Bacterial Utilisation of Aliphatic Organics: Is the Dwarf Planet Ceres Habitable?
Abstract
1. Introduction
2. Materials and Methods
2.1. Culture Conditions
2.2. Experimental Conditions
2.3. Whole-Cell Extraction from Clay Samples
2.4. Cell Enumeration, Intracellular Activity, and Growth Analysis
2.5. Adenosine Triphosphate (ATP) Analysis
2.6. Nicotinamide Adenine Dinucleotide Phosphate (NADP+/NADPH) Analysis
2.7. Protein Content
2.8. Measurement of O2 Consumption
2.9. Elemental Analysis
2.10. Statistical Analysis
3. Results
3.1. Cell Density and Growth
3.2. Physiological Parameters
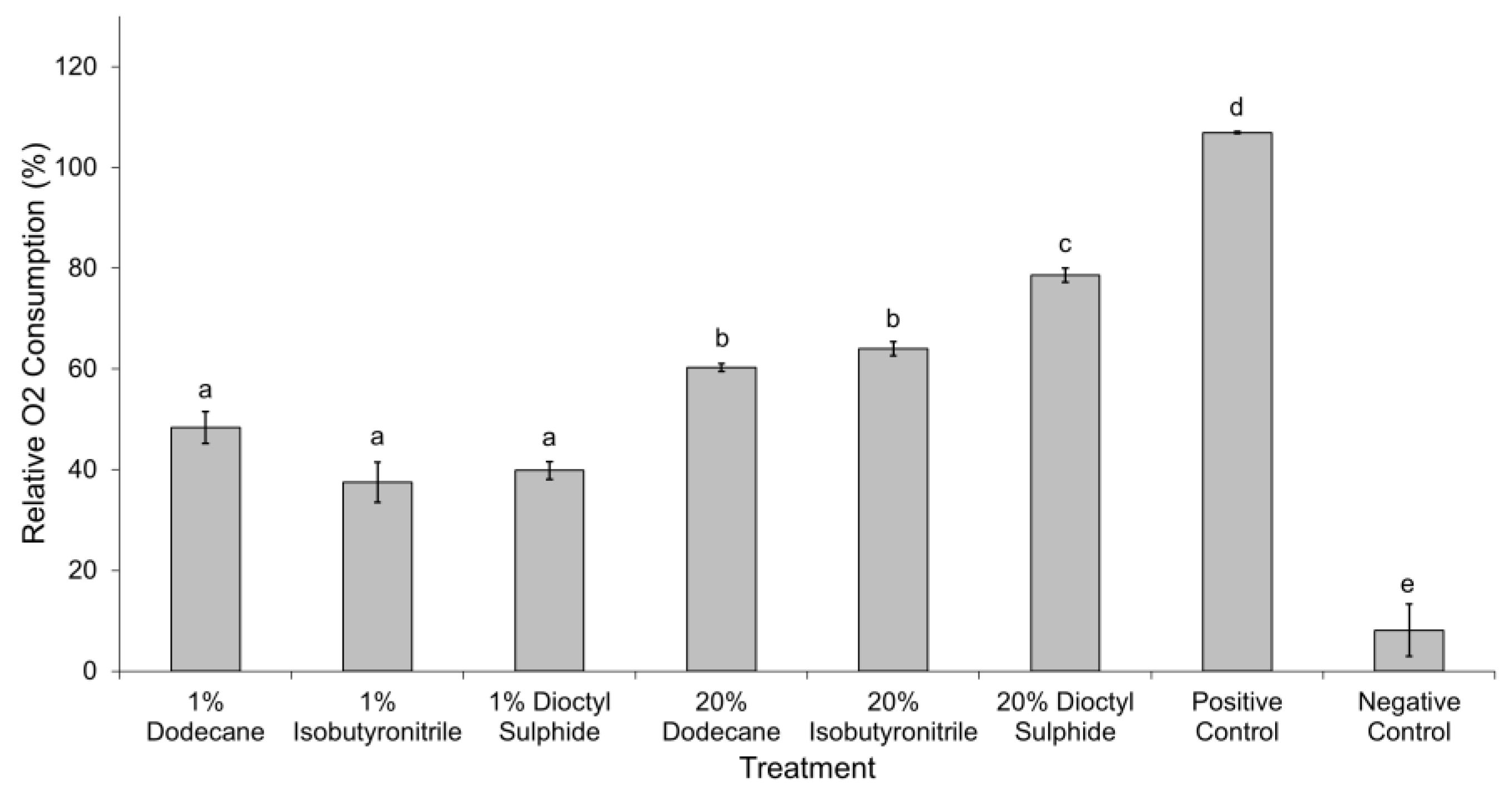
3.3. Oxygen (O2) Consumption
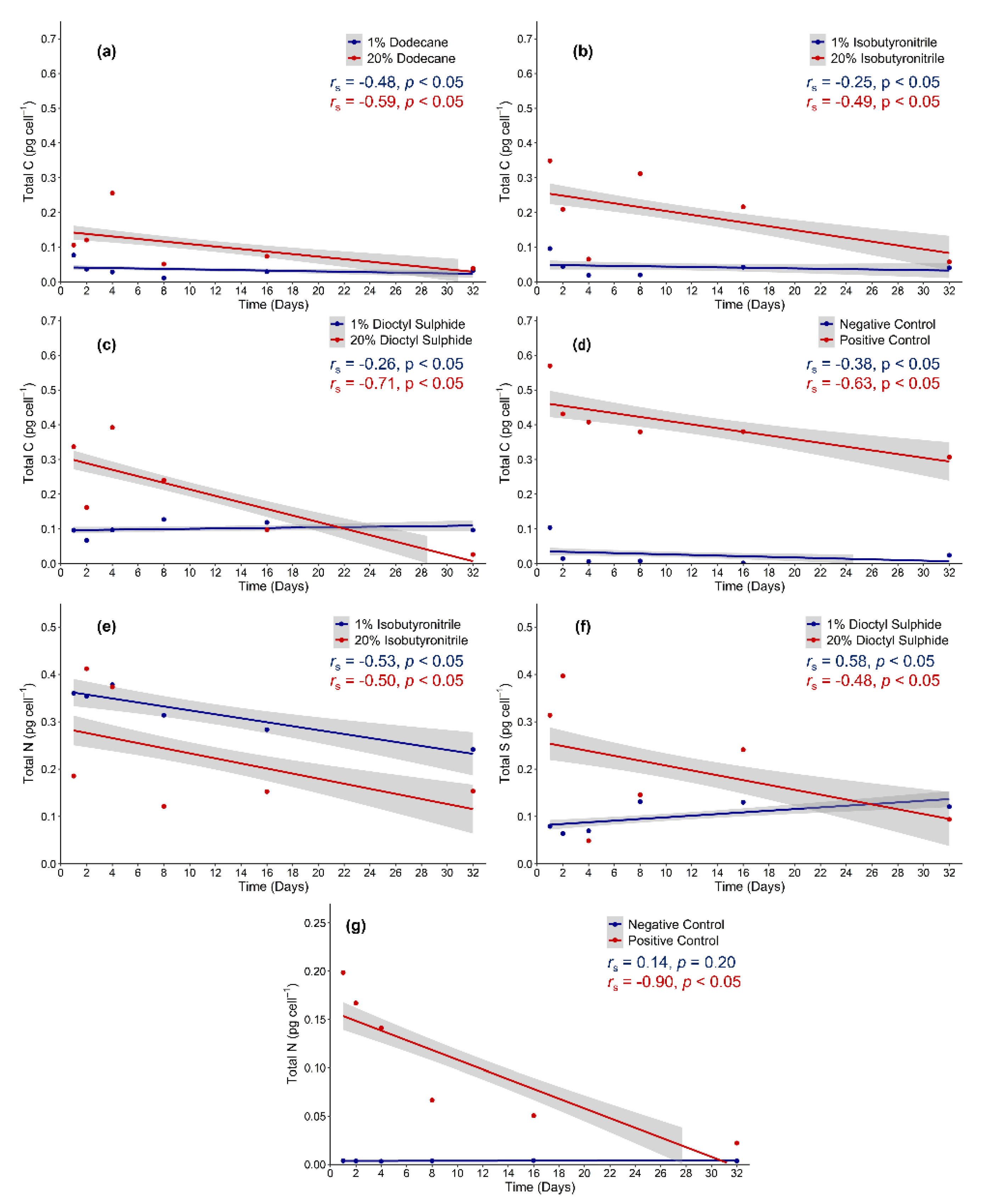
3.4. Elemental Analysis
4. Discussion
4.1. High Aliphatic Substrate Concentration Governs Utilisation Potential
4.2. Dodecane
4.3. Isobutyronitrile
4.4. Dioctyl-Sulphide
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berthe-Corti, L.; Fetzner, S. Bacterial metabolism of n-alkanes and ammonia under oxic, suboxic and anoxic conditions. Acta Biotechnol. 2002, 22, 299–336. [Google Scholar] [CrossRef]
- Mbadinga, S.M.; Wang, L.Y.; Zhou, L.; Liu, J.F.; Gu, J.D.; Mu, B.Z. Microbial communities involved in anaerobic degradation of alkanes. Int. Biodeterior. Biodegrad. 2011, 65, 1–13. [Google Scholar] [CrossRef]
- Xu, X.; Liu, W.; Tian, S.; Wang, W.; Qi, Q.; Jiang, P.; Gao, X.; Li, F.; Li, H.; Yu, H. Petroleum hydrocarbon-degrading bacteria for the remediation of oil pollution under aerobic conditions: A perspective analysis. Front. Microbiol. 2018, 9, 2885. [Google Scholar] [CrossRef] [PubMed]
- Sakshi; Haritash, A.K. A comprehensive review of metabolic and genomic aspects of PAH-degradation. Arch. Microbiol. 2020, 202, 2033–2058. [Google Scholar] [CrossRef]
- Gregson, B.H.; Metodieva, G.; Metodiev, M.V.; McKew, B.A. Differential protein expression during growth on linear versus branched alkanes in the obligate marine hydrocarbon-degrading bacterium Alcanivorax borkumensis SK2T. Environ. Microbiol. 2019, 21, 2347–2359. [Google Scholar] [CrossRef]
- Martins, C.C.; de Abreu-Mota, M.A.; do Nascimento, M.G.; Dauner, A.L.L.; Lourenço, R.A.; Bícego, M.C.; Montone, R.C. Sources and depositional changes of aliphatic hydrocarbons recorded in sedimentary cores from Admiralty Bay, South Shetland Archipelago, Antarctica during last decades. Sci. Total Environ. 2021, 795, 148881. [Google Scholar] [CrossRef]
- Abbasian, F.; Lockington, R.; Mallavarapu, M.; Naidu, R. A comprehensive review of aliphatic hydrocarbon biodegradation by bacteria. Appl. Biochem. Biotechnol. 2015, 176, 670–699. [Google Scholar] [CrossRef]
- Lo Giudice, A.; Casella, P.; Caruso, C.; Mangano, S.; Bruni, V.; de Domenico, M.; Michaud, L. Occurrence and characterization of psychrotolerant hydrocarbon-oxidizing bacteria from surface seawater along the Victoria Land coast (Antarctica). Polar Biol. 2010, 33, 929–943. [Google Scholar] [CrossRef]
