Efficacy of Ficus sycomorus (Sycamore Fig) Extract on Intestinal Coccidiosis in Experimentally Infected Rabbits
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Plant Materials and Preparation of the Extracts
2.3. Rabbits
2.4. Sporulated Oocyst
2.5. Experimental Design
2.6. Fecal Oocyst Examination and Clinical Presentation of Coccidiosis
2.7. Liver Function and Hemogram
2.8. Histopathological Examination
2.9. Growth Performance
2.10. Statistical Analysis
3. Results
3.1. Effects of F. sycomorus Extract on Oocyst Shedding
3.2. Impact of F. sycomorus Extract on the Clinical Presentation of Coccidiosis
3.3. Effects of F. sycomorus Extract on Liver Function and Hemogram
3.4. Pathologically: Medication Tends to Secure Hepato-Intestinal Tissue Architectures
3.5. Impacts of F. sycomorus Extract on the Growth Performance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Szkucik, K.; Pyz-Łukasik, R.; Szczepaniak, K.O.; Paszkiewicz, W. Occurrence of gastrointestinal parasites in slaughter rabbits. Parasitol. Res. 2014, 113, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Duszynski, D.W.; Couch, L. The Biology and Identification of the Coccidia (Apicomplexa) of Rabbits of the World; Newnes: Oxford, UK, 2013. [Google Scholar]
- Licois, D. Domestic rabbit enteropathies. In Proceedings of the 8th Eighth World Rabbit Congress, Puebla, Mexico, 7–10 September 2004; pp. 385–403. [Google Scholar]
- Taylor, M.A.; Coop, R.L.; Wall, R.L. Veterinary Parasitology, 4th ed.; Wiley-Blackwell: Oxford, UK, 2015. [Google Scholar]
- Gu, X.; Liu, H.; Li, C.; Fang, S.; Cui, P.; Liao, Q.; Zhang, S.; Wang, S.; Duan, C.; Yu, F. Selection and characterization of a precocious line of Eimeria media. Parasitol. Res. 2019, 118, 3033–3041. [Google Scholar] [CrossRef] [PubMed]
- Basiaga, M.; Levytska, V.; Kowal, J.; Nosal, P. Coccidiosis—A problem in backyard rabbitries. Ann. Parasitol. 2020, 66, 97–99. [Google Scholar] [PubMed]
- Papeschi, C.; Fichi, G.; Perrucci, S. Oocyst excretion pattern of three intestinal Eimeria species in female rabbits. World Rabbit Sci. 2013, 21, 77–83. [Google Scholar] [CrossRef][Green Version]
- Henneb, M.; Aissi, M. Etude cinétique de l’excrétion oocystale chez la lapine et sa descendance et identification des différentes espèces de coccidies. In Proceedings of the Journées de la Recherche Cunicole, Le Mans, France, 19–20 November 2013; pp. 221–224. [Google Scholar]
- Abbas, R.; Iqbal, Z.; Blake, D.; Khan, M.; Saleemi, M. Anticoccidial drug resistance in fowl coccidia: The state of play revisited. World’s Poult. Sci. J. 2011, 67, 337–350. [Google Scholar] [CrossRef]
- Federation of Veterinarians of Europe FVE. Position Paper on Coccidiostats or Anticoccidials; Federation of Veterinarians of Europe FVE: Avenue Tervueren, Brussels, 2016. Available online: http://www.fve.org/uploads/publications/docs/040_016 (accessed on 2 June 2022).
- Licois, D. Pathologie d’origine bactérienne et parasitaire chez le lapin: Apports de la dernière décennie. Cunicult. Mag. 2010, 37, 35–49. [Google Scholar]
- Kadykalo, S.; Roberts, T.; Thompson, M.; Wilson, J.; Lang, M.; Espeisse, O. The value of anticoccidials for sustainable global poultry production. Int. J. Antimicrob. Agents 2018, 51, 304–310. [Google Scholar] [CrossRef]
- Burke, J.; Miller, J.; Terrill, T.; Orlik, S.; Acharya, M.; Garza, J.; Mosjidis, J. Sericea lespdeza as an aid in the control of Emeria spp. in lambs. Vet. Parasitol. 2013, 193, 39–46. [Google Scholar] [CrossRef]
- Saratsis, A.; Voutzourakis, N.; Theodosiou, T.; Stefanakis, A.; Sotiraki, S. The effect of sainfoin (Onobrychis viciifolia) and carob pods (Ceratonia siliqua) feeding regimes on the control of lamb coccidiosis. Parasitol. Res. 2016, 115, 2233–2242. [Google Scholar] [CrossRef]
- Fraquelli, C.; Zanzani, S.; Gazzonis, A.; Rizzi, R.; Manfredi, M. Effects of condensed tannin on natural coccidian infection in goat kids. Small Rumin. Res. 2015, 126, 19–24. [Google Scholar] [CrossRef]
- Legendre, H.; Saratsi, K.; Voutzourakis, N.; Saratsis, A.; Stefanakis, A.; Gombault, P.; Hoste, H.; Gidenne, T.; Sotiraki, S. Coccidiostatic effects of tannin-rich diets in rabbit production. Parasitol. Res. 2018, 117, 3705–3713. [Google Scholar] [CrossRef] [PubMed]
- Konai, N.; Raidandi, D.; Pizzi, A.; Meva’a, L. Characterization of ficus sycomorus tannin using ATR-FT MIR, MALDI-TOF MS and 13C NMR methods. Eur. J. Wood Wood Prod. 2017, 75, 807–815. [Google Scholar] [CrossRef]
- Al-matani, S.K.; Al-Wahaibi, R.N.S.; Hossain, M.A. In vitro evaluation of the total phenolic and flavonoid contents and the antimicrobial and cytotoxicity activities of crude fruit extracts with different polarities from Ficus sycomorus. Pac. Sci. Rev. A Nat. Sci. Eng. 2015, 17, 103–108. [Google Scholar] [CrossRef]
