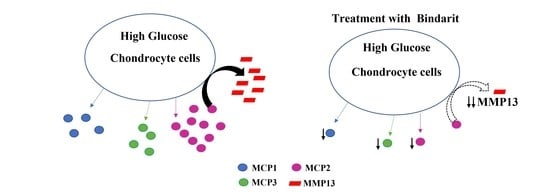
Monocyte Chemotactic Proteins Mediate the Effects of Hyperglycemia in Chondrocytes: In Vitro Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. mRNA Quantification: Quantitative Polymerase Chain Reaction (qPCR) for Gene Expression Evaluation
2.3. Statistical Analysis
3. Results
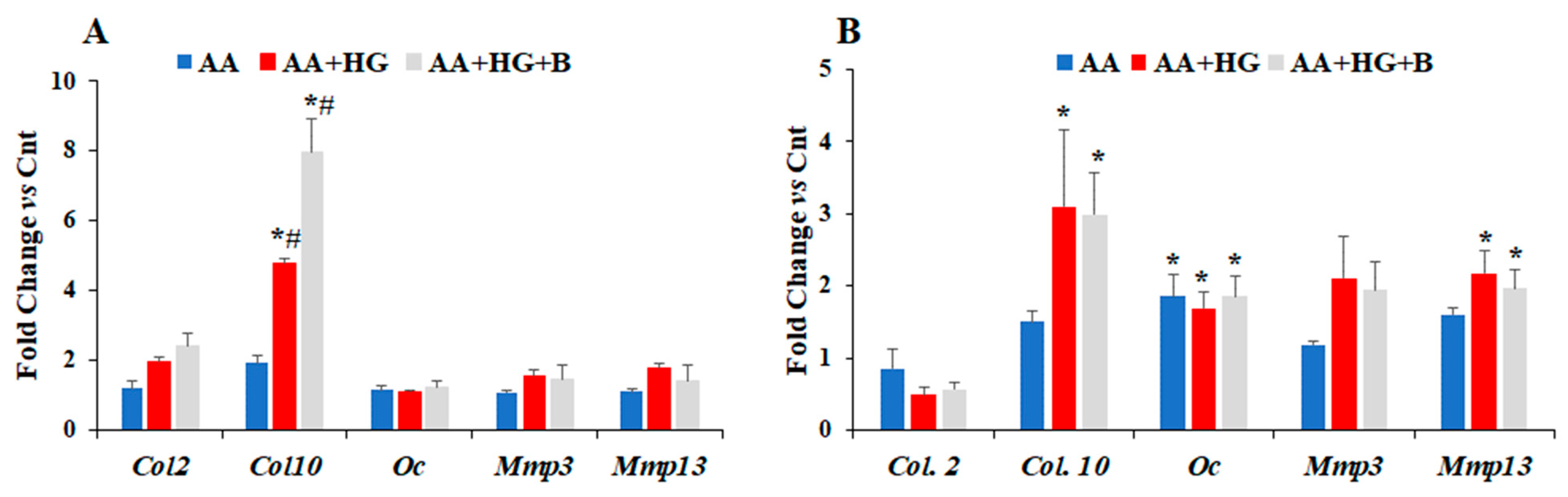
3.1. Effect of HG on the Expression Levels of Different Chemokines and Markers of Chondrogenesis
3.2. Effect of Ascorbic Acid on the Expression of Different Chemokines and Chondrogenesis Markers in Hyperglycemic Conditions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Sarkar, S.A.; Lee, C.E.; Victorino, F.; Nguyen, T.T.; Walters, J.A.; Burrack, A.; Eberlein, J.; Hildemann, S.K.; Homann, D. Expression and regulation of chemokines in murine and human type 1 diabetes. Diabetes 2012, 61, 436–446. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.T.; Chen, J.W. The Role of Chemokines and Chemokine Receptors in Diabetic Nephropathy. Int. J. Mol. Sci. 2020, 21, 3172. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.K.; Ke, Y.; Wang, B.; Lin, J.H. The role of MCP-1-CCR2 ligand-receptor axis in chondrocyte degradation and disease progress in knee osteoarthritis. Biol. Res. 2015, 17, 64. [Google Scholar] [CrossRef] [PubMed]
- Raghu, H.; Lepus, C.M.; Wang, Q.; Wong, H.H.; Lingampalli, N.; Oliviero, F.; Punzi, L.; Giori, N.J.; Goodman, S.B.; Chu, C.R.; et al. CCL2/CCR2, but not CCL5/CCR5, mediates monocyte recruitment, inflammation and cartilage destruction in osteoarthritis. Ann. Rheum. Dis. 2017, 76, 914–922. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.; Edderkaoui, B. Evaluation of CCL21 role in post-knee injury inflammation and early cartilage degeneration. PLoS ONE 2021, 16, e0247913, Erratum in PLoS ONE 2021, 16, e0259553. [Google Scholar] [CrossRef] [PubMed]
- Pang, T.; Gong, M.; Han, J.; Liu, D. Relationship between osteoporosis and expression of Bcl-2 and CXCL12. Exp. Ther. Med. 2018, 15, 1293–1297. [Google Scholar] [CrossRef] [PubMed]
- Lord, P.C.; Wilmoth, L.M.; Mizel, S.B.; McCall, C.E. Expression of interleukin-1 alpha and beta genes by human blood polymorphonuclear leukocytes. J. Clin. Investig. 1991, 87, 1312–1321. [Google Scholar] [CrossRef]
- Wolpe, S.D.; Sherry, B.; Juers, D.; Davatelis, G.; Yurt, R.W.; Cerami, A. Identification and characterization of macrophage inflammatory protein 2. Proc. Natl. Acad. Sci. USA 1989, 86, 612–616. [Google Scholar] [CrossRef]
- Sallusto, F.; Schaerli, P.; Loetscher, P.; Schaniel, C.; Lenig, D.; Mackay, C.R.; Qin, S.; Lanzavecchia, A. Rapid and coordinated switch in chemokine receptor expression during dendritic cell maturation. Eur. J. Immunol. 1998, 28, 2760–2769. [Google Scholar] [CrossRef]
