Mesenchymal-Stem-Cell-Derived Extracellular Vesicles Attenuate Brain Injury in Escherichia coli Meningitis in Newborn Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. MSC-Derived EV Preparation
2.2. Bacterial Preparation
2.3. Animal Model of Neonatal E. coli Meningitis
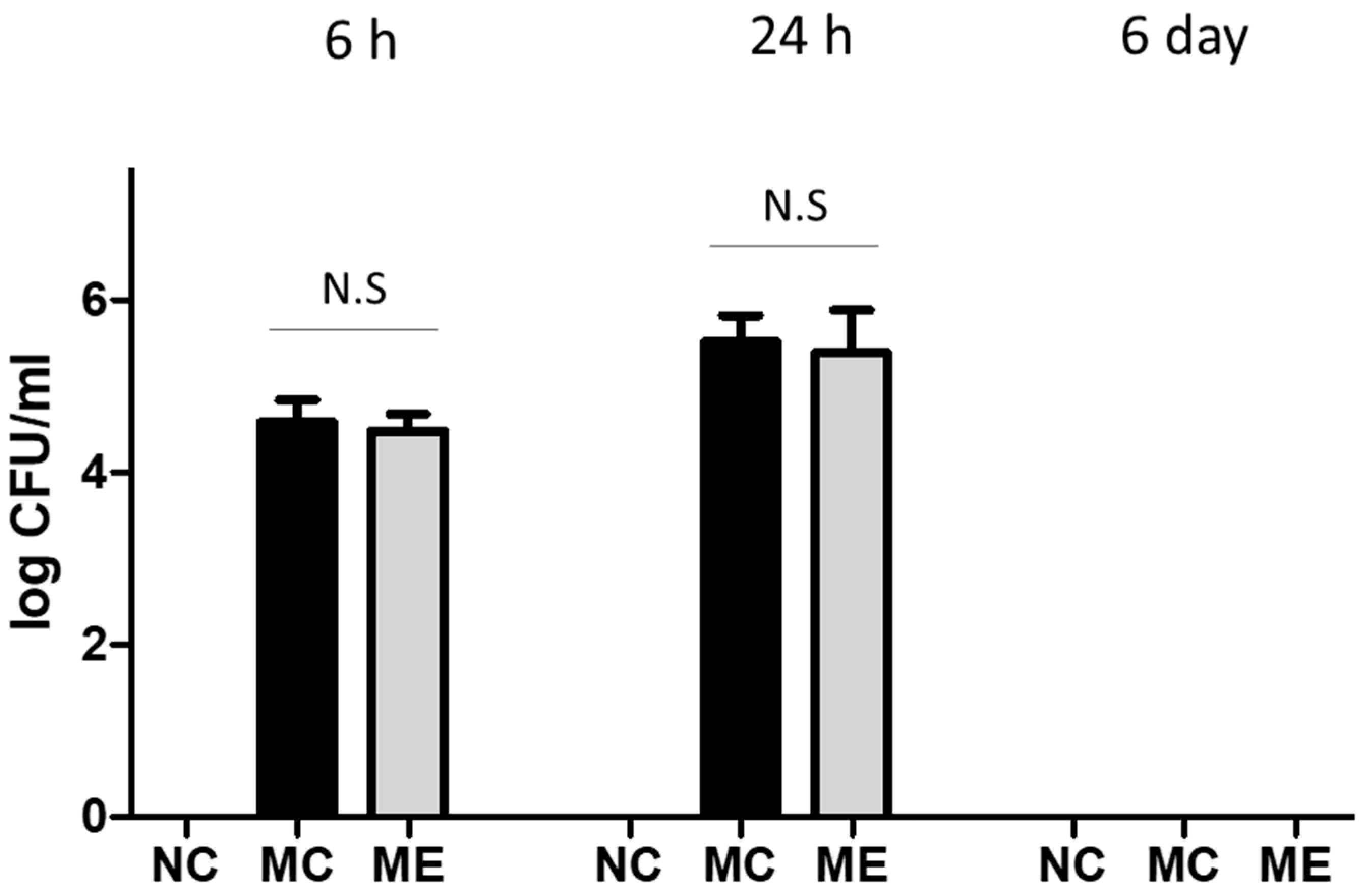
2.4. Bacterial Quantification
2.5. Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling (TUNEL) Assay
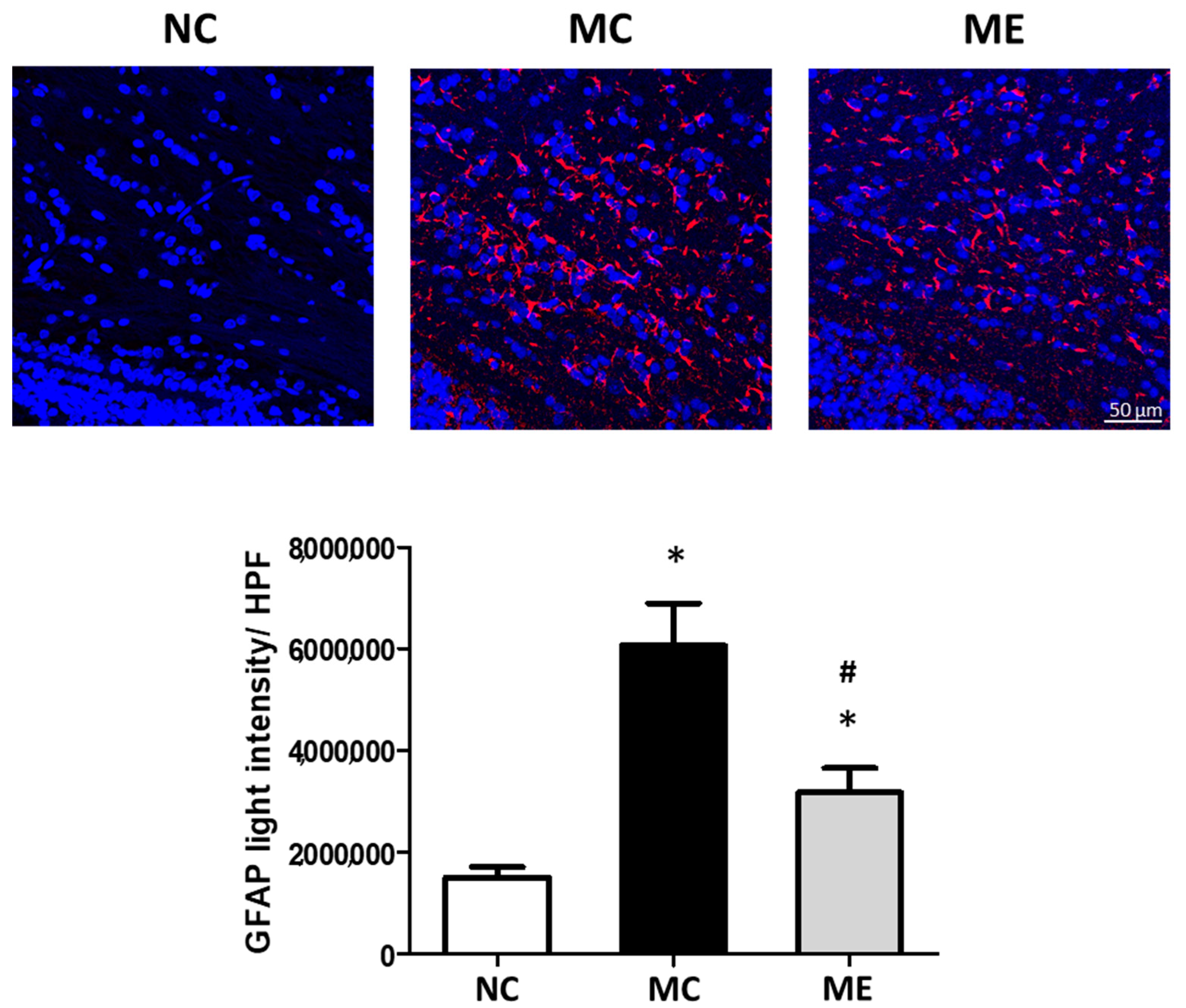
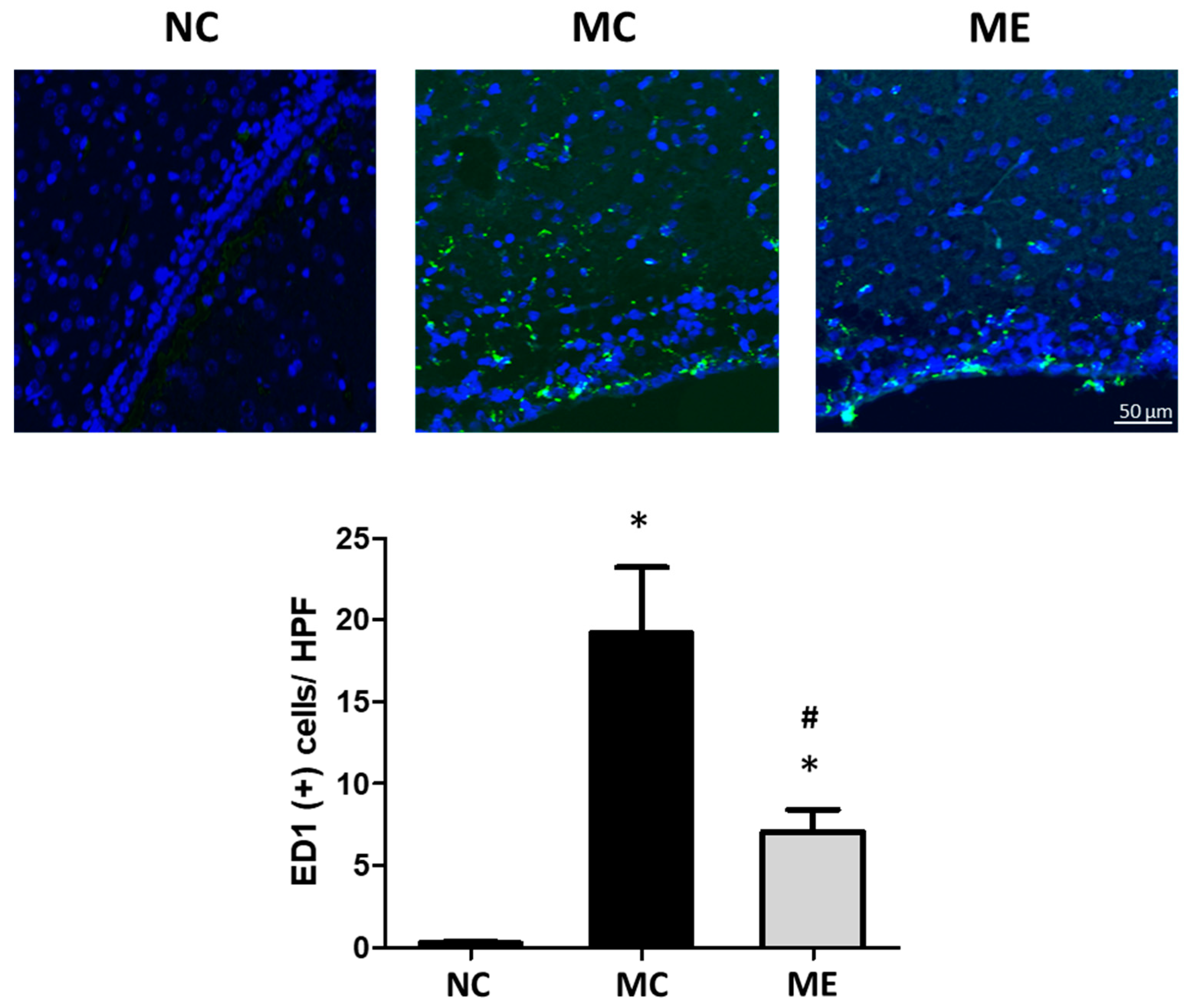
2.6. Immunohistochemistry
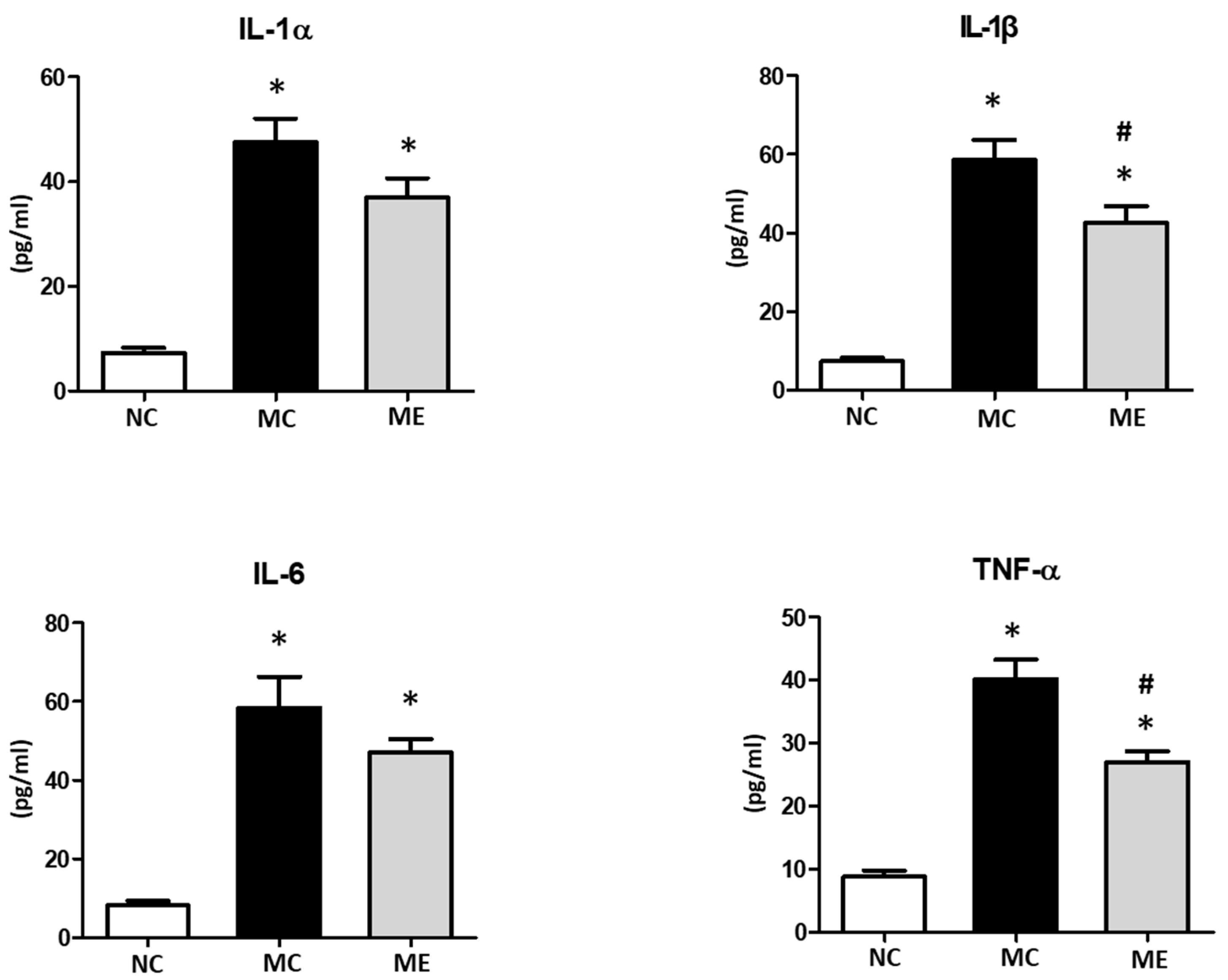
2.7. Enzyme-Linked Immunosorbent Assay
2.8. Statistical Analyses
3. Results
3.1. Bacterial Count
3.2. Cell Death and Reactive Gliosis
3.3. Brain Inflammation
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- El-Naggar, W.; Afifi, J.; McMillan, D.; Toye, J.; Ting, J.; Yoon, E.W.; Shah, P.S. Canadian Neonatal Network, I. Epidemiology of meningitis in canadian neonatal intensive care units. Pediatr. Infect. Dis. J. 2019, 38, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.P.; Eames, M.; Kent, A.; Halket, S.; Holt, D.; Harvey, D. Long term outcome of neonatal meningitis. Arch. Dis. Child. Fetal Neonatal Ed. 2003, 88, F179–F184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheld, W.M.; Koedel, U.; Nathan, B.; Pfister, H.W. Pathophysiology of bacterial meningitis: Mechanism(s) of neuronal injury. J. Infect. Dis. 2002, 186 (Suppl. S2), S225–S233. [Google Scholar] [CrossRef] [PubMed]