- Dawod, A.; Fathalla, S.I.; Elkhatam, A.; Osman, N.; Sheraiba, N.; Hammad, M.A.; El-Seedi, H.R.; Shehata, A.A.; Anis, A. UPLC-QToF Nanospray MS and NMR Analysis of Ficus sycomorus Stem Bark and Its Effects on Rabbit. Processes 2021, 9, 1201. [Google Scholar] [CrossRef]
- Santana-Gálvez, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Chlorogenic acid: Recent advances on its dual role as a food additive and a nutraceutical against metabolic syndrome. Molecules 2017, 22, 358. [Google Scholar] [CrossRef]
- Liu, H.; Chen, P.; Lv, X.; Zhou, Y.; Li, X.; Ma, S.; Zhao, J. Effects of Chlorogenic Acid on Performance, Anticoccidial Indicators, Immunity, Antioxidant Status, and Intestinal Barrier Function in Coccidia-Infected Broilers. Animals 2022, 12, 963. [Google Scholar] [CrossRef]
- Agunloye, O.M.; Oboh, G.; Ademiluyi, A.O.; Ademosun, A.O.; Akindahunsi, A.A.; Oyagbemi, A.A.; Omobowale, T.O.; Ajibade, T.O.; Adedapo, A.A. Cardio-protective and antioxidant properties of caffeic acid and chlorogenic acid: Mechanistic role of angiotensin converting enzyme, cholinesterase and arginase activities in cyclosporine induced hypertensive rats. Biomed. Pharmacother. 2019, 109, 450–458. [Google Scholar] [CrossRef]
- Xu, J.-G.; Hu, Q.-P.; Liu, Y. Antioxidant and DNA-protective activities of chlorogenic acid isomers. J. Agric. Food Chem. 2012, 60, 11625–11630. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Q.; Ci, X.; Chen, S.; Xie, Z.; Li, H.; Zhang, H.; Chen, F.; Xie, Q. Evaluation of the efficacy of chlorogenic acid in reducing small intestine injury, oxidative stress, and inflammation in chickens challenged with Clostridium perfringens type A. Poult. Sci. 2020, 99, 6606–6618. [Google Scholar] [CrossRef]
- Saleh, B.; Al-Mariri, A. Phytochemical constitutes of Ficus sycomorus L. and inhibitory effect of their crude extracts against bacterial pathogens. J. Nat. Prod. 2017, 10, 6–14. [Google Scholar]
- Song, M.A.; Abarshi, M.M.; Ameh, D.A.; Aliyu, M.S.; Mamuda, K.; Nicolas, E.; Isiyaku, A.; Meshak, P.; Mosunmola, I.; Abba, K.; et al. In vitro Antimycobacterial Screening of Ficus sycomorus Extracts on Susceptible Strain of Mycobacterium tuberculosis. J. Adv. Med. Pharm. Sci. 2018, 15, 1–7. [Google Scholar] [CrossRef]
- Hassan, S.; Lawal, M.; Muhammad, B.; Umar, R.; Bilbis, L.; Faruk, U.; Ebbo, A. Antifungal activity and phytochemical analysis of column chromatographic fractions of stem bark extracts of Ficus sycomorus L. (Moraceae). J. Plant Sci. 2007, 2, 209–215. [Google Scholar] [CrossRef][Green Version]
- Igbokwe, N.; Igbokwe, I.; Sandabe, U. Effect of prolonged oral administration of aqueous Ficus sycomorus stem-bark extract on testicular size of growing albino rat. Int. J. Morphol. 2010, 28, 1315–1322. [Google Scholar] [CrossRef][Green Version]
- Foyet, H.S.; Tchinda Deffo, S.; Koagne Yewo, P.; Antioch, I.; Zingue, S.; Asongalem, E.A.; Kamtchouing, P.; Ciobica, A. Ficus sycomorus extract reversed behavioral impairment and brain oxidative stress induced by unpredictable chronic mild stress in rats. BMC Complementary Altern. Med. 2017, 17, 502. [Google Scholar] [CrossRef]
- El-Sayyad, S.; Makboul, M.; Ali, R.; El-Amir, J.; Farag, S. Hepatoprotective activity of Ficus sycomorus L. against N-nitrosodiethylamine and CCL4 induced hepatocarcinogenesis in experimental rats. Res. Rev. J. Pharmacogn. Phytochem. 2015, 3, 1–5. [Google Scholar]
- Slatnar, A.; Klancar, U.; Stampar, F.; Veberic, R. Effect of drying of figs (Ficus carica L.) on the contents of sugars, organic acids, and phenolic compounds. J. Agric. Food Chem. 2011, 59, 11696–11702. [Google Scholar] [CrossRef]
- Ahmadua, A.; Zezi, A.; Yaro, A. Anti-diarrheal activity of the leaf extracts of Daniellia oliveri Hutch and Dalz (Fabaceae) and Ficus sycomorus Miq (Moraceae). Afr. J. Tradit. Complementary Altern. Med. 2007, 4, 524–528. [Google Scholar] [CrossRef]
- Abdel-Haleem, H.M.; Aboelhadid, S.M.; Sakran, T.; El-Shahawy, G.; El-Fayoumi, H.; Al-Quraishy, S.; Abdel-Baki, A.-A.S. Gene expression, oxidative stress and apoptotic changes in rabbit ileum experimentally infected with Eimeria intestinalis. Folia Parasitol. 2017, 64, 12. [Google Scholar] [CrossRef]
- Li, C.; Tao, G.; Gu, X.; Cui, Y.; Wang, Y.; Suo, J.; Lv, Y.; Yu, F.; Mamoun, C.B.; Suo, X. Selection and identification of a precocious line of Eimeria intestinalis with enlarged oocysts and deletion of one generation of schizogony. Parasitol. Res. 2019, 118, 969–976. [Google Scholar] [CrossRef]
- Coudert, P.; Licois, F.; Drouet-Viard, F. Eimeria Species and Strains of Rabbits; Eckert, J., Braun, R., Shirley, M.W., Coudert, P., Eds.; COST. 89/820. Biotechnology: Guidelines on Techniques in Coccidiosis Research; Office for Official Publications of the European Communities: Luxembourg; pp. 52–73.
