The Effect of Colored and White Light on Growth and Phycobiliproteins, Chlorophyll and Carotenoids Content of the Marine Cyanobacteria Phormidium sp. and Cyanothece sp. in Batch Cultures
Abstract
1. Introduction
2. Materials and Methods
3. Results
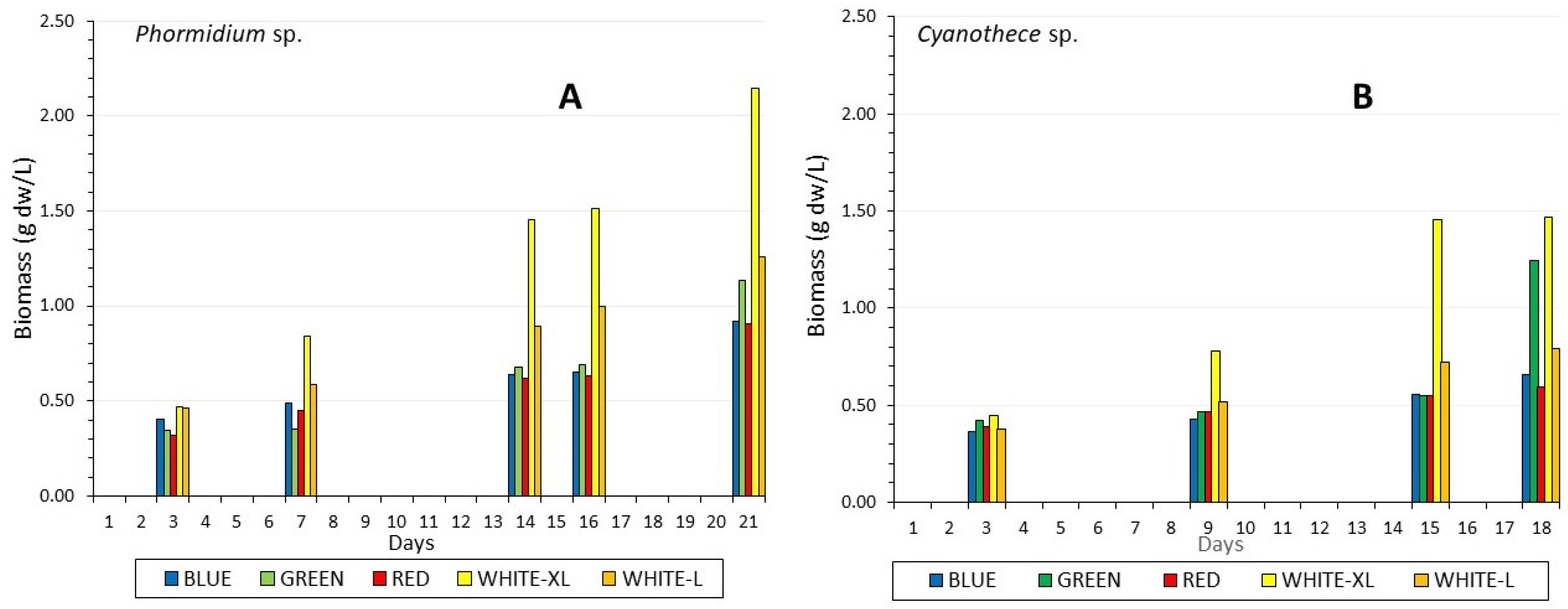
3.1. Biomass
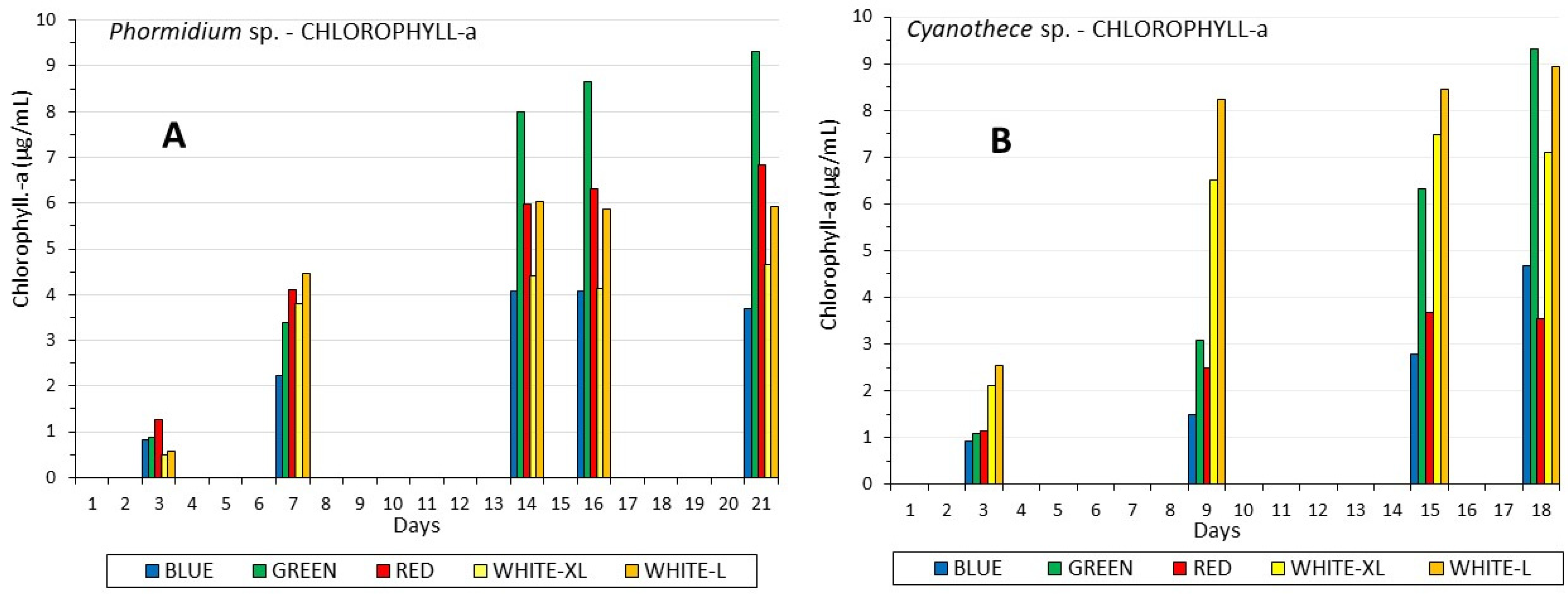
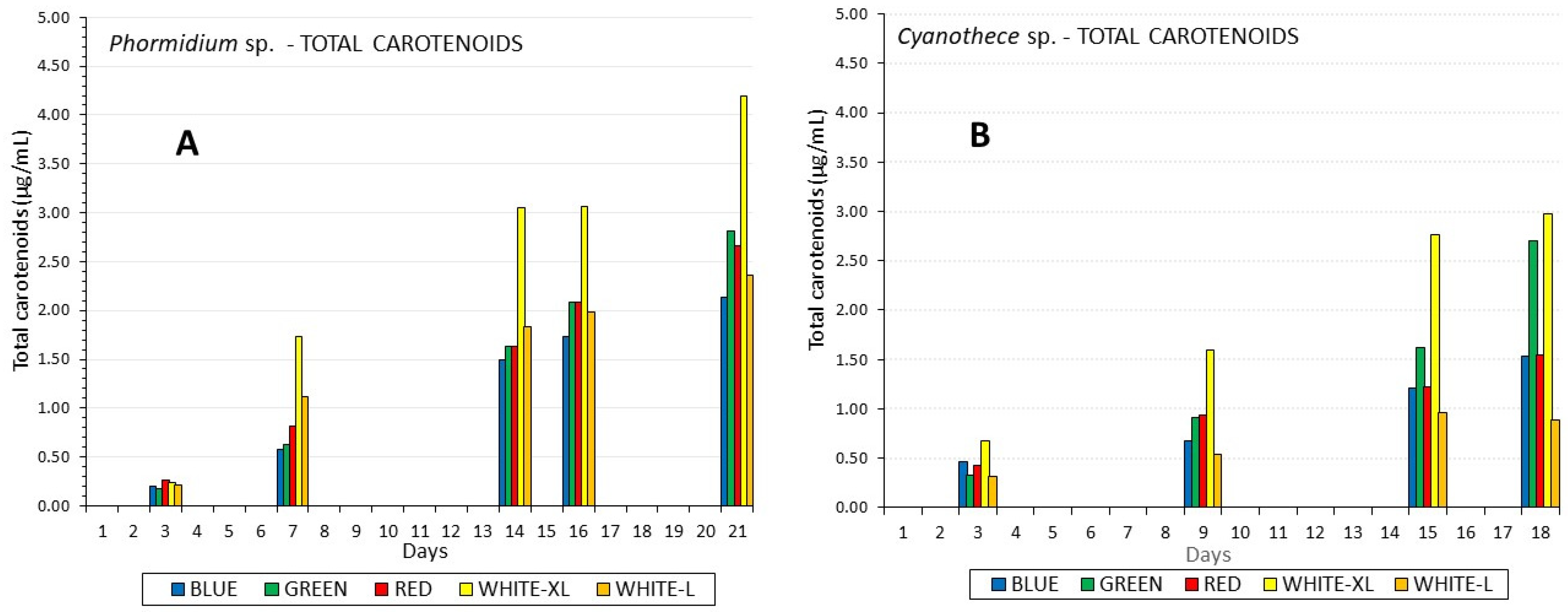
3.2. Chlorophyll and Carotenoids
3.3. Phycobiliproteins
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Venugopal, V.; Prasanna, R.; Sood, A.; Jaiswal, P.; Kaushik, B.D. Stimulation of pigment accumulation in Anabaena azollae strains: Effect of light intensity and sugars. Folia Microbiol. 2006, 51, 50–56. [Google Scholar] [CrossRef]
- Hrouzek, P.; Lukesová, A.; Simek, M. Comparison of light and dark nitrogenase activity in selected soil cyanobacteria. Folia Microbiol. 2004, 49, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Seckbach, J. Algae and Cyanobacteria in extreme environments. In Cellular Origin, Life in Extreme Habitats and Astrobiology; Seckbach, J., Ed.; Springer Science & Business Media: New York, NY, USA, 2007; Volume 11, pp. 1–811. [Google Scholar]
- Dvořák, P.; Poulíčková, A.; Hašler, P.; Belli, M.; Casamatta, D.A.; Papini, A. Species concepts and speciation factors in cyanobacteria, with connection to the problems of diversity and classification. Biodivers. Conserv. 2015, 24, 739–757. [Google Scholar] [CrossRef]
- Kauff, F.; Büdel, B. Phylogeny of cyanobacteria: An overview. Prog. Bot. 2011, 72, 209–224. [Google Scholar] [CrossRef]
- Eriksen, N.T. Production of phycocyanin—A pigment with applications in biology, biotechnology, foods and medicine. Appl. Microbiol. Biotechnol. 2008, 80, 1–14. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, Y.; Yin, Q.; Liu, G.; Liu, H.; Huang, Y.; Li, B. Phycocyanin: A potential drug for cancer treatment. J. Cancer 2017, 8, 3416–3429. [Google Scholar] [CrossRef]
- Klepacz-Smółka, A.; Pietrzyk, D.; Szeląg, R.; Głuszcz, P.; Daroch, M.; Tang, J.; Ledakowicz, S. Effect of light colour and photoperiod on biomass growth and phycocyanin production by Synechococcus PCC 6715. Bioresour. Technol. 2020, 313, 123700. [Google Scholar] [CrossRef]
- Skjånes, K.; Lindblad, P.; Muller, J. BioCO2—A multidisciplinary, biological approach using solar energy to capture CO2 while producing H2 and high value products. Biomol. Eng. 2007, 4, 405–413. [Google Scholar] [CrossRef]
- Satyanarayana, K.G.; Mariano, A.B.; Vargas, J.V.C. A review on microalgae, a versatile source for sustainable energy and materials. Int. J. Energy Res. 2011, 35, 291–311. [Google Scholar] [CrossRef]
- Vo, T.S.; Ngoa, D.H.; Kim, S.K. Marine algae as a potential pharmaceutical source for anti-allergic therapeutics. Process Biochem. 2012, 47, 386–394. [Google Scholar] [CrossRef]
- Bhaskar, S.U.; Gopalaswamy, G.; Raghu, R. A simple method for efficient extraction and purification of c-phycocyanin from Spirulina platensis Geitler. Indian J. Exp. Biol. 2005, 43, 277–279. [Google Scholar]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef]