- Kaczmarek, Ł.; Jakubowska, N.; Celewicz-Gołdyn, S.; Zawierucha, K. The microorganisms of cryoconite holes (algae, archaea, bacteria, cyanobacteria, fungi, and protista): A review. Polar Record 2016, 52, 176–203. [Google Scholar] [CrossRef]
- Castillo-Rogez, J.C.; Neveu, M.; Scully, J.E.C.; House, C.H.; Quick, L.C.; Bouquet, A.; Miller, K.; Bland, M.; De Sanctis, M.C.; Ermakov, A.; et al. Ceres: Astrobiological target and possible ocean world. Astrobiology 2020, 20, 269–291. [Google Scholar] [CrossRef]
- McCord, T.B.; Combe, J.-P.; Castillo-Rogez, J.C.; McSween, H.Y.; Prettyman, T.H. Ceres, a wet planet: The view after Dawn. Geochemistry 2021, 125745. [Google Scholar] [CrossRef]
- Margesin, R.; Gander, S.; Zacke, G.; Gounot, A.M.; Schinner, F. Hydrocarbon degradation and enzyme activities of cold-adapted bacteria and yeasts. Extremophiles 2003, 7, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Bowman, J.P. The family Colwelliaceae. In The Prokaryotes; Springer: Berlin/Heidelberg, Germany, 2014; pp. 179–195. ISBN 9783642389221. [Google Scholar]
- Russell, C.T.; Raymond, C.A.; Ammannito, E.; Buczkowski, D.L.; De Sanctis, M.C.; Hiesinger, H.; Jaumann, R.; Konopliv, A.S.; McSween, H.Y.; Nathues, A.; et al. Dawn arrives at Ceres: Exploration of a small, volatile-rich world. Science 2016, 353, 1008–1010. [Google Scholar] [CrossRef] [PubMed]
- Bland, M.T.; Raymond, C.A.; Schenk, P.M.; Fu, R.R.; Kneissl, T.; Pasckert, J.H.; Hiesinger, H.; Preusker, F.; Park, R.S.; Marchi, S.; et al. Composition and structure of the shallow subsurface of Ceres revealed by crater morphology. Nat. Geosci. 2016, 9, 538–542. [Google Scholar] [CrossRef]
- Prettyman, T.H.; Yamashita, N.; Toplis, M.J.; McSween, H.Y.; Schörghofer, N.; Marchi, S.; Feldman, W.C.; Castillo-Rogez, J.; Forni, O.; Lawrence, D.J.; et al. Extensive water ice within Ceres’ aqueously altered regolith: Evidence from nuclear spectroscopy. Science 2017, 355, 55–59. [Google Scholar] [CrossRef]
- Castillo-Rogez, J. Future exploration of Ceres as an ocean world. Nat. Astron. 2020, 4, 732–734. [Google Scholar] [CrossRef]
- Ruesch, O.; Genova, A.; Neumann, W.; Quick, L.C.; Castillo-Rogez, J.C.; Raymond, C.A.; Russell, C.T.; Zuber, M.T. Slurry extrusion on Ceres from a convective mud-bearing mantle. Nat. Geosci. 2019, 12, 505–509. [Google Scholar] [CrossRef]
- McSween, H.Y.; Emery, J.P.; Rivkin, A.S.; Toplis, M.J.; Castillo-Rogez, J.C.; Prettyman, T.H.; De Sanctis, M.C.; Pieters, C.M.; Raymond, C.A.; Russell, C.T. Carbonaceous chondrites as analogs for the composition and alteration of Ceres. Meteorit. Planet. Sci. 2018, 53, 1793–1804. [Google Scholar] [CrossRef]
- Neveu, M.; Desch, S.J.; Castillo-Rogez, J.C. Aqueous geochemistry in icy world interiors: Equilibrium fluid, rock, and gas compositions, and fate of antifreezes and radionuclides. Geochim. Cosmochim. Acta 2017, 212, 324–371. [Google Scholar] [CrossRef]