- Bazzoni, F.; Cassatella, M.A.; Rossi, F.; Ceska, M.; Dewald, B.; Baggiolini, M. Phagocytosing neutrophils produce and release high amounts of the neutrophil-activating peptide 1/interleukin 8. J. Exp. Med. 1991, 173, 771–774. [Google Scholar] [CrossRef]
- Strieter, R.M.; Kasahara, K.; Allen, R.; Showell, H.J.; Standiford, T.J.; Kunkel, S.L. Human neutrophils exhibit disparate chemotactic factor gene expression. Biochem. Biophys. Res. Commun. 1990, 173, 725–730. [Google Scholar] [CrossRef]
- Sul, O.J.; Ke, K.; Kim, W.K.; Kim, S.H.; Lee, S.C.; Kim, H.J.; Kim, S.Y.; Suh, J.H.; Choi, H.S. Absence of MCP-1 leads to elevated bone mass via impaired actin ring formation. J. Cell Physiol. 2012, 227, 1619–1627. [Google Scholar] [CrossRef] [PubMed]
- Wintges, K.; Beil, F.T.; Albers, J.; Jeschke, A.; Schweizer, M.; Claass, B.; Tiegs, G.; Amling, M.; Schinke, T. Impaired bone formation and increased osteoclastogenesis in mice lacking chemokine (C-C motif) ligand 5 (Ccl5). J. Bone Min. Res. 2013, 28, 2070–2080. [Google Scholar] [CrossRef] [PubMed]
- Edderkaoui, B. Potential role of chemokines in fracture repair. Front. Endocrinol. 2017, 8, 39. [Google Scholar] [CrossRef]
- Lohmann, N.; Schirmer, L.; Atallah, P.; Wandel, E.; Ferrer, R.A.; Werner, C.; Simon, J.C.; Franz, S.; Freudenberg, U. Glycosaminoglycan-based hydrogels capture inflammatory chemokines and rescue defective wound healing in mice. Sci. Transl. Med. 2017, 9, eaai9044. [Google Scholar] [CrossRef]
- Panee, J. Monocyte Chemoattractant Protein 1 (MCP-1) in obesity and diabetes. Cytokine 2012, 60, 1–12. [Google Scholar] [CrossRef]
- Barutta, F.; Bruno, G.; Grimaldi, S.; Gruden, G. Inflammation in diabetic nephropathy: Moving toward clinical biomarkers and targets for treatment. Endocrine 2015, 48, 730–742. [Google Scholar] [CrossRef]
- Ferland-McCollough, D.; Maselli, D.; Spinetti, G.; Sambataro, M.; Sullivan, N.; Blom, A.; Madeddu, P. MCP-1 Feedback Loop Between Adipocytes and Mesenchymal Stromal Cells Causes Fat Accumulation and Contributes to Hematopoietic Stem Cell Rarefaction in the Bone Marrow of Patients with Diabetes. Diabetes 2018, 67, 1380–1394. [Google Scholar] [CrossRef]
- Raina, P.; Sikka, R.; Gupta, H.; Matharoo, K.; Bali, S.K.; Singh, V.; Bhanwer, A. Association of eNOS and MCP-1 genetic variants with type 2 diabetes and diabetic nephropathy susceptibility: A Case-control and meta-analysis study. Biochem. Genet. 2021, 59, 966–996. [Google Scholar] [CrossRef]
- Mulholland, B.S.; Forwood, M.R.; Morrison, N.A. Monocyte Chemoattractant Protein-1 (MCP-1/CCL2) Drives activation of bone remodelling and skeletal metastasis. Curr. Osteoporos. Rep. 2019, 17, 538–547. [Google Scholar] [CrossRef]