- Walter, A.; de Carvalho, J.C.; Soccol, V.T.; de Faria, A.B.B.; Ghiggi, V.; Soccol, C.R. Study of Phycocyanin Production from Spirulina platensis Under Different Light Spectra. Braz. Arch. Biol. Technol. 2011, 54, 675–682. [Google Scholar] [CrossRef]
- Pathak, J.; Rajneesh, M.P.K.; Singh, S.P.; Häder, D.P.; Sinha, R.P. Cyanobacterial Farming for Environment Friendly Sustainable Agriculture Practices: Innovations and Perspectives. Front. Environ. Sci. 2018, 6, 7. [Google Scholar] [CrossRef]
- Saranraj, P.; Sivasakthi, S. Spirulina platensis-Food for future: A review. Asian J. Pharm. Sci. Technol. 2014, 4, 26–33. [Google Scholar]
- Barsanti, L.; Gualtieri, P. Algae: Anatomy, Biochemistry, and Biotechnology, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar] [CrossRef]
- Talbot, P.; Thébault, J.-M.; Dauta, A.; de la Noüe, J. A comparative study and mathematical modeling of temperature, light and growth of three microalgae potentially useful for wastewater treatment. Water Res. 1991, 25, 465–472. [Google Scholar] [CrossRef]
- Hotos, G.N. Culture Growth of the Cyanobacterium Phormidium sp. in Various Salinity and Light Regimes and Their Influence on Its Phycocyanin and Other Pigments Content. J. Mar. Sci. Eng. 2021, 9, 798. [Google Scholar] [CrossRef]
- Richmond, A. (Ed.) CRC Handbook of Microalgal Mass Culture, 1st ed.; CRC Press: Boca Raton, FL, USA, 1986; pp. 83–84. [Google Scholar] [CrossRef]
- Guermazi, W.; Masmoudi, S.; Boukhris, S.; Ayadi, H.; Morant-Manceau, A. Under low irradiation, the light regime modifies growth and metabolite production in various species of microalgae. J. Appl. Phycol. 2014, 26, 2283–2293. [Google Scholar] [CrossRef]
- Singh, S.P.; Singh, P. Effect of temperature and light on the growth of algae species: A review. Renew. Sustain. Energy Rev. 2015, 50, 431–444. [Google Scholar] [CrossRef]
- Maltsev, Y.; Maltseva, K.; Kulikovskiy, M.; Maltseva, S. Influence of Light Conditions on Microalgae Growth and Content of Lipids, Carotenoids, and Fatty Acid Composition. Biology 2021, 10, 1060. [Google Scholar] [CrossRef]
- Silva, S.C.; Ferreira, I.C.F.R.; Dias, M.M.; Barreiro, F.M. Microalgae-derived pigments: A 10-year bibliometric review and industry and market trend analysis. Molecules 2020, 25, 3406. [Google Scholar] [CrossRef]
- Sarada, R.; Pillai, M.G.; Ravishankar, G.A. Phycocyanin from Spirulina sp: Influence of Processing of Biomass on Phycocyanin Yield, Analysis of Efficacy of Extraction Methods and Stability Studies on Phycocyanin. Process Biochem. 1999, 34, 795–801. [Google Scholar] [CrossRef]
- Colla, L.M.; Oliveira Reinehr, C.; Reichert, C.; Costa, J.A. Production of biomass and nutraceutical compounds by Spirulina platensis under different temperature and nitrogen regimes. Bioresour. Technol. 2007, 98, 1489–1493. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Fu, C.-C.; Liu, Y.-C. Effects of using light-emitting diodes on the cultivation of Spirulina Platensis. Biochem. Eng. 2007, 37, 21–25. [Google Scholar] [CrossRef]