- Scully, J.E.C.; Schenk, P.M.; Castillo-Rogez, J.C.; Buczkowski, D.L.; Williams, D.A.; Pasckert, J.H.; Duarte, K.D.; Romero, V.N.; Quick, L.C.; Sori, M.M.; et al. The varied sources of faculae-forming brines in Ceres’ Occator crater emplaced via hydrothermal brine effusion. Nat. Commun. 2020, 11, 3680. [Google Scholar] [CrossRef]
- Raymond, C.A.; Ermakov, A.I.; Castillo-Rogez, J.C.; Marchi, S.; Johnson, B.C.; Hesse, M.A.; Scully, J.E.C.; Buczkowski, D.L.; Sizemore, H.G.; Schenk, P.M.; et al. Impact-driven mobilization of deep crustal brines on dwarf planet Ceres. Nat. Astron. 2020, 4, 741–747. [Google Scholar] [CrossRef]
- De Sanctis, M.C.; Ammannito, E. Organic matter and associated minerals on the dwarf planet ceres. Minerals 2021, 11, 799. [Google Scholar] [CrossRef]
- Kaplan, H.H.; Milliken, R.E.; Alexander, C.M. New constraints on the abundance and composition of organic matter on Ceres. Geophys. Res. Lett. 2018, 45, 5274–5282. [Google Scholar] [CrossRef]
- De Sanctis, M.C.; Ammannito, E.; McSween, H.Y.; Raponi, A.; Marchi, S.; Capaccioni, F.; Capria, M.T.; Carrozzo, F.G.; Ciarniello, M.; Fonte, S.; et al. Localized aliphatic organic material on the surface of Ceres. Science 2017, 355, 719–722. [Google Scholar] [CrossRef]
- Botta, O.; Bada, J.L. Extraterrestrial organic compounds in meteorites. Surv. Geophys. 2002, 23, 411–467. [Google Scholar] [CrossRef]
- Marchi, S.; Raponi, A.; Prettyman, T.H.; De Sanctis, M.C.; Castillo-Rogez, J.; Raymond, C.A.; Ammannito, E.; Bowling, T.; Ciarniello, M.; Kaplan, H.; et al. An aqueously altered carbon-rich Ceres. Nat. Astron. 2019, 3, 140–145. [Google Scholar] [CrossRef]
- De Sanctis, M.C.; Mitri, G.; Castillo-Rogez, J.; House, C.H.; Marchi, S.; Raymond, C.A.; Sekine, Y. Relict ocean worlds: Ceres. Space Sci. Rev. 2020, 216, 60. [Google Scholar] [CrossRef]
- Trigo-Rodríguez, J.M.; Rimola, A.; Tanbakouei, S.; Cabedo-Soto, V.; Lee, M.R. Accretion of water in carbonaceous chondrites: Current evidence and implications for the delivery of water to early Earth. Space Sci. Rev. 2019, 215, 18. [Google Scholar] [CrossRef]
- Shi, X.; Castillo-Rogez, J.; Hsieh, H.; Hui, H.; Ip, W.H.; Lei, H.; Li, J.Y.; Tosi, F.; Zhou, L.; Agarwal, J.; et al. GAUSS-genesis of asteroids and evolution of the solar system. Exp. Astron. 2021. [Google Scholar] [CrossRef]
- Jheeta, S.; Joshi, P.C. Prebiotic RNA synthesis by montmorillonite catalysis. Life 2014, 4, 318. [Google Scholar] [CrossRef]
- Miyakawa, S.; Joshi, P.C.; Gaffey, M.J.; Gonzalez-Toril, E.; Hyland, C.; Ross, T.; Rybij, K.; Ferris, J.P. Studies in the mineral and salt-catalyzed formation of RNA oligomers. Orig. Life Evol. Biosph. 2006, 36, 343–361. [Google Scholar] [CrossRef] [PubMed]
- Ferris, J.P. Montmorillonite-catalysed formation of RNA oligomers: The possible role of catalysis in the origins of life. Philos. Trans. R. Soc. B Biol. Sci. 2006, 361, 1777–1786. [Google Scholar] [CrossRef] [PubMed]