- Long, P.; Millard, B.; Joyner, L.; Norton, C. A guide to laboratory techniques used in the study and diagnosis of avian coccidiosis. Folia Vet. Lat. 1976, 6, 201–217. [Google Scholar]
- MAFF/ADAS. Manual of Veterinary Parasitological Techniques; Reference Book 418: Washington, DC, USA, 1986. [Google Scholar]
- Bancroft, J.D.; Gamble, M. Theory and Practice of Histological Techniques; Elsevier Health Sciences: Philadelphia, PA, USA, 2008. [Google Scholar]
- Gibson-Corley, K.N.; Olivier, A.K.; Meyerholz, D.K. Principles for valid histopathologic scoring in research. Vet. Pathol. 2013, 50, 1007–1015. [Google Scholar] [CrossRef] [PubMed]
- Ogolla, K.O.; Okumu, P.O.; Gathumbi, P.K.; Waruiru, R.M. Effects of anticoccidial drugs on gross and histopathological lesions caused by experimental rabbit coccidiosis. Symbiosis 2018, 4, 2381–2907. [Google Scholar] [CrossRef]
- Steel, R.G.; Torrie, J.H. Principles and Procedures of Statistics: A Biometrical Approach; McGraw-Hill: New York, NY, USA, 1980; Volume 2. [Google Scholar]
- El-Ashram, S.A.; Aboelhadid, S.M.; Abdel-Kafy, E.-S.M.; Hashem, S.A.; Mahrous, L.N.; Farghly, E.M.; Moawad, U.K.; Kamel, A.A. Prophylactic and therapeutic efficacy of prebiotic supplementation against intestinal coccidiosis in rabbits. Animals 2019, 9, 965. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yao, J.; Zhou, B.; Yang, J.; Chaudry, M.T.; Wang, M.; Xiao, F.; Li, Y.; Yin, W. Bacteriostatic effect of quercetin as an antibiotic alternative in vivo and its antibacterial mechanism in vitro. J. Food Prot. 2018, 81, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Alejo-Armijo, A.; Glibota, N.; Frías, M.P.; Altarejos, J.N.; Gálvez, A.; Salido, S.; Ortega-Morente, E. Synthesis and evaluation of antimicrobial and antibiofilm properties of A-type procyanidin analogues against resistant bacteria in food. J. Agric. Food Chem. 2018, 66, 2151–2158. [Google Scholar] [CrossRef] [PubMed]
- Sklenickova, O.; Flesar, J.; Kokoska, L.; Vlkova, E.; Halamova, K.; Malik, J. Selective growth inhibitory effect of biochanin A against intestinal tract colonizing bacteria. Molecules 2010, 15, 1270–1279. [Google Scholar] [CrossRef]