- Chen, H.-B.; Wu, J.-Y.; Wang, C.-F.; Fu, C.-C.; Shieh, C.-J.; Chen, C.-I.; Wang, C.-Y.; Liu, Y.-C. Modeling on chlorophyll-a and phycocyanin production by Spirulina platensis under various light-emitting diodes. Biochem. Eng. J. 2010, 53, 52–56. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, J.E.; Kim, Y.; Lee, S.Y. The Production of High Purity Phycocyanin by Spirulina platensis Using Light Emitting Diodes Based Two-Stage Cultivation. Appl. Biochem. Biotechnol. 2016, 178, 382–395. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Buso, D.; Wang, T.; Lopes, M.; Niangoran, U.; Zissis, G. Effect of Red and Blue LEDs on the Production of Phycocyanin by Spirulina Platensis Based on Photosynthetically Active Radiation. J. Sci. Technol. Lighting 2017, 41, 148–152. [Google Scholar] [CrossRef]
- Lima, G.M.; Teixeira, P.C.N.; Teixeira, C.M.L.L.; Filócomo, D.; Lage, C.L.S. Influence of spectral light quality on the pigment concentrations and biomass productivity of Arthrospira Platensis. Algal Res. 2018, 31, 157–166. [Google Scholar] [CrossRef]
- Moreno, J.; Rodríguez, H.; Vargas, M.A.; Rivas, J.; Guerrero, G.M. Nitrogen-fixing cyanobacteria as source of phycobiliprotein pigments. Composition and growth performance of ten filamentous heterocystous strains. J. Appl. Phycol. 1995, 7, 17–23. [Google Scholar] [CrossRef]
- Adhikary, S.P.; Pattnaik, H. Growth response of Westiellopsis prolifica janet to organic substrates in light and dark. Hydrobiologia 1979, 67, 241–247. [Google Scholar] [CrossRef]
- Prasanna, R.; Pabby, A.; Singh, P.K. Effect of glucose and light-dark environment on pigmentation profiles in the cyanobacterium Calothrix elenkenii. Folia Microbiol. 2004, 49, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Chaneva, G.; Furnadzhieva, S.; Minkova, K.; Lukavsky, J. Effect of light and temperature on the cyanobacterium Arthronema africanum—A prospective phycobiliprotein-producing strain. J. Appl. Phycol. 2007, 19, 537–544. [Google Scholar] [CrossRef]
- Khatoon, H.; Kok Leong, L.; Abdu Rahman, N.; Mian, S.; Begum, H.; Banerjee, S.; Endut, A. Effects of different light source and media on growth and production of phycobiliprotein from freshwater cyanobacteria. Bioresour. Technol. 2018, 249, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Hsieh-Lo, M.; Castillo, G.; Ochoa-Becerra, M.A.; Mojica, L. Phycocyanin and phycoerythrin: Strategies to improve production yield and chemical stability. Algal. Res. 2019, 42, 101600. [Google Scholar] [CrossRef]
- Hotos, G.N. A Preliminary Survey on the Planktonic Biota in a Hypersaline Pond of Messolonghi Saltworks (W. Greece). Diversity 2021, 13, 270. [Google Scholar] [CrossRef]
- Tamary, E.; Kiss, V.; Nevo, R.; Adam, Z.; Bernát, G.; Rexroth, S.; Rögner, M.; Reich, Z. Structural and functional alterations of cyanobacterial phycobilisomes induced by high-light stress. Biochim. Biophys. Acta-Bioenerg. 2012, 1817, 319–327. [Google Scholar] [CrossRef]
- Ho, M.Y.; Soulier, N.T.; Canniffe, D.P.; Shen, G.; Bryant, D.A. Light regulation of pigment and photosystem biosynthesis in cyanobacteria. Curr. Opin. Plant Biol. 2017, 37, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Zavřel, T.; Sinetova, M.A.; Červený, J. Measurement of Chlorophyll a and Carotenoids Concentration in Cyanobacteria. Bio-Protoc. 2015, 5, e1467. [Google Scholar] [CrossRef]
- Kleinegris, D.M.; Janssen, M.; Brandenburg, W.A.; Wijffels, R.H. Continuous production of carotenoids from Dunaliella salina. Enzyme Microb. Technol. 2011, 48, 253–259. [Google Scholar] [CrossRef]