- Nikalje, M.D.; Phukan, P.; Sudalai, A. Recent advances in clay-catalyzed organic transformations. Org. Prep. Proced. Int. 2000, 32, 1–40. [Google Scholar] [CrossRef]
- Karpiński, B.; Szkodo, M. Clay minerals–mineralogy and phenomenon of clay swelling in oil & gas industry. Adv. Mater. Sci. 2015, 15, 37–55. [Google Scholar] [CrossRef]
- Stone, W.; Kroukamp, O.; Moes, A.; McKelvie, J.; Korber, D.R.; Wolfaardt, G.M. Measuring microbial metabolism in atypical environments: Bentonite in used nuclear fuel storage. J. Microbiol. Methods 2016, 120, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Bowman, J.P.; Gosink, J.J.; McCammon, S.A.; Lewis, T.E.; Nichols, D.S.; Nichols, P.D.; Skerratt, J.H.; Staley, J.T.; McMeekin, T.A. Colwellia demingiae sp. nov., Colwellia hornerae sp. nov., Colwellia rossensis sp. nov. and Colwellia psychrotropica sp. nov.: Psychrophilic Antarctic species with the ability to synthesize docosahexaenoic acid (22:ω63). Int. J. Syst. Bacteriol. 1998, 48, 1171–1180. [Google Scholar] [CrossRef]
- Calbrix, R.; Laval, K.; Barray, S. Analysis of the potential functional diversity of the bacterial community in soil: A reproducible procedure using sole-carbon-source utilization profiles. Eur. J. Soil Biol. 2005, 41, 11–20. [Google Scholar] [CrossRef]
- Miller, T.D.; Schroth, M.N. Monitoring the epiphytic populations of Erwinia amylovora on pear with a selective medium. Phytopathology 1972, 62, 1175–1185. [Google Scholar] [CrossRef]
- Bressan, M.; Trinsoutrot Gattin, I.; Desaire, S.; Castel, L.; Gangneux, C.; Laval, K. A rapid flow cytometry method to assess bacterial abundance in agricultural soil. Appl. Soil Ecol. 2015, 88, 60–68. [Google Scholar] [CrossRef]
- Zwietering, M.H.; Jongenburger, I.; Rombouts, F.M.; Van’t Riet, K. Modeling of the bacterial growth curve. Appl. Environ. Microbiol. 1990, 56, 1875–1881. [Google Scholar] [CrossRef]
- Mempin, R.; Tran, H.; Chen, C.; Gong, H.; Kim, H.K.; Lu, S. Release of extracellular ATP by bacteria during growth. BMC Microbiol. 2013, 13, 301. [Google Scholar] [CrossRef] [PubMed]
- Finch, R. Quantitative extraction and estimation of intracellular nucleoside triphosphates of Escherichia coli. Anal. Biochem. 1972, 45, 24–34. [Google Scholar]
- Nordheim, T.A.; Castillo-Rogez, J.C.; Villarreal, M.N.; Scully, J.E.C.; Costello, E.S. The radiation environment of Ceres and implications for surface sampling. Astrobiology 2022, 5, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Varjani, S.J.; Gnansounou, E. Microbial dynamics in petroleum oilfields and their relationship with physiological properties of petroleum oil reservoirs. Bioresour. Technol. 2017, 245, 1258–1265. [Google Scholar] [CrossRef]
- Ma, Y.; Li, X.; Mao, H.; Wang, B.; Wang, P. Remediation of hydrocarbon–heavy metal co-contaminated soil by electrokinetics combined with biostimulation. Chem. Eng. J. 2018, 353, 410–418. [Google Scholar] [CrossRef]