- Wu, A.C.; Morrison, N.A.; Kelly, W.L.; Forwood, M.R. MCP-1 expression is specifically regulated during activation of skeletal repair and remodeling. Calcif. Tissue Int. 2013, 92, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, M.; Ito, H.; Kitaori, T.; Murata, K.; Shibuya, H.; Furu, M.; Yoshitomi, H.; Fujii, T.; Yamamoto, K.; Matsuda, S. MCP/CCR2 signaling is essential for recruitment of mesenchymal progenitor cells during the early phase of fracture healing. PLoS ONE 2014, 9, e104954. [Google Scholar] [CrossRef] [PubMed]
- Hopwood, B.; Tsykin, A.; Findlay, D.M.; Fazzalari, N.L. Gene expression profile of the bone microenvironment in human fragility fracture bone. Bone 2009, 44, 87–101. [Google Scholar] [CrossRef]
- Liuni, F.M.; Rugiero, C.; Feola, M.; Rao, C.; Pistillo, P.; Terracciano, C.; Giganti, M.G.; Tarantino, U. Impaired healing of fragility fractures in type 2 diabetes: Clinical and radiographic assessments and serum cytokine levels. Aging Clin. Exp. Res. 2015, 27 (Suppl. S1), S37–S44. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, B.; Zhang, J.; Wu, Z.; Yu, L.; Sun, Z. Chemokine (C-C Motif) ligand 2/chemokine receptor 2 (CCR2) axis blockade to delay chondrocyte hypertrophy as a therapeutic strategy for osteoarthritis. Med. Sci. Monit. 2021, 27, e930053. [Google Scholar] [CrossRef] [PubMed]
- Von der Mark, K.; Kirsch, T.; Nerlich, A.; Kuss, A.; Weseloh, G.; Gluckert, K.; Stoss, H. Type x collagen synthesis in human osteoarthritic cartilage. indication of chondrocyte hypertrophy. Arthritis Rheum. 1992, 35, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Poole, A.; Nelson, F.; Dahlberg, L.; Tchetina, E.; Kobayashi, M.; Yasuda, T.; Laverty, S.; Squires, G.; Kojima, T.; Wu, W.; et al. Proteolysis of the collagen fibril in osteoarthritis. Biochem. Soc. Symp. 2003, 70, 115–123. [Google Scholar]
- Kuhn, K.; D’Lima, D.D.; Hashimoto, S.; Lotz, M. Cell death in cartilage. Osteoarthr. Cartil. 2004, 12, 1–16. [Google Scholar] [CrossRef]
- Fuerst, M.; Bertrand, J.; Lammers, L.; Dreier, R.; Echtermeyer, F.; Nitschke, Y.; Rutsch, F.; Schafer, F.K.; Niggemeyer, O.; Steinhagen, J.; et al. Calcification of articular cartilage in human osteoarthritis. Arthritis Rheum. 2009, 60, 2694–2703. [Google Scholar] [CrossRef]
- Choi, S.Y.; Ryu, H.M.; Choi, J.Y.; Cho, J.H.; Kim, C.D.; Kim, Y.L.; Park, S.H. The role of Toll-like receptor 4 in high-glucose-induced inflammatory and fibrosis markers in human peritoneal mesothelial cells. Int. Urol. Nephrol. 2017, 49, 171–181. [Google Scholar] [CrossRef]
- Graves, D.T.; Kayal, R.A. Diabetic complications and dysregulated innate immunity. Front. Biosci. 2008, 13, 1227–1239. [Google Scholar] [CrossRef] [PubMed]