- Arashiro, L.T.; Boto-Ordóñez, M.; Van Hulle, S.W.H.; Ferrer, I.; Garfí, M.; Rousseau, D.P.L. Natural pigments from microalgae grown in industrial wastewater. Bioresour. Technol. 2020, 303, 122894. [Google Scholar] [CrossRef]
- Moraes, C.C.; Sala, L.; Cerveira, P.G.; Kalil, J.S. C-Phycocyanin extraction from Spirulina platensis wet biomass. Braz. J. Chem. Eng. 2011, 28, 45–49. [Google Scholar] [CrossRef]
- Liu, L.N.; Chen, X.L.; Zhang, X.Y.; Zhang, Y.Z.; Zhou, B.C. One-step chromatography method for efficient separation and purification of R-phycoerythrin from Polysiphonia Urceolata. J. Biotechnol. 2005, 116, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Vijaya, V.; Anand, N. Blue light enhance the pigment synthesis in cyanobacterium Anabaena ambigua Rao (Nostacales). J. Agric. Biol. Sci. 2009, 4, 36–43. [Google Scholar]
- Bergmann, P.; Trösch, W. Repeated fed-batch cultivation of Thermosynechococcus elongatus BP-1 in flat-panel airlift photobioreactors with static mixers for improved light utilization: Influence of nitrate, carbon supply and photobioreactor design. Algal Res. 2016, 17, 79–86. [Google Scholar] [CrossRef]
- Ho, S.; Liao, J.; Chen, C.; Chang, J. Bioresource Technology Combining lightstrategies with recycled medium to enhance the economic feasibility of phycocyanin production with Spirulina platensis. Bioresour. Technol. 2018, 247, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Hotos, G.N.; Avramidou, D. The Effect of Various Salinities and Light Intensities on the Growth Performance of Five Locally Isolated Microalgae [Amphidinium carterae, Nephroselmis sp., Tetraselmis sp. (var. red pappas), Asteromonas gracilis and Dunaliella sp.] in Laboratory Batch Cultures. J. Mar. Sci. Eng. 2021, 9, 1275. [Google Scholar] [CrossRef]
- Hemlata Fatma, T. Screening of cyanobacteria for phycobiliproteins and effect of different environmental stress on its yield. Bull. Environ. Contam. Toxicol. 2009, 83, 509–515. [Google Scholar] [CrossRef]
- Johnson, M.E.; Kanhaiya, K.; Debabrata, D. Physicochemical parameters optimization, and purification of phycobiliproteins from the isolated Nostoc sp. Bioresour. Technol. 2014, 166, 541–547. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Elvitigala, T.; Welsh, E.; Stöckel, J.; Liberton, M.; Min, H.; Sherman, L.A.; Pakrasi, H.B. Novel metabolic attributes of the genus Cyanothece, comprising a group of unicellular nitrogen-fixing Cyanothece. mBio 2011, 2, e00214-11. [Google Scholar] [CrossRef]
- Kehoe, M.D. Chromatic adaptation and the evolution of light color sensing in cyanobacteria. Proc. Natl. Acad. Sci. USA 2010, 107, 9029–9030. [Google Scholar] [CrossRef] [PubMed]
- Tandeau de Marsac, N. Occurrence and nature of chromatic adaptation in cyanobacteria. J. Bacteriol. 1977, 130, 82–91. [Google Scholar] [CrossRef]
- Rodríguez, H.; Rivas, J.; Guerrero, M.G.; Losada, M. Enhancement of phycobiliprotein production in nitrogen-fixing cyanobacteria. J. Biotechnol. 1991, 20, 263–270. [Google Scholar] [CrossRef]