- Barichello, T.; Fagundes, G.D.; Generoso, J.S.; Elias, S.G.; Simoes, L.R.; Teixeira, A.L. Pathophysiology of neonatal acute bacterial meningitis. J. Med. Microbiol. 2013, 62, 1781–1789. [Google Scholar] [CrossRef] [Green Version]
- Sung, D.K.; Chang, Y.S.; Sung, S.I.; Yoo, H.S.; Ahn, S.Y.; Park, W.S. Antibacterial effect of mesenchymal stem cells against Escherichia coli is mediated by secretion of beta-defensin-2 via toll-like receptor 4 signalling. Cell Microbiol. 2016, 18, 424–436. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Kim, Y.E.; Hong, S.; Kim, K.T.; Sung, D.K.; Lee, Y.; Park, W.S.; Chang, Y.S.; Song, M.R. Reactive microglia and astrocytes in neonatal intraventricular hemorrhage model are blocked by mesenchymal stem cells. Glia 2020, 68, 178–192. [Google Scholar] [CrossRef]
- Ahn, S.Y.; Chang, Y.S.; Sung, D.K.; Sung, S.I.; Park, W.S. Hypothermia broadens the therapeutic time window of mesenchymal stem cell transplantation for severe neonatal hypoxic ischemic encephalopathy. Sci. Rep. 2018, 8, 7665. [Google Scholar] [CrossRef] [Green Version]
- Ahn, S.Y.; Chang, Y.S.; Kim, Y.E.; Sung, S.I.; Sung, D.K.; Park, W.S. Mesenchymal stem cells transplantation attenuates brain injury and enhances bacterial clearance in Escherichia coli meningitis in newborn rats. Pediatr. Res. 2018, 84, 778–785. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Huang, Y.; Han, J.; Yu, L.; Li, Y.; Lu, Z.; Li, H.; Liu, Z.; Shi, C.; Duan, F.; et al. Immunomodulatory effects of mesenchymal stromal cells-derived exosome. Immunol. Res. 2016, 64, 831–840. [Google Scholar] [CrossRef]
- Ahn, S.Y.; Park, W.S.; Kim, Y.E.; Sung, D.K.; Sung, S.I.; Ahn, J.Y.; Chang, Y.S. Vascular endothelial growth factor mediates the therapeutic efficacy of mesenchymal stem cell-derived extracellular vesicles against neonatal hyperoxic lung injury. Exp. Mol. Med. 2018, 50, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Ahn, S.Y.; Chang, Y.S.; Sung, D.K.; Kim, Y.E.; Park, W.S. Developing a newborn rat model of ventriculitis without concomitant bacteremia by intraventricular injection of K1 (-) Escherichia coli. Pediatr. Int. 2020, 62, 347–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sung, D.K.; Sung, S.I.; Ahn, S.Y.; Chang, Y.S.; Park, W.S. Thrombin preconditioning boosts biogenesis of extracellular vesicles from mesenchymal stem cells and enriches their cargo contents via protease-activated receptor-mediated signaling pathways. Int. J. Mol. Sci. 2019, 20, 2899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sung, D.K.; Chang, Y.S.; Sung, S.I.; Ahn, S.Y.; Park, W.S. Thrombin preconditioning of extracellular vesicles derived from mesenchymal stem cells accelerates cutaneous wound healing by boosting their biogenesis and enriching cargo content. J. Clin. Med. 2019, 8, 