- Haesaerts, S.; Rodriguez Buitrago, J.A.; Loris, R.; Baeyens-Volant, D.; Azarkan, M. Crystallization and preliminary X-ray analysis of four cysteine proteases from Ficus carica latex. Acta Crystallogr. Sect. F Struct. Biol. Commun. 2015, 71, 459–465. [Google Scholar] [CrossRef]
- Baidamshina, D.R.; Trizna, E.Y.; Holyavka, M.G.; Bogachev, M.I.; Artyukhov, V.G.; Akhatova, F.S.; Rozhina, E.V.; Fakhrullin, R.F.; Kayumov, A.R. Targeting microbial biofilms using Ficin, a nonspecific plant protease. Sci. Rep. 2017, 7, 46068. [Google Scholar] [CrossRef]
- de Amorin, A.; Borba, H.R.; Carauta, J.P.; Lopes, D.; Kaplan, M.A. Anthelmintic activity of the latex of Ficus species. J. Ethnopharmacol. 1999, 64, 255–258. [Google Scholar] [CrossRef]
- Redondo, L.M.; Chacana, P.A.; Dominguez, J.E.; Fernandez Miyakawa, M.E.D. Perspectives in the use of tannins as alternative to antimicrobial growth promoter factors in poultry. Front. Microbiol. 2014, 5, 118. [Google Scholar] [CrossRef]
- White, E.C.; Houlden, A.; Bancroft, A.J.; Hayes, K.S.; Goldrick, M.; Grencis, R.K.; Roberts, I.S. Manipulation of host and parasite microbiotas: Survival strategies during chronic nematode infection. Sci. Adv. 2018, 4, eaap7399. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Yan, Y.; Jian, F.; Ning, C. Coccidia-microbiota interactions and their effects on the host. Front. Cell. Infect. Microbiol. 2021, 11, 751481. [Google Scholar] [CrossRef] [PubMed]
- Shehata, A.A.; Yalçın, S.; Latorre, J.D.; Basiouni, S.; Attia, Y.A.; Abd El-Wahab, A.; Visscher, C.; El-Seedi, H.R.; Huber, C.; Hafez, H.M.; et al. Probiotics, prebiotics, and phytogenic substances for optimizing gut health in poultry. Microorganisms 2022, 10, 395. [Google Scholar] [CrossRef] [PubMed]
- Islam, A.; Islam, M.S.; Rahman, M.K.; Uddin, M.N.; Akanda, M.R. The pharmacological and biological roles of eriodictyol. Arch. Pharmacal Res. 2020, 43, 582–592. [Google Scholar] [CrossRef]
- Pakandl, M. Coccidia of rabbit: A review. Folia Parasitol. 2009, 56, 153–166. [Google Scholar] [CrossRef]