- Parmar, A.; Singh, N.K.; Kaushal, A.; Sonawala, S.; Madamwar, D. Purification, characterization and comparison of phycoerythrins from three different marine cyanobacterial cultures. Bioresour. Technol. 2011, 102, 1795–1802. [Google Scholar] [CrossRef]
- Ajayan, K.V.; Selvaraju, M.; Thirugnanamoorthy, K. Enrichment of chlorophyll and phycobiliproteins in Spirulina platensis by the use of reflector light and nitrogen sources: An in-vitro study. Biomass Bioenergy 2012, 47, 436–441. [Google Scholar] [CrossRef]
- Hauschild, C.; Heather, J.; McMurter, G.; Pick, F.R. Effect of spectral quality on growth and pigmentation of picocyanobacteria 1. J. Phycol. 1991, 27, 698–702. [Google Scholar] [CrossRef]
- Madhyastha, H.K.; Vatsala, T.M. Pigment production in Spirulina fussiformis in different photophysical conditions. Biomol. Eng. 2007, 24, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.K.; Shrivastav, A.; Maurya, R.R.; Patidar, S.K.; Haldar, S.; Mishra, S. Effect of light quality on the C-phycoerythrin production in marine cyanobacteria Pseudanabaena sp. isolated from Gujarat coast, India. Protein Expr. Purif. 2012, 81, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Kulshreshtha, J.; Singh, G.P. Growth and biopigment accumulation of cyanobacterium Spirulina platensis at different light intensities and temperature. Braz. J. Microbiol. 2011, 42, 1128–1135. [Google Scholar] [CrossRef]
- Tiwari, O.N.; Biswanath, B.; Sagnik, C.; Saswata, G.; Indrama, D. Strategies for improved production of phycobiliproteins (PBPs) by Oscillatoria sp. BTA170 and evaluation of its thermodynamic and kinetic stability. Biochem. Eng. J. 2019, 145, 153–161. [Google Scholar] [CrossRef]
- Takano, H.; Arai, T.; Hirano, M.; Matsunaga, T. Effects of intensity and quality of light on phycocyanin production by a marine cyanobacterium Synechococcus sp. NKBG 042902. Appl. Microbiol. Biotechnol. 1995, 43, 1014–1018. [Google Scholar] [CrossRef]
- Hong, S.J.; Lee, C.G. Statistical optimization of culture media for production of phycobiliproteins by Synechocystis sp. PCC 6701. Biotechnol. Bioprocess Eng. 2008, 13, 491–498. [Google Scholar] [CrossRef]
- Soundarapandian, P.; Vasanthi, B. Effects of chemical parameters on Spirulina platensis biomass production: Optimized method for phycocyanin extraction. Int. J. Zool. Res. 2008, 4, 1–11. [Google Scholar] [CrossRef]
- Kenekar, A.A.; Deodhar, M.A. Effect of varying physicochemical parameters on the productivity and phycobiliprotein content of indigenous isolate Geitlerinema sulphureum. Biotechnology 2013, 12, 146–154. [Google Scholar] [CrossRef]
- de Lorimier, R.M.; Smith, R.L.; Stevens, S.E. Regulation of phycobilisome structure and gene expression by light intensity. Plant Physiol. 1992, 98, 1003–1010. [Google Scholar] [CrossRef]
- Alberte, R.S.; Wood, A.M.; Kursar, T.A.; Guillard, R.R. Novel Phycoerythrins in Marine Synechococcus spp.: Characterization and Evolutionary and Ecological Implications. Plant Physiol. 1984, 75, 732–739. [Google Scholar] [CrossRef]
