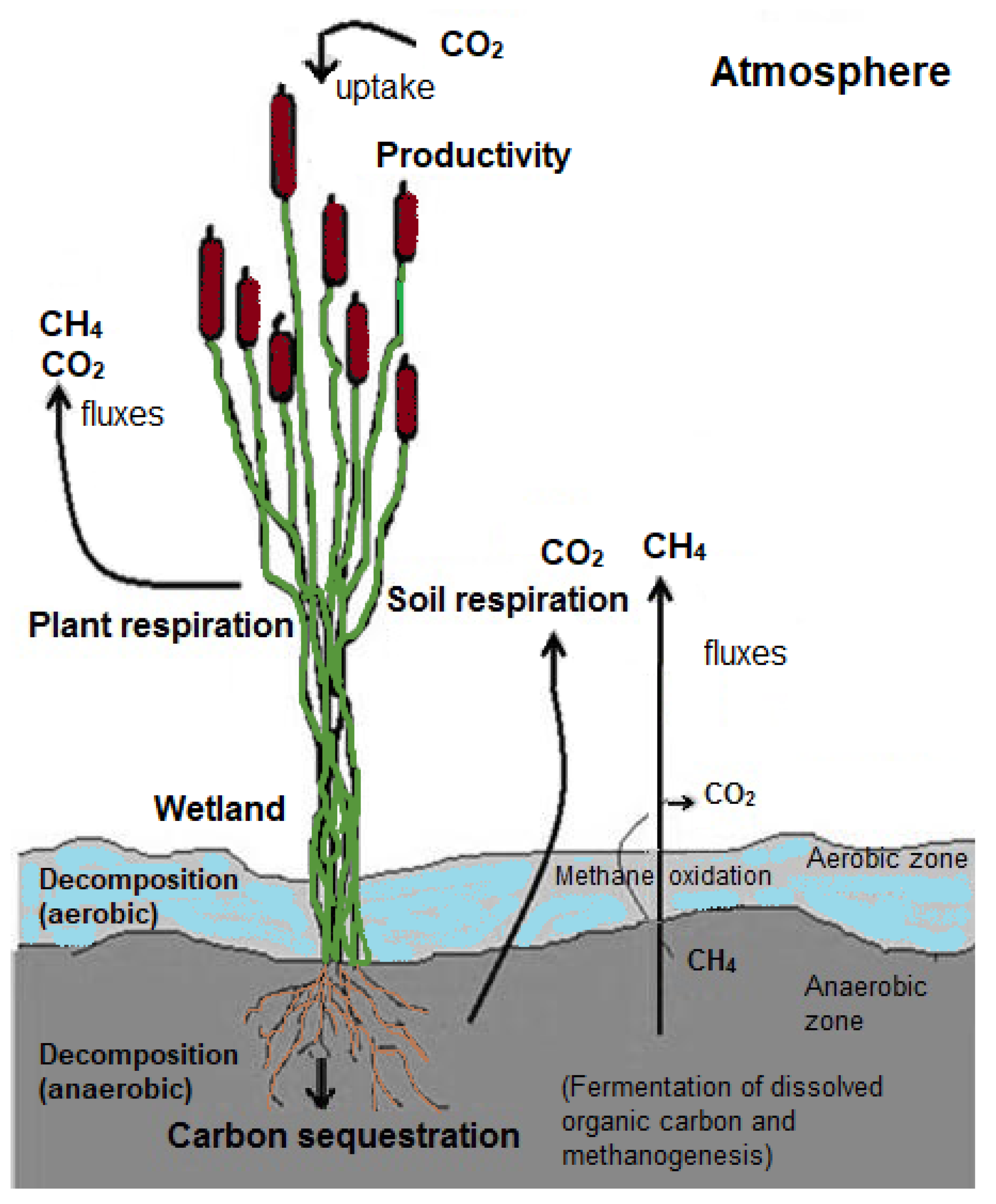
3.3. The Carbon Cycle and Dominant Organisms in Wetland Soils
3.4. Carbon Sequestration in Mexican Wetland Soils
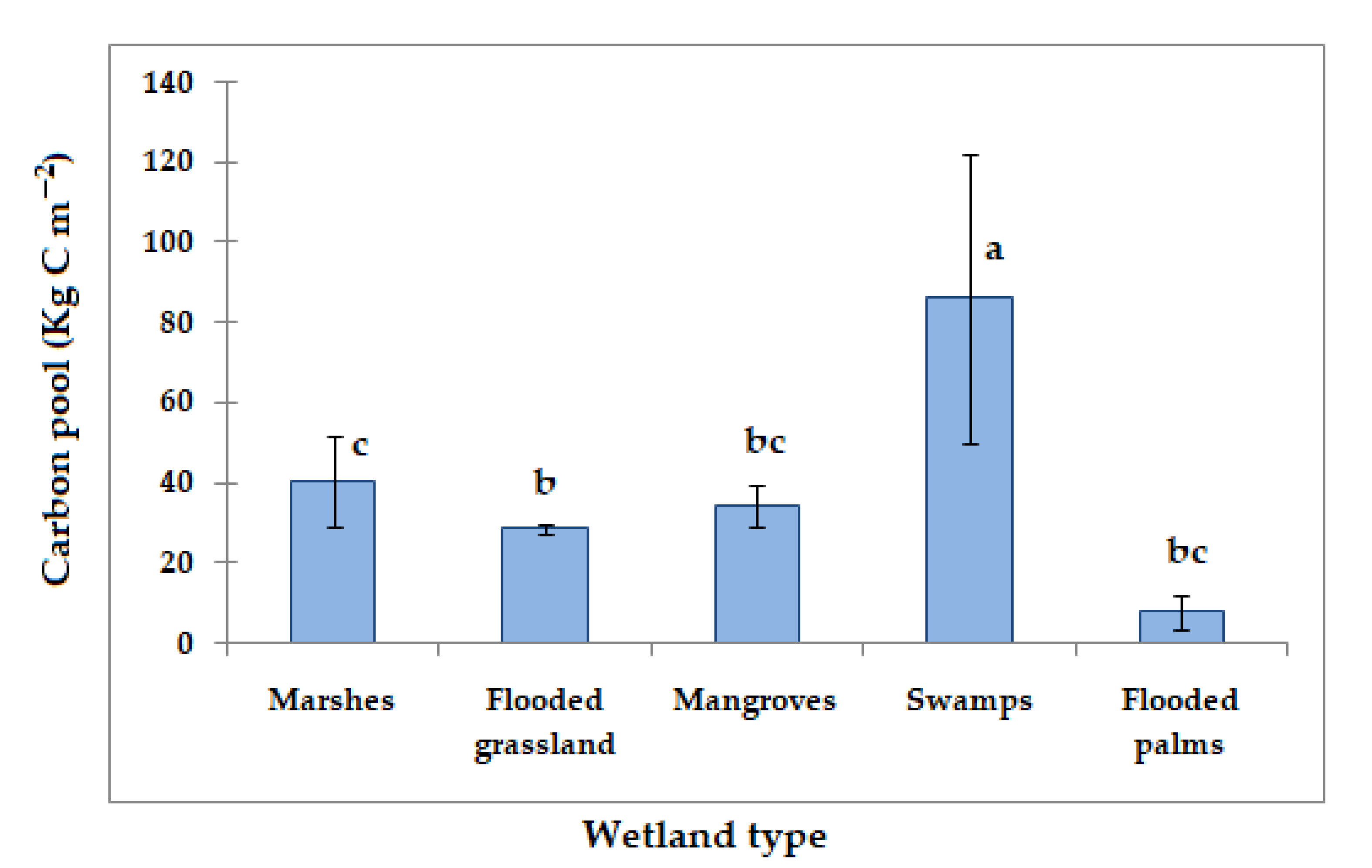
3.5. Mean Carbon Sequestration or Carbon Pool in Mexican Wetland Soils
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Pachauri, R.K.; Allen, M.R.; Barros, V.R.; Broome, J.; Cramer, W.; Christ, R.; Church, J.A.; Clarke, L.; Dahe, Q.; Dasgupta, P.; et al. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Pachauri, R.K., Meyer, L., Eds.; IPCC: Geneva, Switzerland, 2014; p. 151. Available online: https://www.ipcc.ch/site/assets/uploads/2018/02/SYR_AR5_FINAL_full.pdf (accessed on 8 December 2021).
- Mitsch, W.J.; Gosselink, J.G. Wetlands, 5th ed.; John Wiley and Sons Inc.: New York, NY, USA, 2015. [Google Scholar]
- Marín-Muñiz, J.L. Humedales, Riñones del Planeta y Hábitat de MúltiplesEspecies; Editora de Gobierno del Estado de Veracruz: Xalapa, VER, Mexico, 2018; Available online: https://www.sev.gob.mx/servicios/publicaciones/serie_fueraseries/Humedales_Impresion.pdf (accessed on 5 April 2022). (In Spanish)
- Salimi, S.; Almuktar, S.A.; Scholz, M. Impact of climate change on wetland ecosystems: A critical review of experimental wetlands. J. Environ. Manag. 2021, 286, 112160. [Google Scholar] [CrossRef] [PubMed]