- Loffhagen, N.; Härtig, C.; Babel, W. The toxicity of substituted phenolic compounds to a detoxifying and an acetic acid bacterium. Ecotoxicol. Environ. Saf. 1997, 36, 269–274. [Google Scholar] [CrossRef]
- Riis, V.; Stimming, M.; Miethe, D.; Babel, W. Investigations into the toxicity of persistent fractions of mineral oils. Chemosphere 1996, 32, 1435–1443. [Google Scholar] [CrossRef]
- Müller, R.H.; Simon, D.; Große, H.J.; Babel, W. Substrate inhibition under stationary growth conditions-nutristat experiments with Ralstonia eutropha JMP 134 during growth on phenol and 2,4-dichlorophenoxyacetate. Appl. Microbiol. Biotechnol. 1997, 48, 648–655. [Google Scholar] [CrossRef]
- Park, C.; Park, W. Survival and energy producing strategies of Alkane degraders under extreme conditions and their biotechnological potential. Front. Microbiol. 2018, 9, 1081. [Google Scholar] [CrossRef]
- Bælum, J.; Borglin, S.; Chakraborty, R.; Fortney, J.L.; Lamendella, R.; Mason, O.U.; Auer, M.; Zemla, M.; Bill, M.; Conrad, M.E.; et al. Deep-sea bacteria enriched by oil and dispersant from the Deepwater Horizon spill. Environ. Microbiol. 2012, 14, 2405–2416. [Google Scholar] [CrossRef]
- Spaans, S.K.; Weusthuis, R.A.; van der Oost, J.; Kengen, S.W.M. NADPH-generating systems in bacteria and archaea. Front. Microbiol. 2015, 6, 742. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, E. Hydrocarbon-oxidizing bacteria. In The Prokaryotes; Springer: New York, NY, USA, 2006; pp. 564–577. [Google Scholar]
- Widdel, F.; Musat, F. Energetic and other quantitative aspects of microbial hydrocarbon utilization. In Handbook of Hydrocarbon and Lipid Microbiology; Springer: Berlin/Heidelberg, Germany, 2010; pp. 729–763. [Google Scholar]
- Bylund, J.; Brown, K.L.; Movitz, C.; Dahlgren, C.; Karlsson, A. Intracellular generation of superoxide by the phagocyte NADPH oxidase: How, where, and what for? Free Radic. Biol. Med. 2010, 49, 1834–1845. [Google Scholar] [CrossRef] [PubMed]
- Rada, B.; Leto, T.L. Oxidative innate immune defenses by Nox/Duox family NADPH oxidases. In Trends in Innate Immunity; KARGER: Basel, Switzerland, 2008; pp. 164–187. [Google Scholar]
- Heipieper, H.J.; Martínez, P.M. Toxicity of hydrocarbons to microorganisms. Handb. Hydrocarb. Lipid Microbiol. 2010, 1563–1573. [Google Scholar] [CrossRef]
- Dubinsky, E.A.; Conrad, M.E.; Chakraborty, R.; Bill, M.; Borglin, S.E.; Hollibaugh, J.T.; Mason, O.U.; Piceno, Y.M.; Reid, F.C.; Stringfellow, W.T.; et al. Succession of hydrocarbon-degrading bacteria in the aftermath of the Deepwater Horizon oil spill in the Gulf of Mexico. Environ. Sci. Technol. 2013, 47, 10860–10867. [Google Scholar] [CrossRef]
- Overholt, W.A.; Marks, K.P.; Romero, I.C.; Hollander, D.J.; Snell, T.W.; Kostka, J.E. Hydrocarbon-degrading bacteria exhibit a species-specific response to dispersed oil while moderating ecotoxicity. Appl. Environ. Microbiol. 2016, 82, 518–527. [Google Scholar] [CrossRef]