- Nikolajczyk, B.S.; Jagannathan-Bogdan, M.; Shin, H.; Gyurko, R. State of the union between metabolism and the immune system in type 2 diabetes. Genes Immun. 2011, 12, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Xing, Z.; Lu, C.; Hu, D.; Yu, Y.Y.; Wang, X.; Colnot, C.; Nakamura, M.; Wu, Y.; Miclau, T.; Marcucio, R.S. Multiple roles for CCR2 during fracture healing. Dis. Model Mech. 2010, 3, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Scammell, B.E.; Roach, H.I. A new role for the chondrocyte in fracture repair: Endochondral ossification includes direct bone formation by former chondrocytes. J. Bone Miner Res. 1996, 11, 737–745. [Google Scholar] [CrossRef]
- Mora, E.; Guglielmotti, A.; Biondi, G.; Sassone-Corsi, P. Bindarit: An anti-inflammatory small molecule that modulates the NF-κB pathway. Cell Cycle 2012, 11, 159–169. [Google Scholar] [CrossRef]
- Atsumi, T.; Miwa, Y.; Kimata, K.; Ikawa, Y. A chondrogenic cell line derived from a differentiating culture of AT805 teratocarcinoma cells. Cell Differ Dev. 1990, 30, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Rundle, C.H.; Mohan, S.; Edderkaoui, B. Duffy antigen receptor for chemokines regulates post-fracture inflammation. PLoS ONE 2013, 8, e77362. [Google Scholar] [CrossRef][Green Version]
- Dapunt, U.; Maurer, S.; Giese, T.; Gaida, M.M.; Hänsch, G.M. The macrophage inflammatory proteins MIP1α (CCL3) and MIP2α (CXCL2) in implant-associated osteomyelitis: Linking inflammation to bone degradation. Mediat. Inflamm. 2014, 2014, 728619. [Google Scholar] [CrossRef]
- Goto, Y.; Aoyama, M.; Sekiya, T.; Kakita, H.; Waguri-Nagaya, Y.; Miyazawa, K.; Asai, K.; Goto, S. CXCR4+ CD45− Cells are Niche Forming for Osteoclastogenesis via the SDF-1, CXCL7, and CX3CL1 Signaling Pathways in Bone Marrow. Stem Cells 2016, 34, 2733–2743. [Google Scholar] [CrossRef]
- Folk, J.W.; Starr, A.J.; Early, J.S. Early wound complications of operative treatment of calcaneus fractures: Analysis of 190 fractures. J. Orthop. Trauma 1999, 13, 369–372. [Google Scholar] [CrossRef]
- Retzepi, M.; Donos, N. The effect of diabetes mellitus on osseous healing. Clin. Oral. Implants Res. 2010, 21, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Hoff, P.; Gaber, T.; Strehl, C.; Schmidt-Bleek, K.; Lang, A.; Huscher, D.; Burmester, G.R.; Schmidmaier, G.; Perka, C.; Duda, G.N.; et al. Immunological characterization of the early human fracture hematoma. Immunol. Res. 2016, 64, 1195–1206. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Wang, P.; Li, J.; Li, Y.; Wang, S.; Wu, X.; Sun, S.; Cen, S.; Su, H.; Deng, W.; et al. MCP1 triggers monocyte dysfunctions during abnormal osteogenic differentiation of mesenchymal stem cells in ankylosing spondylitis. J. Mol. Med. 2017, 95, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Burrage, P.S.; Mix, K.S.; Brinckerhoff, C.E. Matrix metalloproteinases: Role in arthritis. Front. Biosci. 