- Sood, A.; Vyas, S. Carbon Capture and Sequestration- A Review. IOP Conf. Ser. Earth Environ. Sci. 2017, 83, 12024. [Google Scholar] [CrossRef]
- Xu, T.; Weng, B.; Yan, D.; Wang, K.; Li, X.; Bi, W.; Li, M.; Cheng, X.; Liu, Y. Wetlands of International Importance: Status, Threats, and Future Protection. Int. J. Environ. Res. Public Health 2019, 16, 1818. [Google Scholar] [CrossRef]
- Mitsch, W.J.; Nahlik, A.; Wolski, P.; Bernal, B.; Zhang, L.; Ramberg, L. Tropical wetlands: Seasonal hydrologic pulsing, carbon sequestration, and methane emissions. Wetl. Ecol. Manag. 2009, 18, 573–586. [Google Scholar] [CrossRef]
- Bridgham, S.D.; Megonigal, J.P.; Keller, J.K.; Bliss, N.B.; Trettin, C. The carbon balance of North American wetlands. Wetlands. 2006, 26, 889–916. [Google Scholar] [CrossRef]
- Nahlik, A.M.; Fennessy, M.S. Carbon storage in US wetlands. Nat. Commun. 2016, 7, 13835. [Google Scholar] [CrossRef]
- Mitsch, W.J.; Bernal, B.; Nahlik, A.; Mander, Ü.; Zhang, L.; Anderson, C.J.; Jørgensen, S.E.; Brix, H. Wetlands, carbon, and climate change. Landsc. Ecol. 2012, 28, 583–597. [Google Scholar] [CrossRef]
- Available online: http://www.ramsar.org/ (accessed on 10 December 2021).