- Chapman, H.D.; Barta, J.R.; Blake, D.; Gruber, A.; Jenkins, M.; Smith, N.C.; Suo, X.; Tomley, F.M. A selective review of advances in coccidiosis research. Adv. Parasitol. 2013, 83, 93–171. [Google Scholar]
- Petrova, Y.; Georgieva, T.; Zapryanova, D.; Ivanov, A.; Iliev, P.; Kalkanov, I.; Arabkercyan, K. Red and white blood profile in rabbits after experimentally induced infection with sporulated oocysts of Eimeria stiedae. Tradit. Mod. Vet. Med. 2018, 3, 72–78. [Google Scholar]
- Braide, W.; Dokubo, K.; Adeleye, S.; Uzoh, C.; Akobundu, C. Phytochemical properties, toxicological screening and antibacterial qualities of various parts extracts of Ficus sycomorus. J. Med. Plant Herb. Ther. Res. 2018, 6, 1–8. [Google Scholar]
- Çam, Y.; Atasever, A.; Eraslan, G.; Kibar, M.; Atalay, Ö.; Beyaz, L.; İnci, A.; Liman, B.C. Eimeria stiedae: Experimental infection in rabbits and the effect of treatment with toltrazuril and ivermectin. Exp. Parasitol. 2008, 119, 164–172. [Google Scholar] [CrossRef]
- Freitas, F.L.d.C.; Yamamoto, B.L.; Freitas, W.L.d.C.; Fagliari, J.J.; Almeida, K.d.S.; Machado, R.Z.; Machado, C.R. Systemic inflammatory response indicators in rabbits (Oryctolagus cuniculus) experimentally infected with sporulated oocysts of Eimeria stiedai (Apicomplexa: Eimeriidae). Rev. Bras. Parasitol. Veterinária 2011, 20, 121–126. [Google Scholar] [CrossRef][Green Version]
- Al-Taee, M.N.K.; Al-Zubaidi, M.T.S. Protection against Eimeria stiedae in Rabbits by using sonicated sporulated oocyst vaccine. J. Entomol. Zool. Stud. 2017, 5, 579–585. [Google Scholar]
- El-Sayed, M.M.; Abdel-Hadi, A.M.; Sabra, A.; Mahmoud, M.A.; El-Wakil, E.A.; Ghareeb, M.A. Effect of Ficus sycomorus and Azadirachta indica extracts on liver state of mice infected with Schistosoma mansoni. J. Egypt. Soc. Parasitol. 2011, 41, 77–88. [Google Scholar] [PubMed]
- Garba, S.; Prasad, J.; Sandabe, U. Hepatoprotective effect of the aqueous root bark extract of Ficus sycomorus (Linn) on carbon tetrachloride induced hepatotoxicity in rats. J. Biol. Sci. 2007, 7, 276–281. [Google Scholar] [CrossRef][Green Version]
- Oyewole, O.; Adanlawo, I.; Arise, R. Serum and tissue lipid profile in wistar rats administered leaf extract of Ficus exasperata. Ann. Biol. Res. 2013, 4, 288–291. [Google Scholar]