533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walter, J.; Ware, L.B.; Matthay, M.A. Mesenchymal stem cells: Mechanisms of potential therapeutic benefit in ARDS and sepsis. Lancet Respir. Med. 2014, 2, 1016–1026. [Google Scholar] [CrossRef] [Green Version]
- Eichberger, J.; Resch, E.; Resch, B. Diagnosis of neonatal sepsis: The role of inflammatory markers. Front. Pediatr. 2022, 10, 840288. [Google Scholar] [CrossRef]
- Park, W.S.; Sung, S.I.; Ahn, S.Y.; Sung, D.K.; Im, G.H.; Yoo, H.S.; Choi, S.J.; Chang, Y.S. Optimal timing of mesenchymal stem cell therapy for neonatal intraventricular hemorrhage. Cell Transplant. 2016, 25, 1131–1144. [Google Scholar] [CrossRef] [Green Version]
- Thorsdottir, S.; Henriques-Normark, B.; Iovino, F. The role of microglia in bacterial meningitis: Inflammatory response, experimental models and new neuroprotective therapeutic strategies. Front. Microbiol. 2019, 10, 576. [Google Scholar] [CrossRef]
- Grandgirard, D.; Leib, S.L. Meningitis in neonates: Bench to bedside. Clin. Perinatol. 2010, 37, 655–676. [Google Scholar] [CrossRef]
- Salari, V.; Mengoni, F.; Del Gallo, F.; Bertini, G.; Fabene, P.F. The Anti-inflammatory properties of mesenchymal stem cells in epilepsy: Possible treatments and future perspectives. Int. J. Mol. Sci. 2020, 21, 9683. [Google Scholar] [CrossRef]