- Reid, W.V. Millennium Ecosystem Assessment. In Ecosystems and Human Well-Being: Synthesis; Island Press: Washington, DC, USA, 2005. [Google Scholar]
- Moreno-Casasola, P.; Infante-Mata, D.M. Conociendo los Manglares, las Selvas Inundables y los Humedales Herbáceos; INECOL-OIMT-CONAFOR: Xalapa, Veracruz, Mexico, 2016; 62p, Available online: https://idoc.pub/documents/conociendo-los-manglares-y-selvas-inundables-vnd1j62p9wnx (accessed on 1 May 2022). (In Spanish)
- Joyce, C.; Simpson, M.; Casanova, M. Future wet grasslands: Ecological implications of climate change. Ecosyst. Health Sustain. 2016, 2, e01240. [Google Scholar] [CrossRef]
- Kayranli, B.; Scholz, M.; Mustafa, A.; Hedmark, Å. Carbon storage and fluxes within freshwater wetlands: A critical review. Wetlands. 2010, 30, 111–124. [Google Scholar] [CrossRef]
- Torres-Alvarado, R.; Ramírez-Vives, F.; Fernández, F.; Barriga-Sosa, I. Methanogenesis and methane oxidation in wetlands. Implications in the global carbon cycle. Hidrobiológica. 2005, 15, 327–349. Available online: http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S0188-88972005000300009 (accessed on 11 June 2022). (In Spanish).
- Wilms, R.; Sass, H.; Köpke, B.; Cypionka, H.; Engelen, B. Methane and sulfate profiles within the subsurface of a tidal flat are reflected by the distribution of sulfate-reducing bacteria and methanogenic archaea. FEMS Microbiol. Ecol. 2007, 59, 611–621. [Google Scholar] [CrossRef]
- Struwe, S.; Kjøller, A. Field determination of denitrification in water-logged forest soils. FEMS Microbiol. Lett. 1989, 62, 71–78. [Google Scholar] [CrossRef]
- A Yarwood, S. The role of wetland microorganisms in plant-litter decomposition and soil organic matter formation: A critical review. FEMS Microbiol. Ecol. 2018, 94. [Google Scholar] [CrossRef] [PubMed]
- Vepraskas, M.; Craft, C. Wetland Soils. Genesis, Hydrology, Landscapes, and Classification; CRC Press: Boca Ratón, FL, USA, 2016. [Google Scholar]
- Marin-Muñiz, J.L.; Alarcón, M.E.H.; Rivera, E.S.; Moreno-Casasola, P. Percepciones sobre servicios ambientales y pérdida de humedales arbóreos en la comunidad de Monte Gordo, Veracruz. Madera Bosques. 2016, 22. [Google Scholar] [CrossRef][Green Version]
- Sjögersten, S.; Black, C.R.; Evers, S.; Hoyos-Santillan, J.; Wright, E.L.; Turner, B. Tropical wetlands: A missing link in the global carbon cycle? Glob. Biogeochem. Cycles. 2014, 28, 1371–1386. [Google Scholar] [CrossRef]
- Bernal, B.; Mitsch, W.J. A comparison of soil carbon pools and profiles in wetlands in Costa Rica and Ohio. Ecol. Eng. 2008, 34, 311–323. [Google Scholar] [CrossRef]
- Bernal, B.; Mitsch, W.J. Carbon sequestration in freshwater wetlands in Costa Rica and Botswana. Biogeochemistry. 2013, 115, 77–93. [Google Scholar] [CrossRef]
- Hernandez, M.E.; Marín-Muñiz, J.L.; Casasola, P.M.; Vázquez, V. Comparing soil carbon pools and carbon gas fluxes in coastal forested wetlands and flooded grasslands in Veracruz, Mexico. Int. J. Biodivers. Sci. Ecosyst. Serv. Manag. 2014, 11, 5–16. [Google Scholar] [CrossRef]
- Kumagai, J.A.; Costa, M.T.; Ezcurra, E.; Aburto-Oropeza, O. Prioritizing mangrove conservation across Mexico to facilitate 2020 NDC ambition. Ambio. 2020, 49, 1992–2002. [Google Scholar] [CrossRef]
- Alongi, D.M. Carbon sequestration in mangrove forests. Carbon Manag. 2012, 3, 313–322. [Google Scholar] [CrossRef]
- Harvey, G.E.; Grünwald, M. The Swamp: The Everglades, Florida, and the Politics of Paradise. J. South. Hist. 2007, 73, 766. [Google Scholar] [CrossRef]
- Gonzalez-Marín, R.M.; Moreno-Casasola, P.; Orellana, R.G.; Castillo, A. Palm use and social values in rural communities on the coastal plains of Veracruz, Mexico. Environ. Dev. Sustain. 2012, 14, 541–555. [Google Scholar] [CrossRef]
- González-Marín, R.; Moreno-Casasola, P.; Orellana, R.; Castillo, A. Traditional wetland palm uses in construction and cooking in Veracruz Gulf of Mexico. Indian J. Tradit. Knowl. 2012, 11, 408–413. Available online: http://nopr.niscair.res.in/handle/123456789/14380 (accessed on 20 April 2022).