- Allam, T.; AbdelGaber, M.; Thabet, N.; AbouLaila, M.; Elkhatam, A. Clinicopathological effects of diclazuril prophylaxis and treatment on rabbits experimentally infected with Eimeria stiedae. Damanhour J. Vet. Sci. 2020, 4, 20–28. [Google Scholar] [CrossRef]

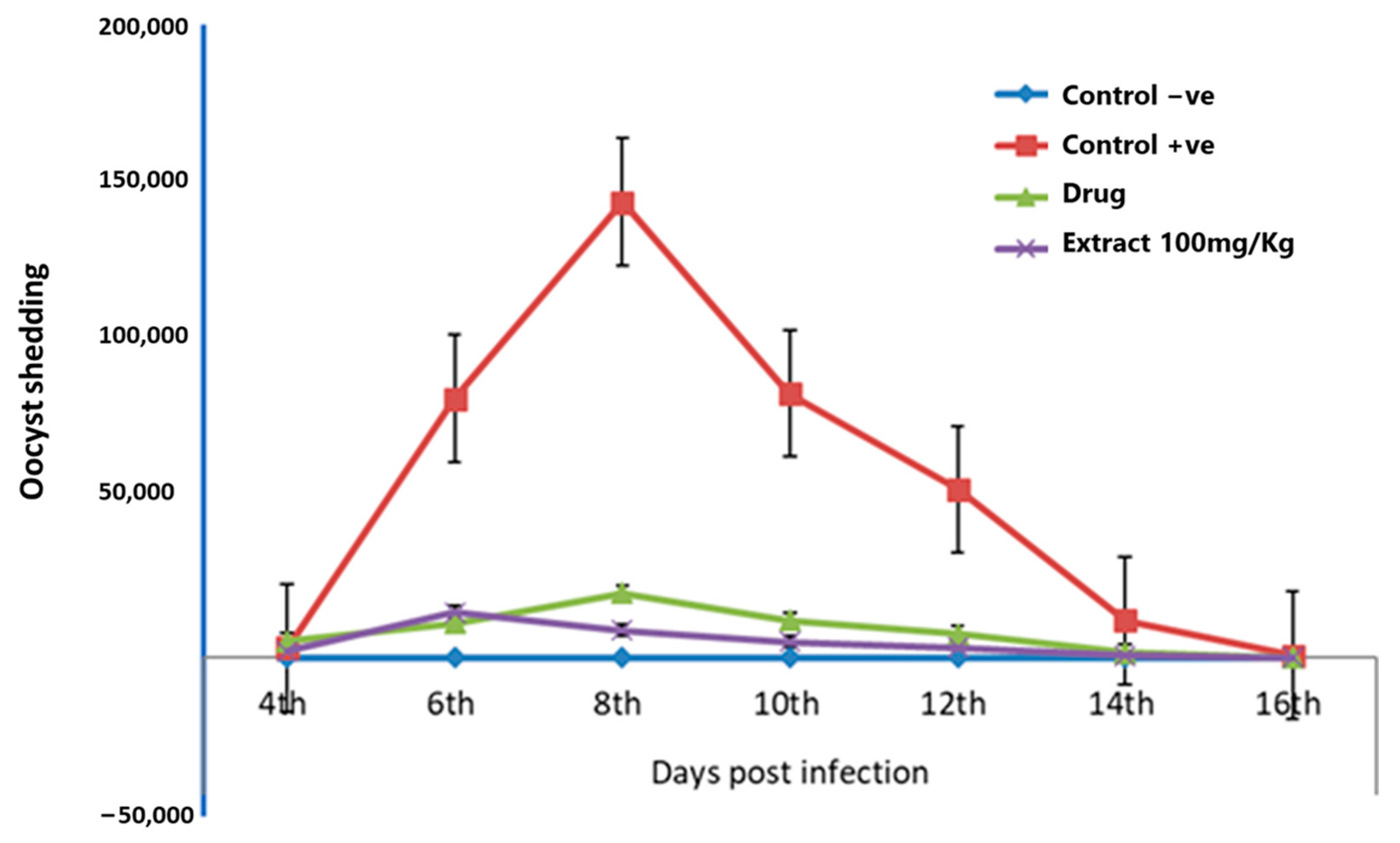

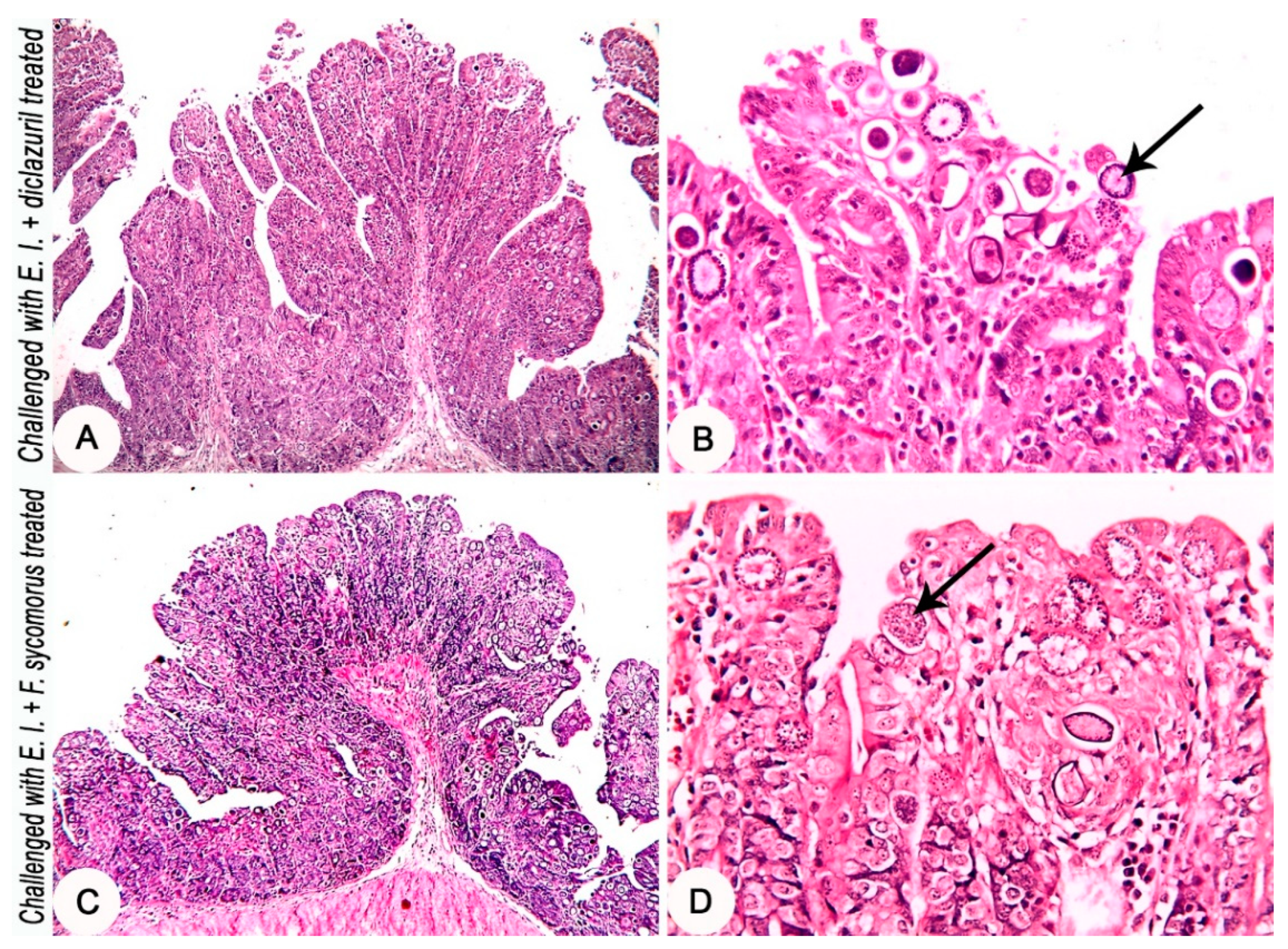
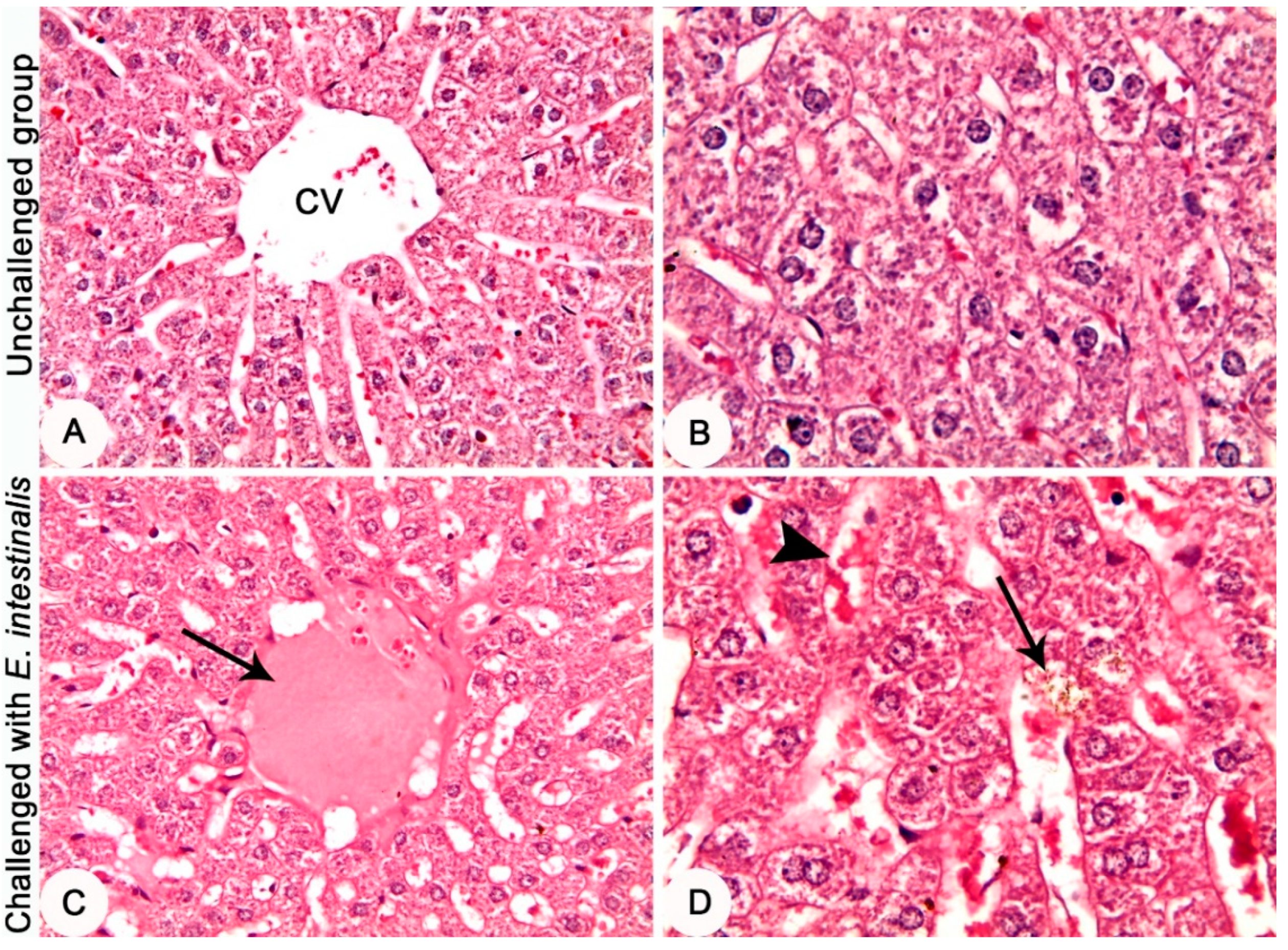
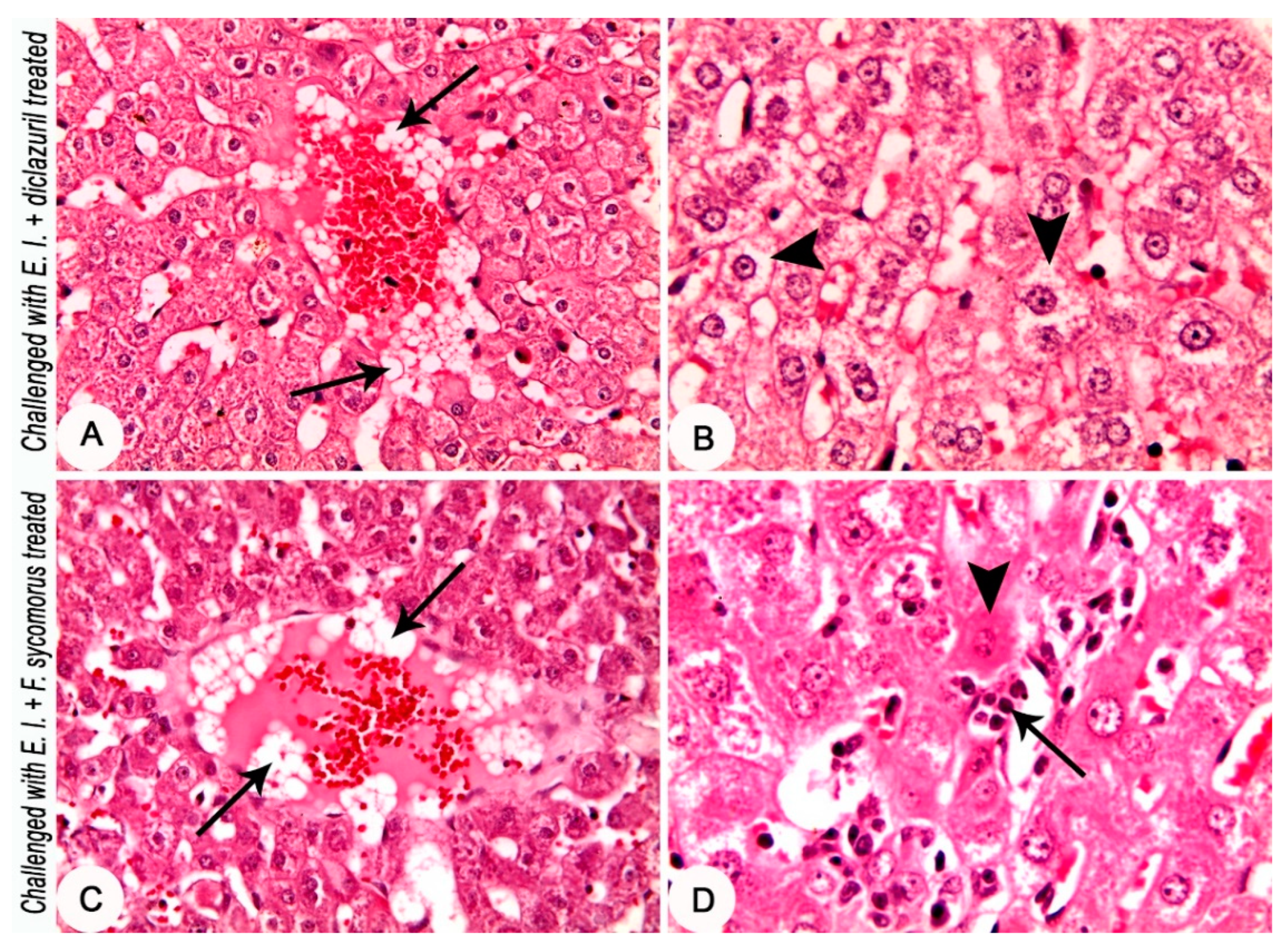
| Ingredients | Composition (%) |
|---|---|
| Maize | 40.00 |
| Soybean meal | 10.00 |
| Dried alfalfa | 20.00 |
| Rice bran | 10.00 |
| White bran | 14.00 |
| Fish meal (72% CP) | 2.00 |
| Bone meal | 2.00 |
| Limestone | 1.00 |
| Premix (Growers) 1 | 0.50 |
| Salt (NaCl) | 0.50 |
| Total | 100.00 |
| Calculated nutrients | |
| Crude protein (%) | 16.73 |
| Crude protein | 16.73 |
| Crude fiber | 9.20 |
| Calcium | 1.25 |
| Available phosphorus | 0.40 |
| Sodium | 0.24 |
| Apparent metabolizable energy (AME; Kcal/kg) | 2600 |
| Digestible methionine | 0.41 |
| Digestible lysine | 1.27 |
| Digestible threonine | 0.73 |
| Choline | 0.75 |
| Digestible total sulfur amino acids (TSAA) | 0.84 |
| Group No. | Number | Challenge with E. intestinalis | Treatment | Assessment Parameters | ||
|---|---|---|---|---|---|---|
| Age | Dose | Drug | Dose | |||
| 1 | 10 | - | - | - | - | 1—Oocyst shedding 2—Clinical symptom 3—Hematological parameters 4—Histopathological findings 5—Growth performance |
| 2 | 10 | 10 weeks | 3 × 104 | - | - | |
| 3 | 10 | 10 weeks | 3 × 104 | Diclazuril 10% | 0.05 mg/kg | |
| 4 | 10 | 10 weeks | 3 × 104 | F. sycomorus | 100 mg/Kg | |
| Clinical Signs | No. of Animals | Control − ve 1 | Control + ve 2 | Diclazuril 0.05 mg/kg 3 | Extract 100 mg/kg 4 | p-Value 5 | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Freq. | % | Freq. | % | Freq. | % | Freq. | % | |||
| Depression | 10 | 0 | 0 | 3 | 30 | 2 | 20 | 2 | 20 | 0.46 |
| Diarrhea | 10 | 0 | 0 | 5 | 50 | 2 | 20 | 0 | 0 | 0.01 |
| Parameters | Number | Control − ve 1 | Control + ve 2 | Diclazuril, 0.05 mg/kg 3 | Extract, 100 mg/Kg 4 | p-Value | |
|---|---|---|---|---|---|---|---|