- Anderson, J.D.; Johansson, H.J.; Graham, C.S.; Vesterlund, M.; Pham, M.T.; Bramlett, C.S.; Montgomery, E.N.; Mellema, M.S.; Bardini, R.L.; Contreras, Z.; et al. Comprehensive proteomic analysis of mesenchymal stem cell exosomes reveals modulation of angiogenesis via nuclear factor-KappaB signaling. Stem Cells 2016, 34, 601–613. [Google Scholar] [CrossRef] [Green Version]
- Cunningham, C.J.; Redondo-Castro, E.; Allan, S.M. The therapeutic potential of the mesenchymal stem cell secretome in ischaemic stroke. J. Cereb. Blood Flow Metab. 2018, 38, 1276–1292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krasnodembskaya, A.; Song, Y.; Fang, X.; Gupta, N.; Serikov, V.; Lee, J.W.; Matthay, M.A. Antibacterial effect of human mesenchymal stem cells is mediated in part from secretion of the antimicrobial peptide LL-37. Stem Cells 2010, 28, 2229–2238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, N.; Krasnodembskaya, A.; Kapetanaki, M.; Mouded, M.; Tan, X.; Serikov, V.; Matthay, M.A. Mesenchymal stem cells enhance survival and bacterial clearance in murine Escherichia coli pneumonia. Thorax 2012, 67, 533–539. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.E.; Sung, S.I.; Chang, Y.S.; Ahn, S.Y.; Sung, D.K.; Park, W.S. Thrombin preconditioning enhances therapeutic efficacy of human Wharton’s jelly-derived mesenchymal stem cells in severe neonatal hypoxic ischemic encephalopathy. Int. J. Mol. Sci. 2019, 20, 2477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, S.Y.; Kim, Y.E.; Park, W.S.; Ahn, S.Y.; Sung, D.K.; Sung, S.I.; Joo, K.M.; Kim, S.G.; Chang, Y.S. Thrombin