- Marín-Muñiz, J.L.; Hernández, M.E.; Moreno-Casasola, P. Comparing soil carbon sequestration in coastal freshwater wetlands with various geomorphic features and plant communities in Veracruz, Mexico. Plant Soil. 2014, 378, 189–203. [Google Scholar] [CrossRef]
- Campos, A.C.; Hernández, M.E.; Moreno-Casasola, P.; Espinosa, E.C.; R., A.R.; Mata, D.I. Soil water retention and carbon pools in tropical forested wetlands and marshes of the Gulf of Mexico. Hydrol. Sci. J. 2011, 56, 1388–1406. [Google Scholar] [CrossRef]
- Sjögersten, S.; de la Barreda-Bautista, B.; Brown, C.; Boyd, D.; Lopez-Rosas, H.; Hernández, E.; Monroy, R.; Rincón, M.; Vane, C.; Moss-Hayes, V.; et al. Coastal wetland ecosystems deliver large carbon stocks in tropical Mexico. Geoderma. 2021, 403, 115173. [Google Scholar] [CrossRef]
- Adame, M.F.; Kauffman, J.B.; Medina, I.; Gamboa, J.N.; Torres, O.; Caamal, J.P.; Reza, M.; Herrera-Silveira, J.A. Carbon Stocks of Tropical Coastal Wetlands within the Karstic Landscape of the Mexican Caribbean. PLoS ONE. 2013, 8, e56569. [Google Scholar] [CrossRef]
- Paredes-García, S.S.; Moreno-Casasola, P.; Barrera, E.d.l.; García-Oliva, F.; Lindig-Cisneros, R. Biomasa y carbonoalmacenadoen un humedal continental enCuitzeo, Michoacán, Mexico. Tecnol. Cienc. Agua. 2021, 12, 416–441. [Google Scholar] [CrossRef]
- Adame, M.F.; Santini, N.S.; Tovilla, C.; Vázquez-Lule, A.; Castro, L.; Guevara, M. Carbon stocks and soil sequestration rates of tropical riverine wetlands. Biogeosciences. 2015, 12, 3805–3818. [Google Scholar] [CrossRef]
- Morales-Ojeda, S.M.; Herrera-Silveira, J.A.; Orellana, R. Almacenes de carbono en un paisaje de humedal cárstico a lo largo de un corredor transversal costero de la Península de Yucatán. Madera Bosques. 2021, 27, 1–18. [Google Scholar] [CrossRef]
- Hernández, M.E.; Campos, A.; Marín-Muñiz, J.L.; Moreno-Casasola, P. Almacenes de carbono en selvas inundables, manglares, humedales herbáceos y potreros inundables. In Servicios Ecosistémicos de las Selvas y Bosques Costeros de Veracruz; Moreno Casasola, P., Ed.; Inecol ITTO Conafor INECC: Xalapa, VER, Mexico, 2016; pp. 121–129. Available online: https://www.itto.int/files/itto_project_db_input/3000/Technical/Servicios_Ecosostemicos_de_las_selvas_y_bosques_costeros.pdf (accessed on 6 May 2022). (In Spanish)
- Herrera, J.; Camacho, A.; Pech, E.; Pech, M.; Ramírez, J.; Teutli, C. Dinámica del carbono (almacenes y flujos) en manglares de Mexico. Terra Latinoamericana. 2016, 34, 61–72. [Google Scholar]
- Cáliz, E.M.; Peña, A.G.; Castorena, M.D.C.G.; Solorio, C.A.O.; López, D.J.P. Los manglares de Tabasco, una reserva natural de carbono. Madera Bosques. 2016, 8, 115–128. [Google Scholar] [CrossRef]
- Moreno-May, G.; Cerón, J.; Cerón, R.; Guerra, J.; Amador, L.; Endañú, E. Evaluation of carbon storage potential in mangrove soils of Isla del Carmen. Unacar Tecociencia. 2010, 4, 23–39. Available online: https://www.academia.edu/2568197/Estimaci%C3%B3n_del_potencial_de_captura_de_carbono_en_suelos_de_manglar_de_isla_del_Carmen (accessed on 1 November 2021).
- Cerón-Bretón, J.G.; Cerón-Bretón, R.M.; Rangel-Marrón, M.; Estrella-Cahuich, A. Evaluation of carbon sequestration potential in undisturbed mangrove forest in Términos Lagoon Campeche. Dev. Energy Environ. Econ. 2010, 1, 295–300. Available online: https://www.researchgate.net/publication/279903142_Evaluation_of_carbon_sequestration_potential_in_undisturbed_mangrove_forest_in_Terminos_Lagoon_Campeche (accessed on 25 November 2019).
- Kauffman, J.B.; Trejo, H.H.; Del Carmen Jesus Garcia, M.; Heider, C.; Contreras, W.M. Carbon stocks of mangroves and losses arising from their conversion to cattle pastures in the Pantanos de Centla, Mexico. Wetl. Ecol. Manag. 2015, 24, 203–216. [Google Scholar] [CrossRef]
- Adame, M.F.; Fry, B. Source and stability of soil carbon in mangrove and freshwater wetlands of the Mexican Pacific coast. Wetl. Ecol. Manag. 2016, 24, 129–137. [Google Scholar] [CrossRef]
- Moreno-Casasola, P.; Hernández, M.E.; Campos, A.C. Hydrology, Soil Carbon Sequestration and Water Retention along a Coastal Wetland Gradient in the Alvarado Lagoon System, Veracruz, Mexico. J. Coast. Res. 2017, 77, 104–115. [Google Scholar] [CrossRef]
- Santiago, L. Estimación del Potencial de Captura de Carbono (c) del Bosque de Manglar de Tumilco de Tuxpan, Veracruz, México: Tesis Maestría en Manejo de Ecosistemas Marinos y Costeros. Universidad Veracruzana: Tuxpan, Veracruz, México, 2018; Available online: https://www.uv.mx/pozarica/mmemc/files/2020/02/LuisAlbertoSantiagoMolina.pdf (accessed on 3 June 2022).
- Bautista-Olivas, A.I.; Mendoza-Cariño, M.; Cesar-Rodriguez, J.; Colado-Amador, C.E.; Robles-Zazueta, C.A.; Meling-López, A.E. Above-ground biomass and carbon sequestration in mangroves in the arid area of the northwest of Mexico: Bahía del Tóbari and Estero El Sargento, Sonora. Rev. Chapingo Ser. Cienc. For. Ambient. 2018, 24, 387–403. [Google Scholar] [CrossRef]
- Valdés, V.E.; Valdés, J.I.; Ordaz, V.M.; Gallardo, J.F.; Pérez, J.; Ayala, C. Evaluación del carbono orgánico en los suelos de manglares de Nayarit. Rev. Mex. Cienc. Forestales. 2011, 2, 807–815. Available online: http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S2007-11322011000600005 (accessed on 28 October 2021). (In Spanish).