- Singh, N.K.; Parmar, A.; Madamwar, D. Optimization of medium components for increased production of C-phycocyanin from Phormidium ceylanicum and its purification by single step process. Bioresour. Technol. 2009, 100, 1663–1669. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-W.; Zhang, Y.-M.; Chen, F. Kinetic models for phycocyanin production by high cell density mixotrophic culture of the microalga Spirulina platensis. J. Ind. Microbiol. Biotechnol. 1998, 21, 283–288. [Google Scholar] [CrossRef]
- Zeng, X.; Danquah, M.K.; Zhang, S.; Zhang, X.; Wu, M.; Chen, X.D.; Ng, I.-S.; Jing, K.; Lu, Y. Autotrophic cultivation of Spirulina platensis for CO2 fixation and phycocyanin production. Chem. Eng. J. 2012, 183, 192–197. [Google Scholar] [CrossRef]
- Kovač, D.; Babić, O.; Milovanović, I.; Mišan, A.; Simeunović, J. The production of biomass and phycobiliprotein pigments in filamentous cyanobacteria: The impact of light and carbon sources. Appl. Biochem. Microbiol. 2017, 53, 539–545. [Google Scholar] [CrossRef]
- Singh, N.K.; Parmar, A.; Sonani, R.R.; Madamwar, D. Isolation, identification and characterization of novel thermotolerant Oscillatoria sp. N9DM: Change in pigmentation profile in response to temperature. Process Biochem. 2012, 47, 2472–2479. [Google Scholar] [CrossRef]
- Becker, E.W.; Venkataraman, L.V. Production and utilization of the blue-green alga Spirulina in India. Biomass 1984, 4, 105–125. [Google Scholar] [CrossRef]
- Patel, A.; Mishra, S.; Pawar, R.; Ghosh, P.K. Purification and characterization of C-Phycocyanin from cyanobacterial species of marine and freshwater habitat. Protein Expr. Purif. 2005, 40, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Bogorad, L. Phycobiliproteins and Complementary Chromatic Adaptation. Annu. Rev. Plant Physiol. 1975, 26, 369–401. [Google Scholar] [CrossRef]
- Luimstra, V.M.; Schuurmans, J.M.; Verschoor, A.M.; Hellingwerf, K.J.; Huisman, J.; Matthijs, H.C.P. Blue light reduces photosynthetic efficiency of cyanobacteria through an imbalance between photosystems I and II. Photosynth. Res. 2018, 138, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Gutu, A.; Kehoe, D.M. Emerging Perspectives on the Mechanisms, Regulation, and Distribution of Light Color Acclimation in Cyanobacteria. Mol. Plant 2012, 5, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Stowe, W.C.; Brodie-Kommit, J.; Stowe-Evans, E. Characterization of complementary chromatic adaptation in Gloeotrichia UTEX 583 and identification of a transposon-like insertion in the cpeBA operon. Plant Cell Physiol. 2011, 52, 553–562. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vijaya, V.; Anand, N.; Karunai, S.B. Enhancing the phycobilin pigment synthesis in Calothrix elenkinii through optimization of light conditions. Intern. J. Sci. Nat. 2015, 6, 88–91. [Google Scholar]
- Kim, N.N.; Hyun, S.S.; Heum, G.P.; Jehee, L.; Gyung-Suk, K.; Cheol, Y.C. Profiles of photosynthetic pigment accumulation and expression of photosynthesis-related genes in the marine cyanobacteria Synechococcus sp.: Effects of LED wavelengths. Biotechnol. Bioprocess Eng. 2014, 19, 250–256. [Google Scholar] [CrossRef]
- Ashokkumar, P.; Narayanaswamy, A. Studies on growth and Phycobilin pigments of the Cyanobacterium Westiellopsis iyengarii. Int. J. Biotechnol. Biochem. 2010, 6, 315–323. [Google Scholar]