preconditioning improves the therapeutic efficacy of mesenchymal stem cells in severe intraventricular hemorrhage induced neonatal rats. Int. J. Mol. Sci. 2022, 23, 4447. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.-E.; Ahn, S.-Y.; Park, W.-S.; Sung, D.-K.; Sung, S.-I.; Yang, M.-S.; Chang, Y.-S. Mesenchymal-Stem-Cell-Derived Extracellular Vesicles Attenuate Brain Injury in Escherichia coli Meningitis in Newborn Rats. Life 2022, 12, 1030. https://doi.org/10.3390/life12071030
Kim Y-E, Ahn S-Y, Park W-S, Sung D-K, Sung S-I, Yang M-S, Chang Y-S. Mesenchymal-Stem-Cell-Derived Extracellular Vesicles Attenuate Brain Injury in Escherichia coli Meningitis in Newborn Rats. Life. 2022; 12(7):1030. https://doi.org/10.3390/life12071030
Chicago/Turabian StyleKim, Young-Eun, So-Yoon Ahn, Won-Soon Park, Dong-Kyung Sung, Se-In Sung, Mi-Sun Yang, and Yun-Sil Chang. 2022. "Mesenchymal-Stem-Cell-Derived Extracellular Vesicles Attenuate Brain Injury in Escherichia coli Meningitis in Newborn Rats" Life 12, no. 7: 1030. https://doi.org/10.3390/life12071030
APA StyleKim, Y.-E., Ahn, S.-Y., Park, W.-S., Sung, D.-K., Sung, S.-I., Yang, M.-S., & Chang, Y.-S. (2022). Mesenchymal-Stem-Cell-Derived Extracellular Vesicles Attenuate Brain Injury in Escherichia coli Meningitis in Newborn Rats. Life, 12(7), 1030. https://doi.org/10.3390/life12071030