- Ochoa-Gómez, J.G.; Lluch-Cota, S.E.; Rivera-Monroy, V.H.; Lluch-Cota, D.B.; Troyo-Diéguez, E.; Oechel, W.; Serviere-Zaragoza, E. Mangrove wetland productivity and carbon stocks in an arid zone of the Gulf of California (La Paz Bay, Mexico). For. Ecol. Manag. 2019, 442, 135–147. [Google Scholar] [CrossRef]
- Hernández, M.E.; Junca-Gómez, D. Carbon stocks and greenhouse gas emissions (CH4 and N2O) in mangroves with different vegetation assemblies in the central coastal plain of Veracruz Mexico. Sci. Total Environ. 2020, 741, 140276. [Google Scholar] [CrossRef] [PubMed]
- Arias, X. Carbono, Nitrógeno y Azufre en Manglares de Paraíso Tabasco; Tesis Ingeniero en Restauración Forestal; Universidad Au-tónoma: Chapingo, Mexico, 2018; Available online: http://dicifo.chapingo.mx/pdf/tesislic/2018/Arias_Vel%C3%A1zquez_Xochitl_Rosario.pdf (accessed on 9 November 2021).
- Gutiérrez-Mendoza, J.; Herrera-Silveira, J. Almacenes de Carbono en manglares de tipo Chaparro en un escenario cárstico. In Estado Actual del Conocimiento del Ciclo del Carbono Y Sus Interacciones en México: Síntesis A 2014; Paz, F., Wong, J., Eds.; Programa Mexicano del Carbono; Centro de investigación y estudios avanzados del Instituto Politécnico Nacional, unidad Mérida y Centro de investigación y asistencia en tecnología y diseño del estado de Jalisco: Jalisco, México, 2014; pp. 405–414. Available online: http://pmcarbono.org/pmc/publicaciones/Libro_Merida_2014_PMC_ISBN-web.pdf (accessed on 20 October 2021). (In Spanish)
- Herrera-Silveira, J.; Teutli-Hernández, C.; Caamal-Sosa, J.; Pech-Cardenas, M.; Pech-Poot, E.; Carrillo-Baeza, L.; Zenteno, K.; Erosa, J.; Pérez, O.; Gamboa, S. Almacenes y flujos de carbono en diferentes tipos ecológicos de manglares en Celestun, Yucatán. In Estado Actual del Conocimiento del Ciclo del Carbono y sus Interacciones en México: Síntesis a 2018; Paz, F., Torres, R., Velázquez, Eds.; Programa Mexicano del Carbono; Centro de investigación científica y de educación superior de Ensenada, Universidad Autónoma de Baja California: Baja, México, 2018; pp. 219–225. [Google Scholar]
- Ezcurra, P.; Ezcurra, E.; Garcillán, P.P.; Costa, M.T.; Aburto-Oropeza, O. Coastal landforms and accumulation of mangrove peat increase carbon sequestration and storage. Environ. Sci. 2016, 113, 4404–4409. [Google Scholar] [CrossRef] [PubMed]
- Barreras-Apodaca, A.; Sánchez-Mejía, Z.; Bejarano, M.; Méndez-Barroso, L.; Borquez-Olguín, R. Carbono almacenado en la capa superficial de suelo de dos manglares geográficamente contrastantes. In Estado actual del Conocimiento del Ciclo del Carbono y sus Interacciones en México: Síntesis a 2017; Paz, F., Velázquez, A., Rojo, M., Eds.; Programa Mexicano del Carbono; InstitutoTecnológico de Sonora: Álamos, Mexico, 2017; pp. 258–264. (In Spanish) [Google Scholar]
- Castillo-Cruz, I.; De la Rosa-Meza, K. Cuantificación de carbono en manglares en El Rabón, dentro de la RB Marismas Nacionales, Nayarit. In Estado Actual del Conocimiento del Ciclo del Carbono y sus Interacciones en México: Síntesis a 2017; Paz, F., Velázquez, A., Rojo, M., Eds.; (In Spanish). Programa Mexicano del Carbono; InstitutoTecnológico de Sonora: Álamos, México, 2017; pp. 252–257. [Google Scholar]
- Pech-Poot, E.; Herrera-Silveira, J.; Caamal-Sosa, J.; Cortes-Balan, O.; Carrillo-Baeza, L.; Teutli-Hernández, C. Carbono en se-dimentos de manglares de ambientes cársticos: La Península de Yucatán. In Estado Actual del Conocimiento del Ciclo del Carbono y susInteraccionesen México: Síntesis a 2016; Paz, F., Torres, M., Eds.; Programa Mexicano del Carbono; Universidad Autónoma del Estado de Hidalgo: Campo de Tiro, México, 2016; pp. 336–343. (In Spanish) [Google Scholar]
- Velázquez-Pérez, C.; Tovilla-Hernández, C.; Romero-Berny, E.I.; De Jesús-Navarrete, A. Estructura del manglar y su influencia en el almacén de carbono en la Reserva La Encrucijada, Chiapas, México. Madera Bosques. 2019, 25. [Google Scholar] [CrossRef]
- Marín-Muñiz, J.L.; Hernández, M.E.; Moreno-Casasola, P. Soil carbon sequestration in coastal freshwater wetlands of Veracruz. Trop. Subtrop. Agroecosyst. 2011, 13, 365–372. Available online: http://www.revista.ccba.uady.mx/ojs/index.php/TSA/article/view/1336 (accessed on 25 May 2022).