- Ma, R.; Lu, F.; Bi, Y.; Hu, Z. Effects of light intensity and quality on phycobiliprotein accumulation in the cyanobacterium Nostoc sphaeroides Kützing. Biotechnol. Lett. 2015, 37, 1663–1669. [Google Scholar] [CrossRef]
- Lonneborg, A.; Lind, K.L.; Kalla, R.S.; Gustafsson, P.; Oquist, G. Acclimation Processes in the Light-Harvesting System of the Cyanobacterium Anacystis nidulans following a Light Shift from White to Red Light. Plant Physiol. 1985, 78, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Liotenberg, S.; Campbell, D.; Rippka, R.; Houmard, J.; de Marsac, N.T. Effect of the nitrogen source on phycobiliprotein synthesis and cell reserves in a chromatically adapting filamentous cyanobacterium. Microbiology 1996, 142, 611–622. [Google Scholar] [CrossRef]
- Poza-Carrión, C.; Fernández-Valiente, E.; Fernández Piñas, F.; Leganés, F. Acclimation of photosynthetic pigments and photosynthesis of the cyanobacterium Nostoc sp. strain UAM206 to combined fluctuations of irradiance, pH, and inorganic carbon availability. J. Plant Phys. 2001, 158, 1455–1461. [Google Scholar] [CrossRef]
- Ranjitha, K.; Kaushik, B.D. Purification of phycobiliproteins from Nostoc muscorum. J. Sci. Ind. Res. 2005, 64, 372–375. [Google Scholar]
- Aman, J.; Koli, D.; Kumar, A.; Kumar, S.; Sagar, S. Pigments analysis of cyanobacterial strains. Int. J. Chem. Stud. 2018, 6, 1248–1251. [Google Scholar]
- Nicklisch, A. Growth and light absorption of some planktonic cyanobacteria, diatoms and Chlorophyceae under simulated natural light fluctuations. J. Plankton Res. 1998, 20, 105–119. [Google Scholar] [CrossRef]
- Raqiba, H.; Sibi, G. Light emitting diode (LED) illumination for enhanced growth and cellular composition in three microalgae. Adv. Microb. Res. 2019, 3, 007. [Google Scholar] [CrossRef]
- Khajepour, F.; Hosseini, S.A.; Ghorbani Nasrabadi, R.; Markou, G. Effect of light intensity and photoperiod on growth and biochemical composition of a local isolate of Nostoc calcicola. Appl. Biochem. Biotechnol. 2015, 176, 2279–2289. [Google Scholar] [CrossRef]
- Xu, Y.; Ibrahim, I.M.; Wosu, C.I.; Ben-Amotz, A.; Harvey, P.J. Potential of New Isolates of Dunaliella Salina for Natural β-Carotene Production. Biology 2018, 7, 14. [Google Scholar] [CrossRef]
- Inomura, K.; Masuda, T.; Eichner, M.; Rabouille, S.; Zavřel, T.; Červený, J.; Vancová, M.; Bernát, G.; Armin, G.; Claquin, P.; et al. Quantifying Cyanothece growth under DIC limitation. Comput. Struct. Biotechnol. J. 2021, 19, 6456–6464. [Google Scholar] [CrossRef] [PubMed]
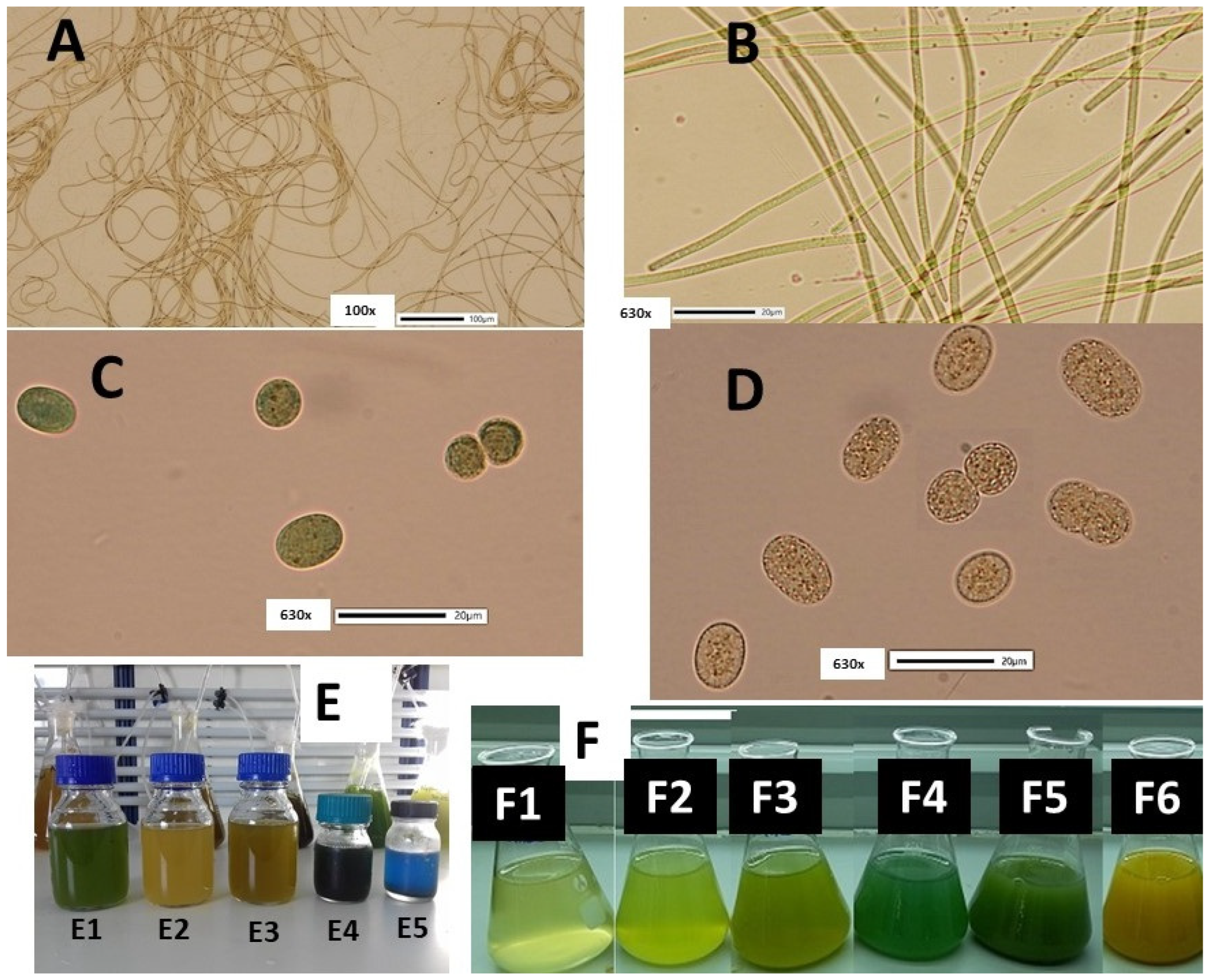

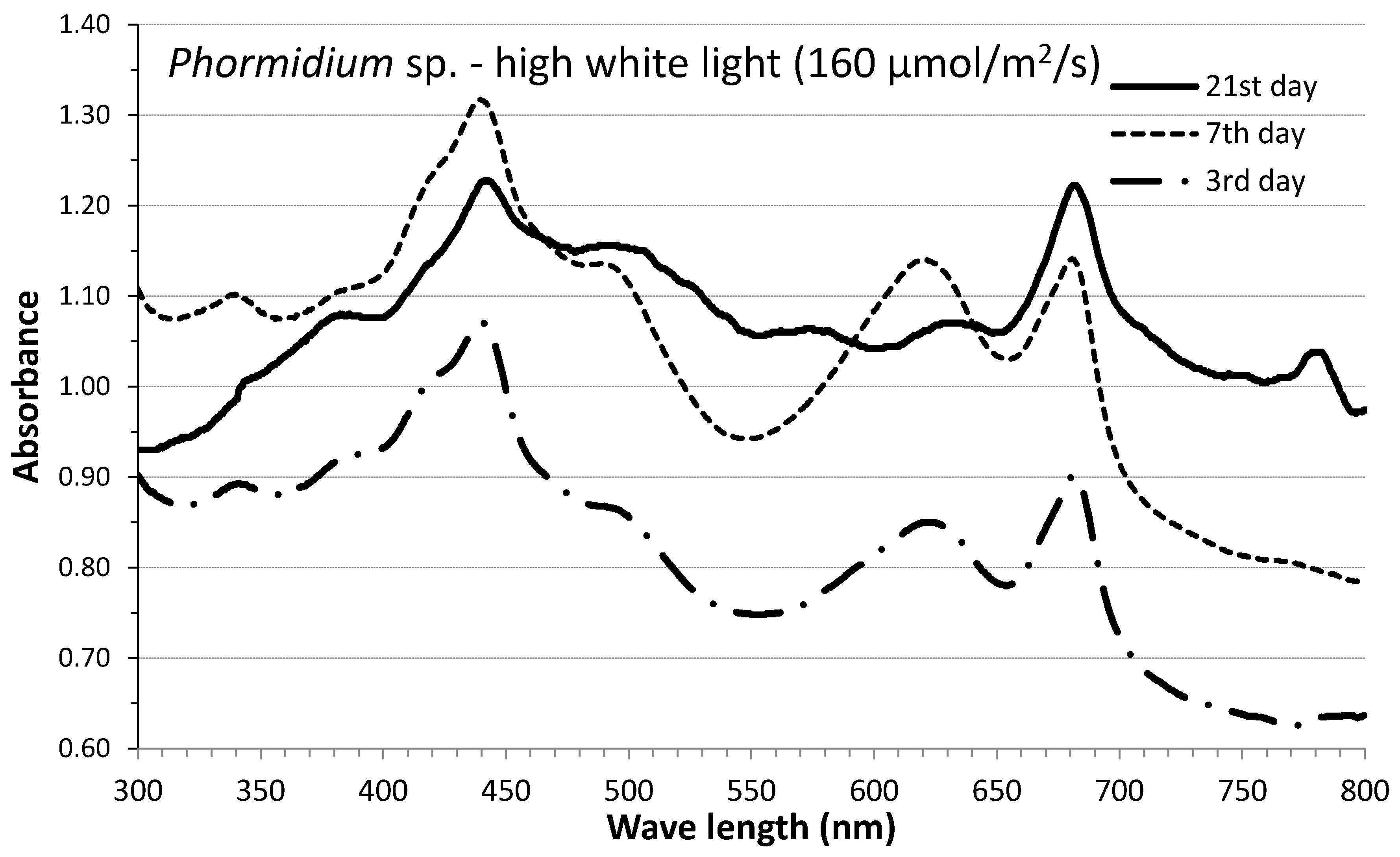

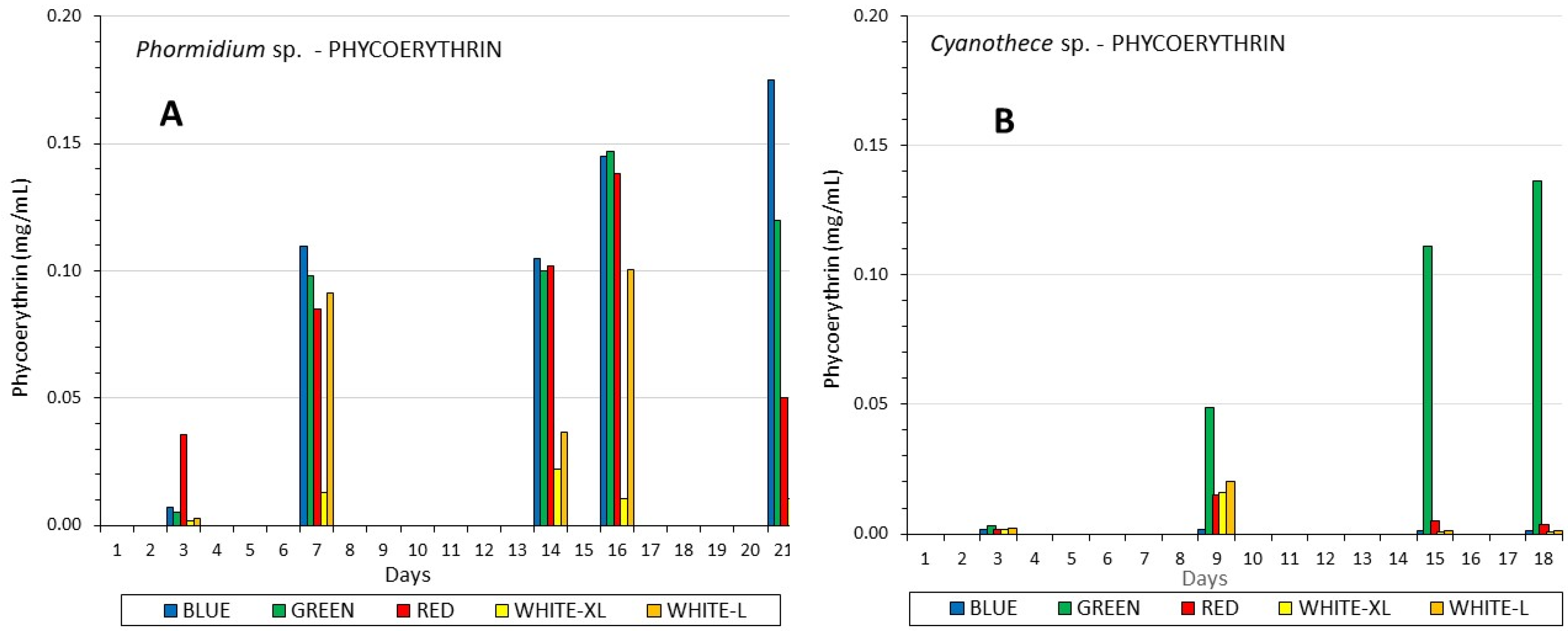
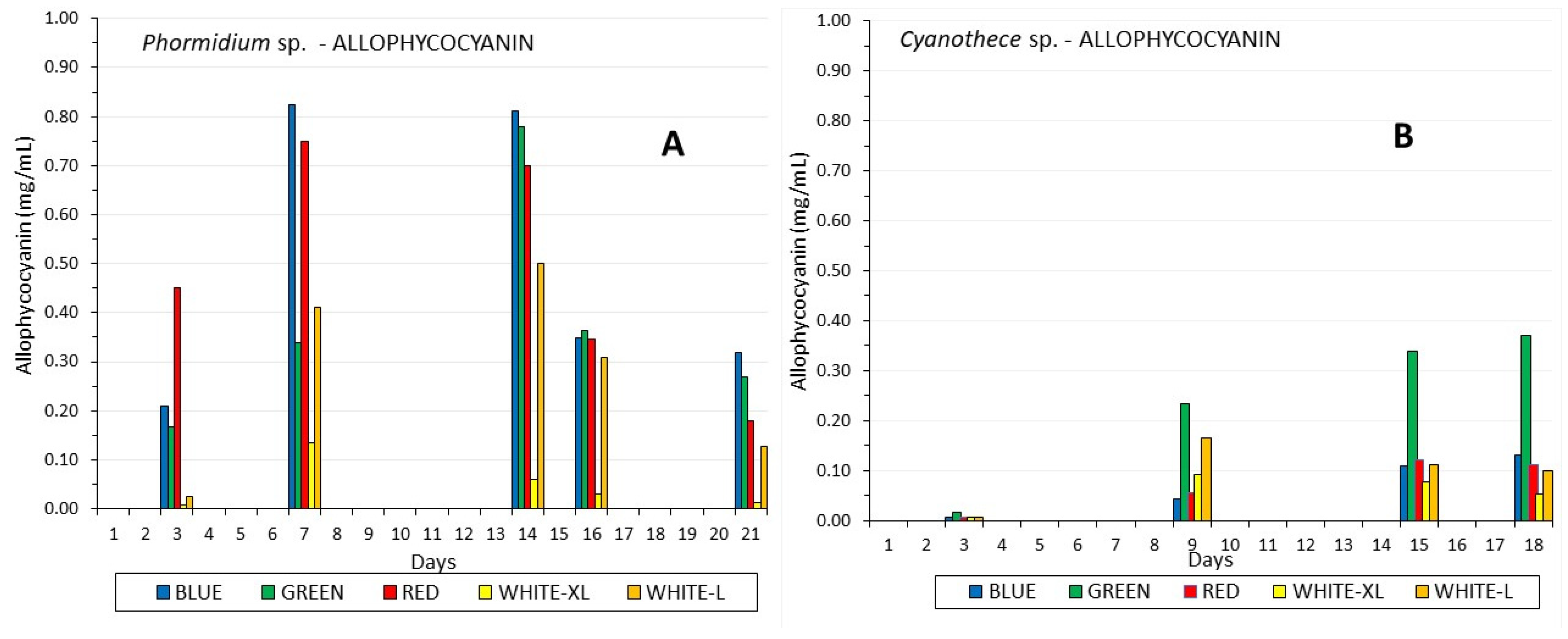
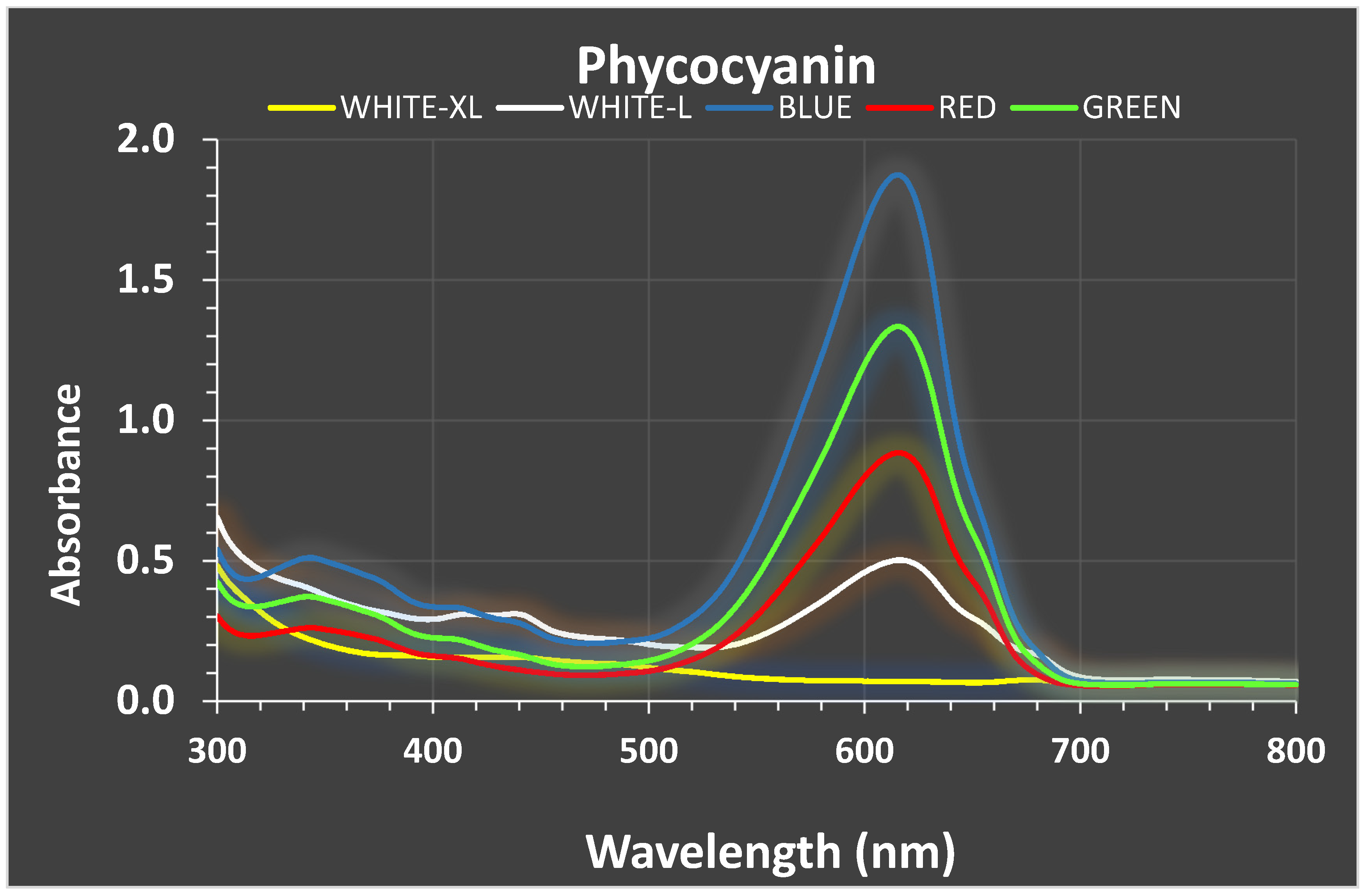
| Mean Values ± SE | Ph-W-XL | Ph-W-L | Ph-BLUE | Ph-GREEN | Ph-RED | Cy-W-XL | Cy-W-L | Cy-BLUE | Cy-GREEN | Cy-RED |
|---|---|---|---|---|---|---|---|---|---|---|
| SGR (doubl./d) | 0.085 a ±0.0003 | 0.062 b ±0.0004 | 0.041 c,e ±0.0005 | 0.102 d ±0.0004 | 0.044 e,c ±0.0009 | 0.083 f ±0.001 | 0.038 g ±0.0002 | 0.037 h ±0.0007 | 0.023 i ±0.0005 | 0.019 j ±0.0016 |
| Tg (days) | 8.16 | 11.12 | 17.06 | 6.78 | 15.7 | 8.37 | 18.35 | 18.6 | 30.64 | 35.92 |