2006, 11, 529–543. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zheng, S.; Liu, T.; Liu, Q.; Chen, Y.; Tan, D.; Ma, R.; Lu, X. MCP2 activates NF-κB signaling pathway promoting the migration and invasion of ESCC cells. Cell Biol. Int. 2018, 42, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Grassia, G.; Maddaluno, M.; Guglielmotti, A.; Mangano, G.; Biondi, G.; Maffia, P.; Ialenti, A. The anti-inflammatory agent bindarit inhibits neointima formation in both rats and hyperlipidaemic mice. Cardiovasc. Res. 2009, 84, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Kaźmierczak-Barańska, J.; Boguszewska, K.; Adamus-Grabicka, A.; Karwowski, B.T. Two Faces of Vitamin C-Antioxidative and Pro-Oxidative Agent. Nutrients 2020, 12, 1501. [Google Scholar] [CrossRef]
- Chang, Z.; Huo, L.; Li, P.; Wu, Y.; Zhang, P. Ascorbic acid provides protection for human chondrocytes against oxidative stress. Mol. Med. Rep. 2015, 12, 7086–7092. [Google Scholar] [CrossRef]
- Levenson, G.E. Behavior in culture of three types of chondrocytes, and their response to ascorbic acid. Exp. Cell Res. 1970, 62, 271–285. [Google Scholar] [CrossRef]
- Leboy, P.S.; Vaias, L.; Uschmann, B.; Golub, E.; Adams, S.L.; Pacifici, M. Ascorbic acid induces alkaline phosphatase, type X collagen, and calcium deposition in cultured chick chondrocytes. J. Biol. Chem. 1989, 264, 17281–17286. [Google Scholar] [CrossRef]
- Temu, T.M.; Wu, K.Y.; Gruppuso, P.A.; Phornphutkul, C. The mechanism of ascorbic acid-induced differentiation of ATDC5 chondrogenic cells. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E325–E334. [Google Scholar] [CrossRef] [PubMed]
- Newton, P.T.; Staines, K.A.; Spevak, L.; Boskey, A.L.; Teixeira, C.C.; Macrae, V.E.; Canfield, A.E.; Farquharson, C. Chondrogenic ATDC5 cells: An optimised model for rapid and physiological matrix mineralisation. Int. J. Mol. Med. 2012, 30, 1187–1193. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Wu, X.; Song, M.; Li, P.; Wang, L. Oxidative damage induces MCP-1 secretion and macrophage aggregation in age-related macular degeneration (AMD). Graefe’s Arch. Clin. Exp. Ophthalmol. 2016, 254, 2469–2476. [Google Scholar] [CrossRef] [PubMed]
- Alblowi, J.; Tian, C.; Siqueira, M.F.; Kayal, R.A.; McKenzie, E.; Behl, Y.; Gerstenfeld, L.; Einhorn, T.A.; Graves, D.T. Chemokine expression is upregulated in chondrocytes in diabetic fracture healing. Bone 2013, 53, 294–300. [Google Scholar] [CrossRef]
- Ding, Z.C.; Zeng, W.N.; Rong, X.; Liang, Z.M.; Zhou, Z.K. Do patients with diabetes have an increased risk of impaired fracture healing? A systematic review and meta-analysis. ANZ J. Surg. 2020, 90, 1259–1264. [Google Scholar] [CrossRef]