- Kleindienst, S.; Grim, S.; Sogin, M.; Bracco, A.; Crespo-Medina, M.; Joye, S.B. Diverse, rare microbial taxa responded to the Deepwater Horizon deep-sea hydrocarbon plume. ISME J. 2016, 10, 400–415. [Google Scholar] [CrossRef]
- Techtmann, S.M.; Zhuang, M.; Campo, P.; Holder, E.; Elk, M.; Hazen, T.C.; Conmy, R.; Santo Domingo, J.W. Corexit 9500 enhances oil biodegradation and changes active bacterial community structure of oil-enriched microcosms. Appl. Environ. Microbiol. 2017, 83, e03462-16. [Google Scholar] [CrossRef]
- Lofthus, S.; Bakke, I.; Greer, C.W.; Brakstad, O.G. Biodegradation of weathered crude oil by microbial communities in solid and melted sea ice. Mar. Pollut. Bull. 2021, 172, 112823. [Google Scholar] [CrossRef]
- Owen, D.J.; Eggink, G.; Hauer, B.; Kok, M.; McBeth, D.L.; Yang, Y.L.; Shapiro, J.A. Physical structure, genetic content and expression of the alkBAC operon. Mol. Gen. Genet. MGG 1984, 197, 373–383. [Google Scholar] [CrossRef]
- van Beilen, J.B.; Wubbolts, M.G.; Witholt, B. Genetics of alkane oxidation by Pseudomonas oleovorans. Biodegradation 1994, 5, 161–174. [Google Scholar] [CrossRef]
- Rojo, F. Degradation of alkanes by bacteria: Minireview. Environ. Microbiol. 2009, 11, 2477–2490. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.E.; Finnerty, W.R. Fatty aldehyde dehydrogenases in Acinetobacter sp. strain HO1-N: Role in hexadecanol metabolism. J Bacteriol. 1985, 164, 1011–1016. [Google Scholar] [CrossRef] [PubMed]
- Maeng, J.H.; Sakai, Y.; Tani, Y.; Kato, N. Isolation and characterization of a novel oxygenase that catalyzes the first step of n-alkane oxidation in Acinetobacter sp. strain M-1. J. Bacteriol. 1996, 178, 3695–3700. [Google Scholar] [CrossRef] [PubMed]
- Sakai, Y.; Maeng, J.H.; Kubota, S.; Tani, A.; Tani, Y.; Kato, N. A non-conventional dissimilation pathway for long chain n-alkanes in Acinetobacter sp. M-1 that starts with a dioxygenase reaction. J. Ferment. Bioeng. 1996, 81, 286–291. [Google Scholar] [CrossRef]
- Hazen, T.C.; Dubinsky, E.A.; DeSantis, T.Z.; Andersen, G.L.; Piceno, Y.M.; Singh, N.; Jansson, J.K.; Probst, A.; Borglin, S.E.; Fortney, J.L.; et al. Deep-sea oil plume enriches indigenous oil-degrading bacteria. Science 2010, 330, 204–208. [Google Scholar] [CrossRef]
- Sorokin, D.Y.; Van Pelt, S.; Tourova, T.P.; Muyzer, G. Microbial isobutyronitrile utilization under haloalkaline conditions. Appl. Environ. Microbiol. 2007, 73, 5574–5579. [Google Scholar] [CrossRef][Green Version]
- Sorokin, D.Y.; van Pelt, S.; Tourova, T.P.; Evtushenko, L.I. Nitriliruptor alkaliphilus gen. nov., sp. nov., a deeplineage haloalkaliphilic actinobacterium from soda lakes capable of growth on aliphatic nitriles, and proposal of Nitriliruptoraceae fam. nov. and Nitriliruptorales ord. nov. Int. J. Syst. Evol. Microbiol. 2009, 59, 248–253. [Google Scholar] [CrossRef]
- Kobayashi, M. Nitrile hydrolases. Curr. Opin. Chem. Biol. 2000, 4, 95–102. [Google Scholar] [CrossRef]