| Max. Values ± SE | Ph-BLUE | Ph-GREEN | Ph-RED | Ph-W-XL | Ph-W-L | Cy-BLUE | Cy-GREEN | Cy-RED | Cy-W-XL | Cy-W-L |
|---|---|---|---|---|---|---|---|---|---|---|
| Chl.-a (μg/mL) | 4.08 c ±0.023 | 9.3 d ±0.07 | 6.85 e ±0.034 | 4.65 a,h ±0.08 | 5.93 b ±0.045 | 4.7 h,a ±0.078 | 9.31 i,d,g ±0.13 | 3.67 j ±0.05 | 7.47 f ±0.11 | 8.94 g,d,i ±0.6 |
| Tcar (μg/mL) | 2.14 c ±0.03 | 2.8 d ±0.021 | 2.66 e ±0.006 | 4.9 a ±0.05 | 2.36 b ±0.006 | 1.54 h, j ±0.0261 | 2.71 i ±0.036 | 1.55 j, h ±0.0261 | 2.97 f ±0.025 | 0.96 g ±0.0115 |
| PC (mg/mL) | 0.31 c, b ±0.009 | 0.447 d ±0.003 | 0.315 e,b,c,j ±0.0065 | 0.256 a ±0.006 | 0.317 b,c ±0.0004 | 0.305 h,b,c,g ±0.0025 | 0.296 i ±0.0147 | 0.327 j,b,c,e,g ±0.0148 | 0.195 f ±0.0134 | 0.321 g,b,c ±0.025 |
| PE (mg/mL) | 0.175 c ±0.002 | 0.147 d ±0.004 | 0.138 e,i ±0.003 | 0.022 a ±0.001 | 0.1 b ±0.0001 | 0.0017 h ±0.00007 | 0.136 i,e ±0.0014 | 0.015 j ±0.0002 | 0.016 f ±0.00031 | 0.02 g ±0.00038 |
| APC (mg/mL) | 0.824 c ±0.008 | 0.78 d ±0.003 | 0.751 e ±0.005 | 0.134 a,h ±0.003 | 0.51 b,i ±0.056 | 0.131 h,a ±0.0005 | 0.371 i,b ±0.0056 | 0.121 j ±0.0008 | 0.092 f ±0.0015 | 0.165 g ±0.0035 |
| Total PBP (mg/mL) | 1.084 | 1.145 | 0.940 | 0.403 | 0.854 | 0.437 | 0.768 | 0.452 | 0.303 | 0.506 |
| PCyield (mg/g dw) | 18.52 c,a,f,h ±0.71 | 31.76 d ±0.48 | 22.54 e ±0.47 | 19.41 a,c,f,h ±0.44 | 20.68 b ±0.37 | 19.26 h,a,c,f ±0.89 | 27.43 i ±0.98 | 34.6 j ±0.88 | 18.34 f,a,c ±0.98 | 29.16 g ±1.95 |
| PEyield (mg/g dw) | 10.62 c ±0.11 | 10.44 d ±0.005 | 10.87 e ±0.057 | 1.09 a ±0.0006 | 6.091 b ±0.005 | 0.122 h ±0.0015 | 12.07 i ±1.031 | 0.151 j ±0.0137 | 1.16 f ±0.075 | 1.83 g ±0.078 |
| Chl.: Tcar | 4.18 | 5.44 | 5.04 | 2.2 | 3.99 | 3.04 | 3.9 | 3.01 | 4.1 | 15.24 |
| PBP: Chl.-a | 616 | 428 | 620 | 106 | 173 | 109 | 187 | 109 | 47 | 62 |
| PBP:Tcar | 1735 | 2096 | 3028 | 233 | 690 | 331 | 632 | 371 | 190 | 938 |
| PC: PE | 40.3 | 39.3 | 8.4 | 20 | 18.1 | 304 | 15 | 67 | 150 | 174 |
| PC: APC | 1.4 | 1.6 | 1.7 | 2.0 | 2.0 | 2.77 | 2.71 | 3.51 | 2.12 | 2.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hotos, G.N.; Antoniadis, T.I. The Effect of Colored and White Light on Growth and Phycobiliproteins, Chlorophyll and Carotenoids Content of the Marine Cyanobacteria Phormidium sp. and Cyanothece sp. in Batch Cultures. Life 2022, 12, 837. https://doi.org/10.3390/life12060837
Hotos GN, Antoniadis TI. The Effect of Colored and White Light on Growth and Phycobiliproteins, Chlorophyll and Carotenoids Content of the Marine Cyanobacteria Phormidium sp. and Cyanothece sp. in Batch Cultures. Life. 2022; 12(6):837. https://doi.org/10.3390/life12060837
Chicago/Turabian StyleHotos, George N., and Theodoros I. Antoniadis. 2022. "The Effect of Colored and White Light on Growth and Phycobiliproteins, Chlorophyll and Carotenoids Content of the Marine Cyanobacteria Phormidium sp. and Cyanothece sp. in Batch Cultures" Life 12, no. 6: 837. https://doi.org/10.3390/life12060837
APA StyleHotos, G. N., & Antoniadis, T. I. (2022). The Effect of Colored and White Light on Growth and Phycobiliproteins, Chlorophyll and Carotenoids Content of the Marine Cyanobacteria Phormidium sp. and Cyanothece sp. in Batch Cultures. Life, 12(6), 837. https://doi.org/10.3390/life12060837