- Alamilla, S. Gradientes de Carbono por Tipo de Suelo y Vegetación en Quintana Roo; Tesis Licenciatura en Manejo de Recursos Naturales. Chetumal, Quitana: Roo, México, 2018; Available online: http://risisbi.uqroo.mx/bitstream/handle/20.500.12249/1973/S590.2018-1973.pdf?sequence=1&isAllowed=y (accessed on 1 October 2021). (In Spanish)
- Sánchez, E. Caracterización de tres Propiedades del Suelo en Humedales Transformados a Potreros, en el Municipio de Jamapa, Veracruz y su Entorno. Tesis especialista en diagnóstico y gestión Ambiental; Facultad de Ciencias Químicas; Universidad Veracruzana: Xalapa, Veracruz, México, 2015; Available online: https://cdigital.uv.mx/bitstream/handle/123456789/42319/SanchezGarciaEdgar.pdf?sequence=2&isAllowed=y (accessed on 6 October 2021). (In Spanish)
- Bernal, B.; Mitsch, W.J. Carbon Sequestration in Two Created Riverine Wetlands in the Midwestern United States. J. Environ. Qual. 2013, 42, 1236–1244. [Google Scholar] [CrossRef]
- Marin-Muñiz, J.L. Carbon balance in tropical freshwater wetland on the coastal plain of the Gulf of Mexico. Limnetica 2020, 39, 653–665. [Google Scholar] [CrossRef]
- Ji, H.; Han, J.; Xue, J.; Hatten, J.A.; Wang, M.; Guo, Y.; Li, P. Soil organic carbon pool and chemical composition under different types of land use in wetland: Implication for carbon sequestration in wetlands. Sci. Total Environ. 2020, 716, 136996. [Google Scholar] [CrossRef]
- Zitácuaro-Contreras, I. Administración de Humedales Artificiales con Perspectiva de Género como Estrategia Sustentable para el Saneamiento de Aguas Residuales Municipales. Tesis de Doctorado, El Colegio de Veracruz, Veracruz, Mexico, 2021. [Google Scholar]
- Mitsch, W.J.; Hernandez, M.E. Landscape and climate change threats to wetlands of North and Central America. Aquat. Sci. 2012, 75, 133–149. [Google Scholar] [CrossRef]
- Martínez, A.; Manzanilla, S.; Hidalgo, J. Vulnerability to Climate Change of Marine and Coastal Fisheries in Mexico. Atmosfera. 2011, 24, 103–123. Available online: http://www.scielo.org.mx/pdf/atm/v24n1/v24n1a8.pdf (accessed on 5 May 2022).
- Vega-López, E. Valor Económico Potencial de las Áreas Naturales Protegidas Federales de México como Sumideros de Carbono. The Nature Conservancy-Mexico. Available online: https://docplayer.es/67251566-Valor-economico-potencial-de-las-areas-naturales-protegidas-federales-de-mexico-como-sumideros-de-carbono.html (accessed on 11 June 2022). (In Spanish).
- Crooks, S.; Rybczyk, J.; O’Connell, K.; Devier, D.L.; Poppe, K.; Emmett-Mattox, S. Coastal Blue Carbon Opportunity Assessment for the Snohomish Estuary: The Climate Benefits of Estuary Restoration; Report by Environmental Science Associates, Western Washington University, Earth Corps, and Restore America’s Estuaries: Bellingham, WA, USA, 2014. [Google Scholar] [CrossRef]
- Chastain, S.; Kohfeld, K.E. Blue Carbon in tidal wetlands of the pacific coast of Canada: Commission for Environmental Cooperation’s (CEC’s) 2015–2016 project, North American Blue Carbon: Next Steps in Science for Policy. 2017. Available online: http://www.cec.org/publications/blue-carbon-in-tidal-wetlands-of-the-pacific-coast-of-canada/ (accessed on 20 May 2022).
- Raw, J.; Julie, C.; Adams, J. A comparison of soil carbon pools across a mangrove-salt marsh ecotone at the southern African warm-temperate range limit. S. Afr. J. Bot. 2019, 127, 301–307. [Google Scholar] [CrossRef]
- Abdul, D.; Marín, A.; Trombetti, M.; San Roman, S. Carbon Pools and Sequestration Potential of Wetlands in the European Union; European Topic Centre on Urban, Land and Soil Systems, Viena and Malaga: Catalonia, Spain, 2021; ISBN 9783200074330. Available online: https://www.eionet.europa.eu/etcs/etc-di/products/etc-uls-report-10-2021-carbon-pools-and-sequestration-potential-of-wetlands-in-the-european-union (accessed on 1 June 2022).