- Kayal, R.A.; Alblowi, J.; McKenzie, E.; Krothapalli, N.; Silkman, L.; Gerstenfeld, L.; Einhorn, T.; Graves, D.T. Diabetes Causes the Accelerated Loss of Cartilage During Fracture Repair Which Is Reversed by Insulin Treatment. Bone 2009, 44, 357–363. [Google Scholar] [CrossRef]



| R Values | Col2 | Col10 | Oc | Mmp3 | Mmp13 | Mcp1/ccl2 | Mcp2/Ccl8 | Mcp3/Ccl7 | Mip-1a | Mip-1g | Rantes |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Col2 | 1 | 0.27 | −0.67 | −0.06 | −0.62 | −0.39 | −0.65 | −0.62 | 0.60 | −0.67 | 0.42 |
| Col10 | 1 | −0.38 | −0.21 | −0.20 | −0.44 | −0.25 | −0.47 | −0.27 | −0.23 | −0.12 | |
| Oc | 1 | 0.78 | 0.98 | 0.88 | 0.98 | 0.93 | −0.40 | −0.50 | −0.45 | ||
| Mmp3 | 1 | −0.48 | 0.62 | 0.61 | 0.65 | 0.70 | −0.50 | −0.16 | |||
| Mmp13 | 1 | 0.83 | 0.97 | 0.87 | 0.60 | −0.67 | −0.27 | ||||
| Mcp-1 | 1 | 0.88 | 0.91 | 0.69 | −0.4 | −0.35 | |||||
| Mcp-2 | 1 | 0.91 | 0.70 | −0.56 | −0.26 | ||||||
| Mcp-3 | 1 | 0.55 | −0.24 | −0.28 | |||||||
| Mip-1a | 1 | −0.60 | 0.15 | ||||||||
| Mip-1g | 1 | 0.25 |
| p Values | Col2 | Col10 | Oc | Mmp3 | Mmp13 | Mcp1/Ccl2 | Mcp2/Ccl8 | Mcp3/Ccl7 | Mip-1a | Mip-1g | Rantes |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Col2 | <0.01 | 0.39 | 0.03 | 0.85 | 0.032 | 0.34 | 0.08 | 0.10 | 0.04 | 0.03 | 0.15 |
| Col10 | <0.01 | 0.23 | 0.51 | 0.52 | 0.15 | 0.44 | 0.12 | 0.31 | 0.12 | 0.22 | |
| Oc | <0.01 | 0.01 | <0.01 | <0.01 | <0.01 | <0.01 | 0.11 | 0.11 | 0.70 | ||
| Mmp3 | <0.01 | 0.11 | 0.04 | 0.04 | 0.04 | 0.03 | 0.06 | 0.12 | |||
| Mmp13 | <0.01 | <0.01 | <0.01 | <0.01 | 0.04 | 0.03 | 0.63 | ||||
| Mcp-1 | <0.01 | <0.01 | <0.01 | 0.03 | 0.12 | 0.31 | |||||
| Mcp-2 | <0.01 | <0.01 | 0.04 | 0.05 | 0.32 | ||||||
| Mcp-3 | <0.01 | 0.05 | 0.06 | 0.30 | |||||||
| Mip-1a | <0.01 | 0.07 | 0.31 | ||||||||
| Mip-1g | <0.01 | 0.50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quincey, A.; Mohan, S.; Edderkaoui, B. Monocyte Chemotactic Proteins Mediate the Effects of Hyperglycemia in Chondrocytes: In Vitro Studies. Life 2022, 12, 836. https://doi.org/10.3390/life12060836
Quincey A, Mohan S, Edderkaoui B. Monocyte Chemotactic Proteins Mediate the Effects of Hyperglycemia in Chondrocytes: In Vitro Studies. Life. 2022; 12(6):836. https://doi.org/10.3390/life12060836
Chicago/Turabian StyleQuincey, Adam, Subburaman Mohan, and Bouchra Edderkaoui. 2022. "Monocyte Chemotactic Proteins Mediate the Effects of Hyperglycemia in Chondrocytes: In Vitro Studies" Life 12, no. 6: 836. https://doi.org/10.3390/life12060836
APA StyleQuincey, A., Mohan, S., & Edderkaoui, B. (2022). Monocyte Chemotactic Proteins Mediate the Effects of Hyperglycemia in Chondrocytes: In Vitro Studies. Life, 12(6), 836. https://doi.org/10.3390/life12060836