| Liver enzymes | ALT (U/L) | 7 | 27.26 ± 3.55 b | 34.01 ± 3.55 a | 21.10 ± 3.27 b | 20.17 ± 3.29 b | 0.03 |
| AST (U/L) | 7 | 16.44 ± 3.50 | 13.67 ± 3.39 | 18.40 ± 3.22 | 12.22 ± 3.25 | 0.18 | |
| ALP (IU/L) | 7 | 80.49 ± 11.76 | 106.67 ± 12.99 | 94.87 ± 11.76 | 88.13 ± 14.71 | 0.56 | |
| Erythrogram | RBCs (×106) | 7 | 5.64 ± 0.06 | 5.44 ± 0.06 | 5.49 ± 0.06 | 5.47 ± 0.06 | 0.98 |
| Hb (g/dL) | 7 | 12.46 ± 0.28 | 11.92 ± 0.29 | 12.18 ± 0.25 | 12.18 ± 0.26 | 0.45 | |
| MCV (fL) | 7 | 43.81 ± 0.63 a | 41.28 ± 0.64 b | 40.88 ± 0.56 b | 40.90 ± 0.58 b | 0.03 | |
| MCH (pg) | 7 | 22.02 ± 0.26 | 21.85 ± 0.27 | 22.15 ± 0.24 | 22.23 ± 0.25 | 0.19 | |
| MCHC (%) | 7 | 51.75 ± 1.09 b | 54.40 ± 1.12 ab | 56.11 ± 0.98 a | 56.18 ± 1.02 a | 0.05 | |
| Leukogram | WBCs (×103) | 7 | 7.81 ± 0.03 c | 9.80 ± 0.03 b | 10.21 ± 0.02 a | 9.70 ± 0.03 b | 0.05 |
| Lymphocytes % | 7 | 70.34 ± 2.71 a | 53.83 ± 2.78 c | 61.76 ± 2.44 b | 55.06 ± 2.52 bc | 0.05 | |
| Monocytes % | 7 | 10.11 ± 0.73 a | 10.49 ± 0.73 a | 9.25 ± 0.73 c | 9.37 ± 0.73 b | 0.04 | |
| Granulocytes% | 7 | 25.83 ± 2.34 c | 39.79 ± 2.40 a | 33.97 ± 2.10 b | 39.74 ± 2.17 a | 0.03 | |
| Lesions | No. | Lesion Score 1 | Chi-Square | p-Value | |||
|---|---|---|---|---|---|---|---|
| Control − ve 2 | Control + ve 3 | Diclazuril, 0.05 mg/kg 4 | Extract, 100 mg/kg 5 | ||||
| Intestine | |||||||
| Mucosal hyperplasia | 3 | 0.00 ± 0.00 b | 3.33 ± 0.33 a | 2.83 ± 0.17 b | 3.00 ± 0.00 b | 8.92 | 0.03 |
| The intensity of coccidia parasite in the intestinal mucosa | 3 | 0.00 ± 0.00 b | 4.00 ± 0.00 a | 3.17 ± 0.17 b | 1.83 ± 0.17 b | 10.76 | 0.01 |
| Liver | |||||||
| Hydropic degeneration of hepatocytes | 3 | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 2.83 ± 0.17 a | 1.00 ± 0.29 ab | 10.87 | 0.01 |
| Coagulative necrosis of hepatocytes | 3 | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.83 ± 0.17 a | 10.80 | 0.01 |
| Dilatation and engorgement of blood sinusoids | 3 | 0.00 ± 0.00 b | 2.83 ± 0.17 a | 1.83 ± 0.17 ab | 1.00 ± 0.00 ab | 10.76 | 0.01 |
| Dilatation and engorgement of bile duct | 3 | 0.00 ± 0.00 b | 1.67 ± 0.33 a | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 10.80 | 0.01 |