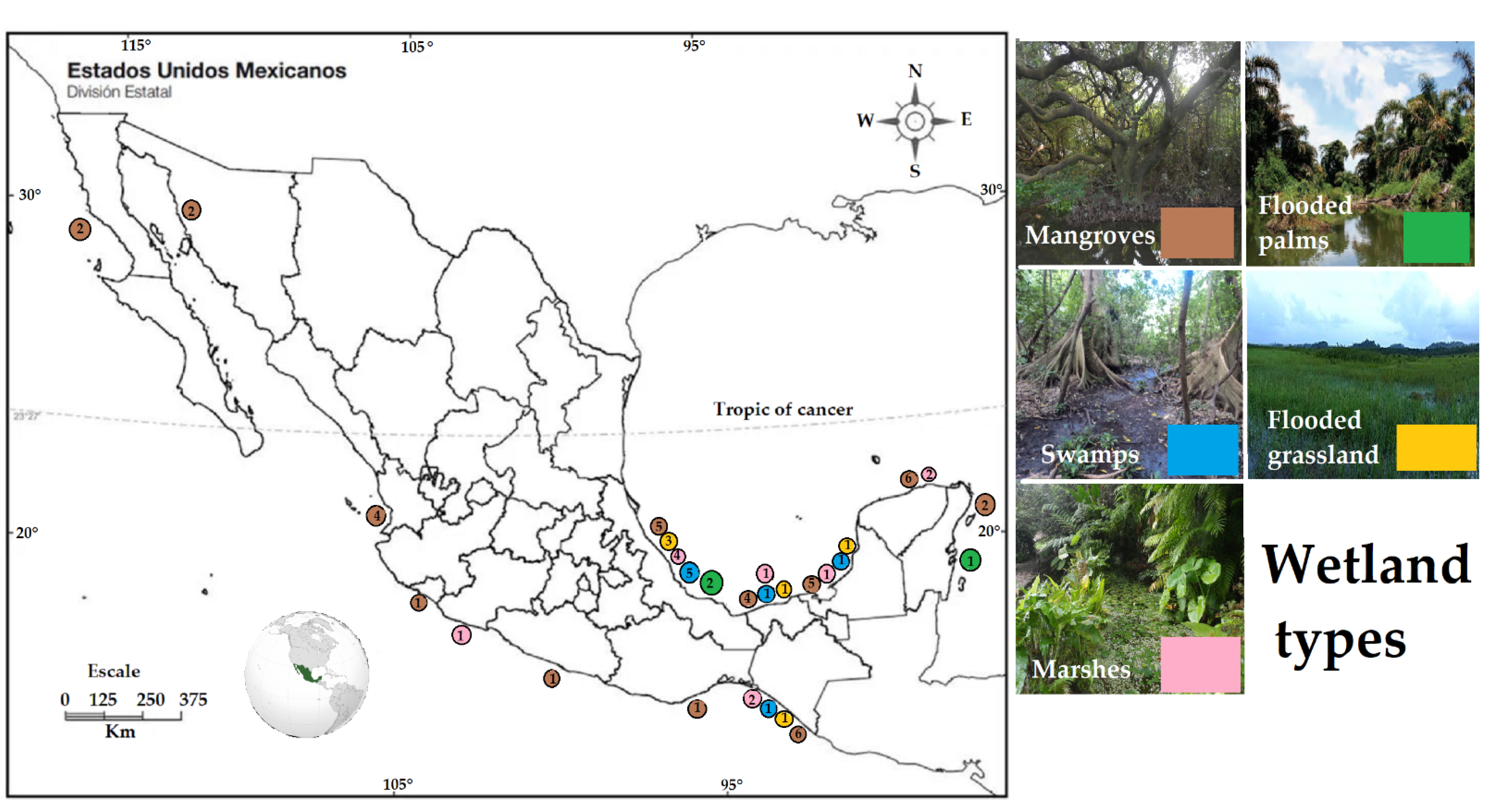
| Forested Wetland Type | Site (Municipality or Area, State) | Carbon Stock (Kg C m−2) | Location in the Map (Figure 2) | Reference |
|---|---|---|---|---|
| Marshes | Tecolutla and Vega de Alatorre, Veracruz | 25.9 | D | Marín-Muñiz [31] |
| Marshes | Alto Lucero and Tecolutla, Veracruz | 31.0 | D | Campos [32] |
| Marshes | Veracruz, Tabasco/Campeche, Chiapas | 110 | D, G, E, F | Sjögersten et al. [33] |
| Marshes | Yucatán Peninsula | 17.8 | H | Adame et al. [34] |
| Marshes | Cuitzeo, Michoacán | 16.8 | K | Paredes-García et al. [35] |
| Marshes | La Encrucida, Biosphere Reserve, Chiapas | 33.7 | F | Adame et al. [36] |
| Marshes | Yucatán Peninsula | 21.2 | H | Morales-Ojeda et al. [37] |
| Marshes | Río Blanco, Veracruz | 68 | D | Hernández et al. [38] |
| Flooded grassland | Jamapa y Yagual, Veracruz | 28 | D | Hernández et al. [38] |
| Flooded grassland | Veracruz, Tabasco/Campeche, Chiapas | 27.1 | D, E, G, F | Sjögersten et al. [33] |
| Flooded grassland | Estero Dulce and Boquilla de Oro, Veracruz | 30.6 | D | Hernández et al. [25] |
| Mangrove | Yucatán Peninsula | 28.0 | H | Morales-Ojeda et al. [37] |
| Mangrove | Veracruz, Tabasco/Campeche, Chiapas | 93 | D | Sjögersten et al. [33] |
| Mangrove | Oaxaca and Guerrero | 66.3 | M, L | Herrera et al. [39] |
| Mangrove | Huimanguillo and Cárdenas, Tabasco | 64.7 | E | Moreno et al. [40] |
| Mangrove | Laguna de Términos, Campeche | 25.2 | G | Moreno-May et al. [41] |
| Mangrove | Carmen city, Campeche | 11.7 | G | Ceron-breton et al. [42] |
| Mangrove | Yucatán Peninsula | 66.4 | H | Adame et al. [34] |
| Mangrove | La Encrucida, Biosphere Reserve, Chiapas | 78.5 | F | Adame et al. [36] |
| Mangrove | Pantanos de Centla, Tabasco and Campeche | 45.8 | E, G | Kauffman et al. [43] |
| Mangrove | Vega de Alatorre, Veracruz | 22 | D | Hernández et al. [38] |
| Mangrove | La Encrucida, Biosphere Reserve, Chiapas | 28.4 | F | Adame and Fry. [44] |
| Mangroves | Alvarado, Veracruz | 16 | D | Moreno-Casasola et al. [45] |
| Mangrove | Tuxpan, Veracruz | 14.7 | D | Santiago [46] |
| Mangrove | Agua Brava Lagooon, Nayarit | 4.2 | C | Herrera-Silveira et al. [39] |
| Mangrove | Bahía Tóbari, Sonora | 7.9 | B | Bautista-Olivas et al. [47] |
| Mangrove | Cuyutlán, Colima | 10.2 | J | Herrera-Silveira et al. [39] |
| Mangrove | Nayarit | 12 | C | Valdés et al. [48] |
| Mangrove | La Paz Bay, Baja California | 17.5 | A | Ochoa-Gómez et al. [49] |
| Mangrove | Central coastal plain of Veracruz | 37.5 | D | Hernández and Junca-Gómez [50] |
| Mangrove | Paraíso Tabasco | 20 | E | Arias [51] |
| Mangrove | Península Yucatán | 28.7 | H | Gutiérrez-Mendoza and Herrera-Silveira[52] |
| Mangrove | Celestun, Yucatán | 61.6 | H | Herrera-Silveira et al. [53] |
| Mangrove | Nayarit | 10 | C | Valdés et al. [48] |
| Mangrove | Magdalena and Malandra bay. Baja California | 22.5 | A | Ezcurra et al. [54] |
| Mangrove | Sian Ka’an, Quintana Roo | 45 | I | Herrera-Silveira et al. [39] |
| Mangrove | Puerto Morelos, Yucatán | 36 | H | Herrera-Silveira et al. [39] |