- Brady, D.; Beeton, A.; Zeevaart, J.; Kgaje, C.; van Rantwijk, F.; Sheldon, R.A. Characterisation of nitrilase and nitrile hydratase biocatalytic systems. Appl. Microbiol. Biotechnol. 2004, 64, 76–85. [Google Scholar] [CrossRef]
- Belloche, A.; Garrod, R.T.; Müller, H.S.; Meuten, K.M. Detection of a branched alkyl molecule in the interstellar medium: Iso-propyl cyanide. Science 2014, 345, 1584–1587. [Google Scholar] [CrossRef]
- Öberg, K.I.; Guzmán, V.V.; Furuya, K.; Qi, C.; Aikawa, Y.; Andrews, S.M.; Loomis, R.; Wilner, D.J. The comet-like composition of a protoplanetary disk as revealed by complex cyanides. Nature 2015, 520, 198–201. [Google Scholar] [CrossRef] [PubMed]
- Cronin, J.R.; Pizzarello, S. Amino acids in meteorites. Adv. Sp. Res. 1983, 3, 5–18. [Google Scholar] [CrossRef]
- Aponte, J.C.; Elsila, J.E.; Glavin, D.P.; Milam, S.N.; Charnley, S.B.; Dworkin, J.P. Pathways to meteoritic glycine and methylamine. ACS Earth Sp. Chem. 2017, 1, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Dopson, M.; Johnson, D.B. Biodiversity, metabolism and applications of acidophilic sulfur-metabolizing microorganisms. Environ. Microbiol. 2012, 14, 2620–2631. [Google Scholar] [CrossRef]
- Methe, B.A.; Nelson, K.E.; Deming, J.W.; Momen, B.; Melamud, E.; Zhang, X.J.; Moult, J.; Madupu, R.; Nelson, W.C.; Dodson, R.J.; et al. The psychrophilic lifestyle as revealed by the genome sequence of Colwellia psychrerythraea 34H through genomic and proteomic analyses. Proc. Natl. Acad. Sci. USA 2005, 102, 10913–10918. [Google Scholar] [CrossRef]
- Moran, M.A. Genomics and metagenomics of marine prokaryotes. In Microbial Ecology of the Oceans; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008; pp. 91–129. [Google Scholar]
- Muyzer, G.; Stams, A.J.M. The ecology and biotechnology of sulphate-reducing bacteria. Nat. Rev.-Microbiol. 2008, 6, 441–454. [Google Scholar] [CrossRef]
- Borgne, S.L.; Ayala, M. Microorganisms Utilizing Sulfur-Containing Hydrocarbons. In Handbook of Hydrocarbon and Lipid Microbiology; Timmis, K.N., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 2129–2141. ISBN 9783540775874. [Google Scholar]
- Duarte, G.F.; Rosado, A.S.; Seldin, L.; de Araujo, W.; van Elsas, J.D. Analysis of bacterial community structure in sulfurous-oil-containing soils and detection of species carrying dibenzothiophene desulfurization (dsz) genes. Appl. Environ. Microbiol. 2001, 67, 1052–1062. [Google Scholar] [CrossRef]
- Kirkwood, K.M.; Ebert, S.; Foght, J.M.; Fedorak, P.M.; Gray, M.R. Bacterial biodegradation of aliphatic sulfides under aerobic carbon- or sulfur-limited growth conditions. J. Appl. Microbiol. 2005, 99, 1444–1454. [Google Scholar] [CrossRef]
- Stipanuk, M.H. Metabolism of sulfur-containing amino acids. Annu. Rev. Nutr. 1986, 6, 179–209. [Google Scholar] [CrossRef]
- Roy, K.-M. Thiols and organic sulfides. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2000. [Google Scholar]