| Presence of fat globules in the bloodstream of central veins | 3 | 0.00 ± 0.00 b | 2.33 ± 0.33 b | 4.00 ± 0.00 a | 4.00 ± 0.00 a | 10.87 | 0.01 |
| Group | No. of Animals | Initial Body Weight | Covariate Final Body Weight (g) | Body Gain (g) | Feed Intake (g) | Feed Conversion |
|---|---|---|---|---|---|---|
| Control − ve 1 | 7 | 2410.33 ± 52.26 | 2568.33 ± 72.17 a | 158.00 ± 21.82 a | 1523.33 ± 104.91 a | 10.89 ± 1.67 c |
| Control + ve 2 | 7 | 2340.00 ± 46.51 | 2386.25 ± 63.72 b | 46.25 ± 20.66 b | 971.50 ± 77.93 b | 38.97 ± 8.39 a |
| Diclazuril-treated, 0.05 mg/kg 3 | 7 | 2290.67 ± 37.60 | 2386.67 ± 54.81 b | 96.00 ± 25.53 ab | 1367.67 ± 82.68 ab | 21.07 ± 5.19 b |
| Extract-treated 100 mg/kg 4 | 7 | 2311.00 ± 52.64 | 2371.25 ± 58.46 b | 60.25 ± 14.67 b | 1067.38 ± 129.78 b | 26.89 ± 7.53 b |
| p-value | 0.39 | 0.01 | 0.01 | 0.01 | 0.05 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dawod, A.; Fathalla, S.; El-Seedi, H.R.; Hammad, M.A.; Osman, N.; Abosheriba, N.; Anis, A.; Shehata, A.A.; Elkhatam, A. Efficacy of Ficus sycomorus (Sycamore Fig) Extract on Intestinal Coccidiosis in Experimentally Infected Rabbits. Life 2022, 12, 917. https://doi.org/10.3390/life12060917
Dawod A, Fathalla S, El-Seedi HR, Hammad MA, Osman N, Abosheriba N, Anis A, Shehata AA, Elkhatam A. Efficacy of Ficus sycomorus (Sycamore Fig) Extract on Intestinal Coccidiosis in Experimentally Infected Rabbits. Life. 2022; 12(6):917. https://doi.org/10.3390/life12060917
Chicago/Turabian StyleDawod, Ahmed, Said Fathalla, Hesham R. El-Seedi, Mohamed A. Hammad, Noha Osman, Nagwa Abosheriba, Anis Anis, Awad A. Shehata, and Ahmed Elkhatam. 2022. "Efficacy of Ficus sycomorus (Sycamore Fig) Extract on Intestinal Coccidiosis in Experimentally Infected Rabbits" Life 12, no. 6: 917. https://doi.org/10.3390/life12060917
APA StyleDawod, A., Fathalla, S., El-Seedi, H. R., Hammad, M. A., Osman, N., Abosheriba, N., Anis, A., Shehata, A. A., & Elkhatam, A. (2022). Efficacy of Ficus sycomorus (Sycamore Fig) Extract on Intestinal Coccidiosis in Experimentally Infected Rabbits. Life, 12(6), 917. https://doi.org/10.3390/life12060917