| Mangrove | Aguiabampo, Sonora | 3.5 | B | Barreras-Apodaca et al. [55] |
| Mangrove | El Rabón, Nayarit | 30 | C | Castillo-Cruz and Rosa-Meza [56] |
| Mangrove | La Encrucijada, Chiapas | 17.9 | F | Barreras-Apodaca et al. [55] |
| Mangrove | Isla Arena, Campeche | 30.5 | G | Pech-Poot et al. [57] |
| Mangrove | Celestún, Yucatán | 22.4 | H | Pech-Poot et al. [57] |
| Mangrove | Cancún, Quintana Roo | 26.4 | I | Pech-Poot et al. [57] |
| Mangrove | La Encrucijada, Chiapas | 6.3 | F | Velázquez-Pérez et al.[58] |
| Mangrove | La Encrucijada, Chiapas | 140 | Sjögersten et al. [33] | |
| Swamp | La Encrucida, Biosphere Reserve, Chiapas | 72.2 | F | Adame et al. [36] |
| Swamp | Jamapa, Veracruz | 39 | D | Hernández et al. [44] |
| Swamp | Alvarado, Veracruz | 60 | D | Moreno-Casasola et al. [45] |
| Swamp | Campeche y Tabasco | 300 | E, G | Sjögersten et al. [33] |
| Swamp | Tecolutla, Actopan, and Alto Lucero, Veracruz | 45 | D | Marín-Muñiz et al. [59] |
| Swamp | Alto Lucero and Tecolutla, Veracruz | 52 | D | Campos et al. [32] |
| Swamp | Tecolutla and Vega de Alatorre, Veracruz | 35 | D | Marín-Muñiz et al. [31] |
| Flooded Palm | Sian Ka’an, Quintana Roo | 6.5 | I | Alamilla, [60] |
| Flooded Palm | Alvarado, Veracruz | 16 | D | Moreno-Casasola et al. [45] |
| Flooded Palm | Jamapa, Veracruz | 1.5 | D | Sánchez [61] |
| Ecosystem | Carbon Pool (Kg Cm−2) | Reference |
|---|---|---|
| Mexican terrestrial ecosystem | 6.26 | Vega-López [68]. |
| Everette USA | 7.81 | Crooks et al. [69]. |
| Clayoquot Sound marsh soils Canada | 8.06 | Chastain and Kohfeld[70]. |
| African Salt Marshes | 10.9 | Raw et al. [71]. |
| Okavango Delta, riverine marsh, Botswana, África | 1.5 | Bernal and Mitsch[24]. |
| Wetlands of Europe | 15–30 | Abdul et al. [72]. |
| Swamps | 86.17 | This study |
| Flooded grassland | 28.57 | |
| Mangroves | 34.1 | |
| Flooded palms | 8.0 | |
| Marshes | 40.55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zamora, S.; Zitácuaro-Contreras, I.; Betanzo-Torres, E.A.; Herazo, L.C.S.; Sandoval-Herazo, M.; Vidal-Álvarez, M.; Marín-Muñiz, J.L. Carbon Pool in Mexican Wetland Soils: Importance of the Environmental Service. Life 2022, 12, 1032. https://doi.org/10.3390/life12071032
Zamora S, Zitácuaro-Contreras I, Betanzo-Torres EA, Herazo LCS, Sandoval-Herazo M, Vidal-Álvarez M, Marín-Muñiz JL. Carbon Pool in Mexican Wetland Soils: Importance of the Environmental Service. Life. 2022; 12(7):1032. https://doi.org/10.3390/life12071032
Chicago/Turabian StyleZamora, Sergio, Irma Zitácuaro-Contreras, Erick Arturo Betanzo-Torres, Luis Carlos Sandoval Herazo, Mayerlin Sandoval-Herazo, Monserrat Vidal-Álvarez, and José Luis Marín-Muñiz. 2022. "Carbon Pool in Mexican Wetland Soils: Importance of the Environmental Service" Life 12, no. 7: 1032. https://doi.org/10.3390/life12071032
APA StyleZamora, S., Zitácuaro-Contreras, I., Betanzo-Torres, E. A., Herazo, L. C. S., Sandoval-Herazo, M., Vidal-Álvarez, M., & Marín-Muñiz, J. L. (2022). Carbon Pool in Mexican Wetland Soils: Importance of the Environmental Service. Life, 12(7), 1032. https://doi.org/10.3390/life12071032