- Brzóska, K.; Mȩczyńska, S.; Kruszewski, M. Iron-sulfur cluster proteins: Electron transfer and beyond. Acta Biochim. Pol. 2006, 53, 685–691. [Google Scholar] [CrossRef]
- Hendrix, A.R.; Vilas, F.; Li, J.-Y. Ceres: Sulfur deposits and graphitized carbon. Geophys. Res. Lett. 2016, 43, 8920–8927. [Google Scholar] [CrossRef]
- Castillo-Rogez, J.; Neveu, M.; McSween, H.Y.; Fu, R.R.; Toplis, M.J.; Prettyman, T. Insights into Ceres’s evolution from surface composition. Meteorit. Planet. Sci. 2018, 53, 1820–1843. [Google Scholar] [CrossRef]
- Bu, C.; Rodriguez, G.; Dukes, C.A.; Ruesch, O.; McFadden, L.A.; Li, J.-Y. Search for sulfates on the surface of Ceres. Meteorit. Planet Sci. 2018, 53, 1946–1960. [Google Scholar] [CrossRef]
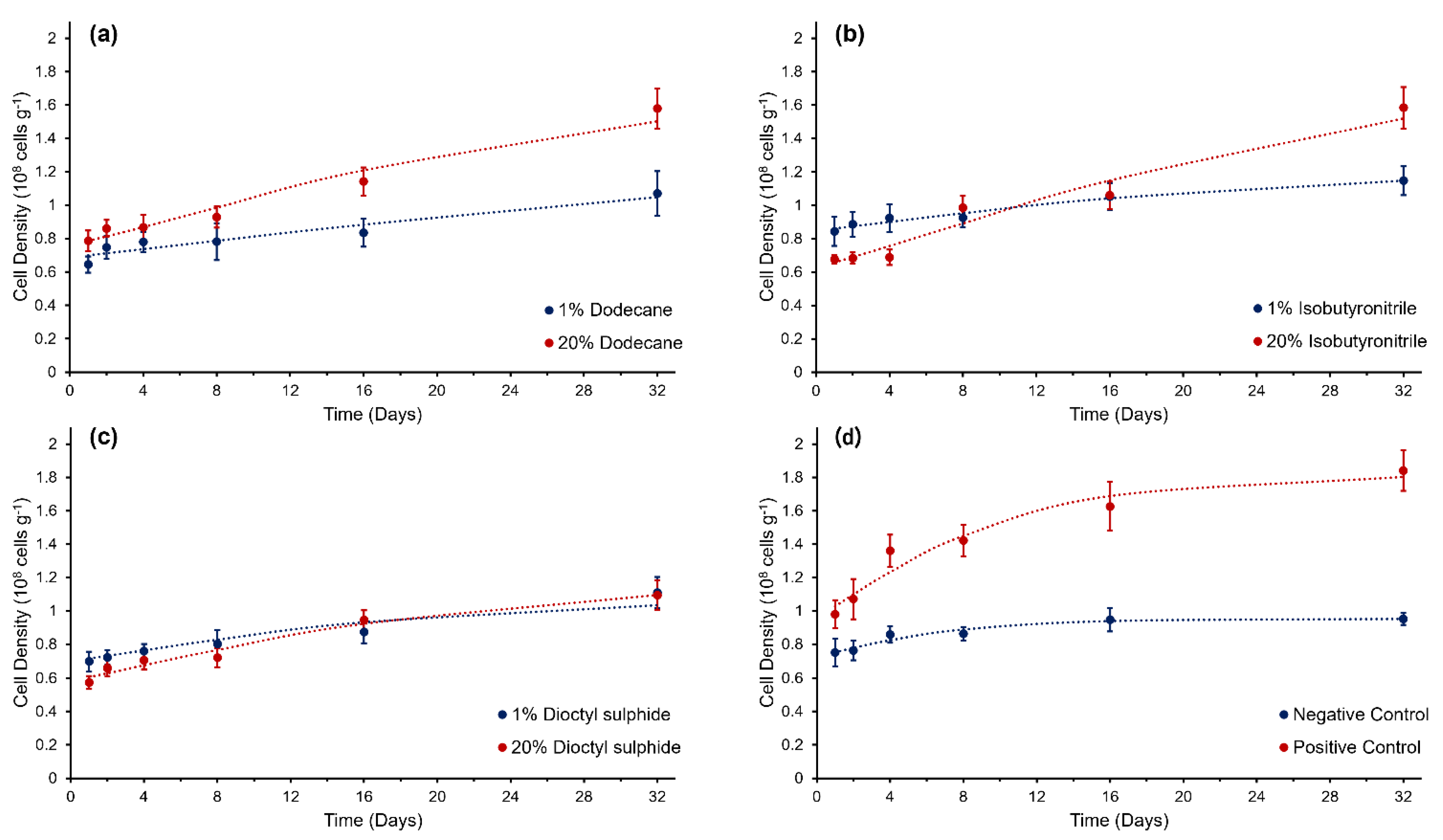

| Substrate | Y0 (n × 108 cells g−1) | Ymax (n × 108 cells g−1) | RGRmax (day−1) | M | rmax (day−1) | Λ (days) | R2 | SSE | RMSE | |
|---|---|---|---|---|---|---|---|---|---|---|
| Dodecane | 1% | 0.4 (−30.7, 31.5) | 1.3 (−17.9, 20.6) | 0.04 (−1.9, 2.0) | 5.4 (−1036, 1047) | 0.012 | 3.3 × 10−10 | 0.91 | 0.009 | 0.067 |
| 20% | 0.6 (−5.8, 6.9) | 1.7 (−1.3, 4.7) | 0.1 (−0.1, 0.7) | 7.6 (−104, 119) | 0.029 | 1.7 × 10−3 | 0.97 | 0.016 | 0.089 | |
| Isobutyronitrile | 1% | 0.7 (−3.6, 5.1) | 1.2 (0.3, 2.1) | 0.2 (−0.5, 0.7) | 3.2 (−185.4, 191.9) | 0.013 | 1.9 × 10−5 | 0.97 | 0.002 | 0.030 |
| 20% | 0.3 (−15.4, 15.9) | 1.8 (−5.5, 9.1) | 0.1 (−0.8, 0.9) | 6.5 (−222.2, 235.2) | 0.034 | 2.3 × 10−5 | 0.96 | 0.026 | 0.114 | |
| Dioctyl Sulphide | 1% | 0.6 (−6.2, 7.4) | 1.1 (−0.04, 2.2) | 0.1 (−1.0, 1.2) | 3.5 (−245.2, 252.3) | 0.016 | 4.2 × 10−4 | 0.93 | 0.010 | 0.071 |
| 20% | 0.38 (−5.0, 5.7) | 1.2 (0.04, 2.3) | 0.1 (−0.5, 0.6) | 4.1 (−130.8, 138.9) | 0.023 | 2.1 × 10−4 | 0.97 | 0.005 | 0.052 | |
| Control | Negative | 0.6 (0.451, 0.738) | 1.0 (0.9, 1.1) | 0.2 (−0.02, 0.4) | 1.1 × 10−11 (fixed at bound) | 0.027 | 7.3 × 10−3 | 0.94 | 0.002 | 0.026 |
| Positive | 0.5 (−16.7, 17.7) | 1.8 (1.2, 2.5) | 0.2 (−0.7, 0.9) | 1.1 × 10−7 (−138.3, 138.3) | 0.073 | 1.0 × 10−3 | 0.95 | 0.026 | 0.113 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jayasinghe, S.A.; Kennedy, F.; McMinn, A.; Martin, A. Bacterial Utilisation of Aliphatic Organics: Is the Dwarf Planet Ceres Habitable? Life 2022, 12, 821. https://doi.org/10.3390/life12060821
Jayasinghe SA, Kennedy F, McMinn A, Martin A. Bacterial Utilisation of Aliphatic Organics: Is the Dwarf Planet Ceres Habitable? Life. 2022; 12(6):821. https://doi.org/10.3390/life12060821
Chicago/Turabian StyleJayasinghe, Sahan A., Fraser Kennedy, Andrew McMinn, and Andrew Martin. 2022. "Bacterial Utilisation of Aliphatic Organics: Is the Dwarf Planet Ceres Habitable?" Life 12, no. 6: 821. https://doi.org/10.3390/life12060821
APA StyleJayasinghe, S. A., Kennedy, F., McMinn, A., & Martin, A. (2022). Bacterial Utilisation of Aliphatic Organics: Is the Dwarf Planet Ceres Habitable? Life, 12(6), 821. https://doi.org/10.3390/life12060821







