Multidrug Resistance in Cancer Cells: Focus on a Possible Strategy Plan to Address Colon Carcinoma Cells
Abstract
:1. Introduction
1.1. General Approach and Evolution of Multidrug Resistance
- Within the primary group of the tumor cells, there are limited cells, perhaps small subgroups, which are already resistant to the treatment methodology. There are indications of this in acute myelogenous leukemia, and it is certainly the case in melanoma. When tumors are treated and remission occurs, the cells that regrow are originally derived from the parent tumor cells. This has been demonstrated using sophisticated molecular techniques [12].
- There may be resistant insensitive cells in the initial group, but during treatment, an additional mechanism of resistance can become evolved, which may become fixed during treatment, leading to mixed resistance [13].
- They play a major role in e multidrug resistance in tumor cells.
- They play a significant role in drug pharmacokinetics (uptake, distribution, and excretion).
- They play a significant role in drug toxicity.
- They play a key role in development (stem cells, morphogenesis).
- To acquire knowledge about the biology of all transport systems.
1.2. Multidrug Resistance and Its Mechanistic Approaches
- (1)
- The specificity of the individual cells concerning the absorption, metabolism, and delivery of the drugs to the specific targeted tissues. These factors are dependent on the individual’s genetic patterns, which help to regulate the cellular responses, which, in turn, prevent the drug from reaching its threshold levels, which is usually required for pharmacological action to take place [42].
- (2)
- The specificity of the tumor in terms of its origin, vasculature, and tissue function. Resistance to chemotherapy is observed when the tumor is located in the parts of the body where the drug cannot be accessed or the tumor comprises compromised vasculature [43].
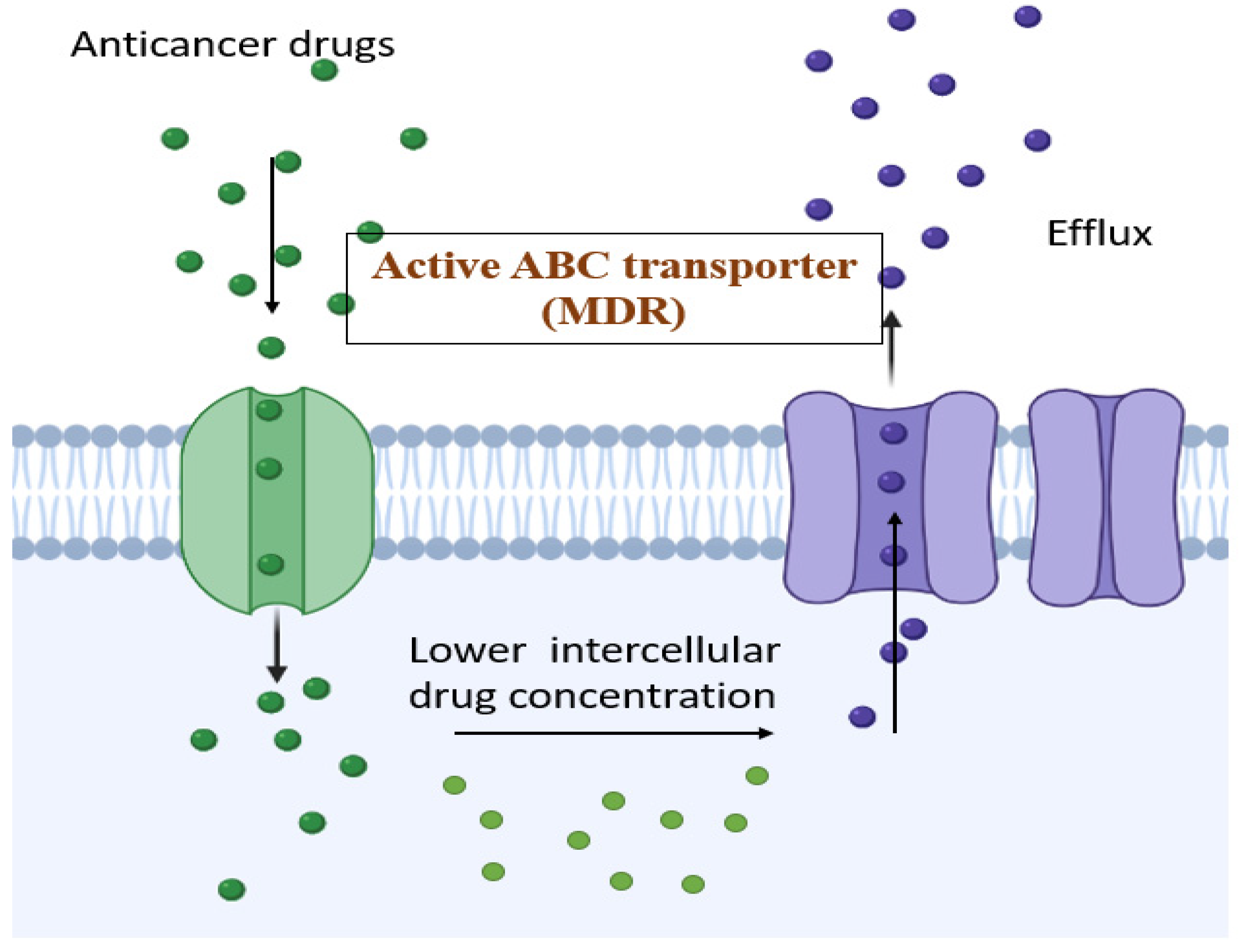
2. MDR Modulators and the Mechanisms of Inhibitors
2.1. ATP Binding Cassette (ABC)
- ABCB1 (P-gp)
- ABCC1 (MRP-1)
- ABCG2 (BCRP, MXR)
2.2. BCRP
2.3. Proteasomes
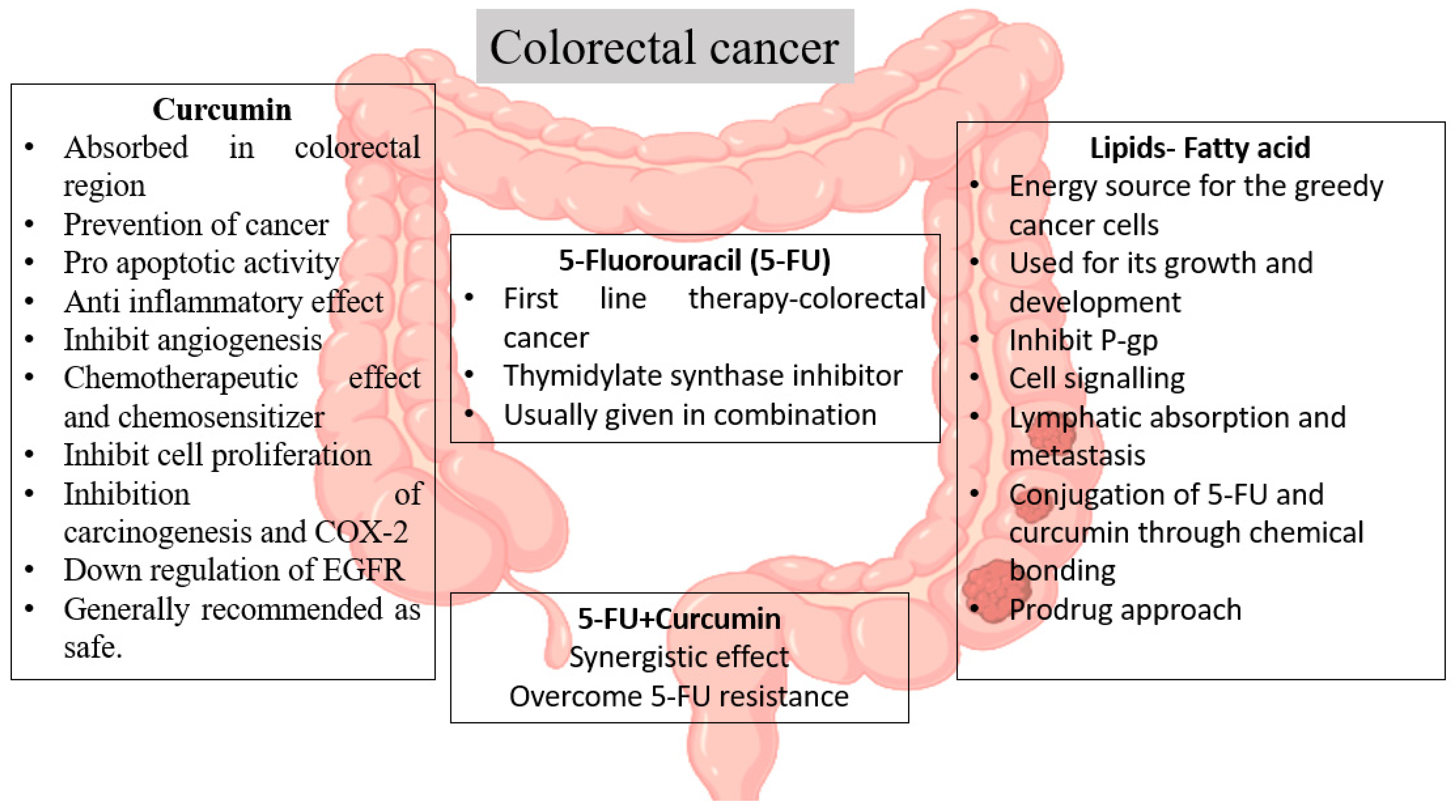
3. Colorectal Cancer: Understanding the Background
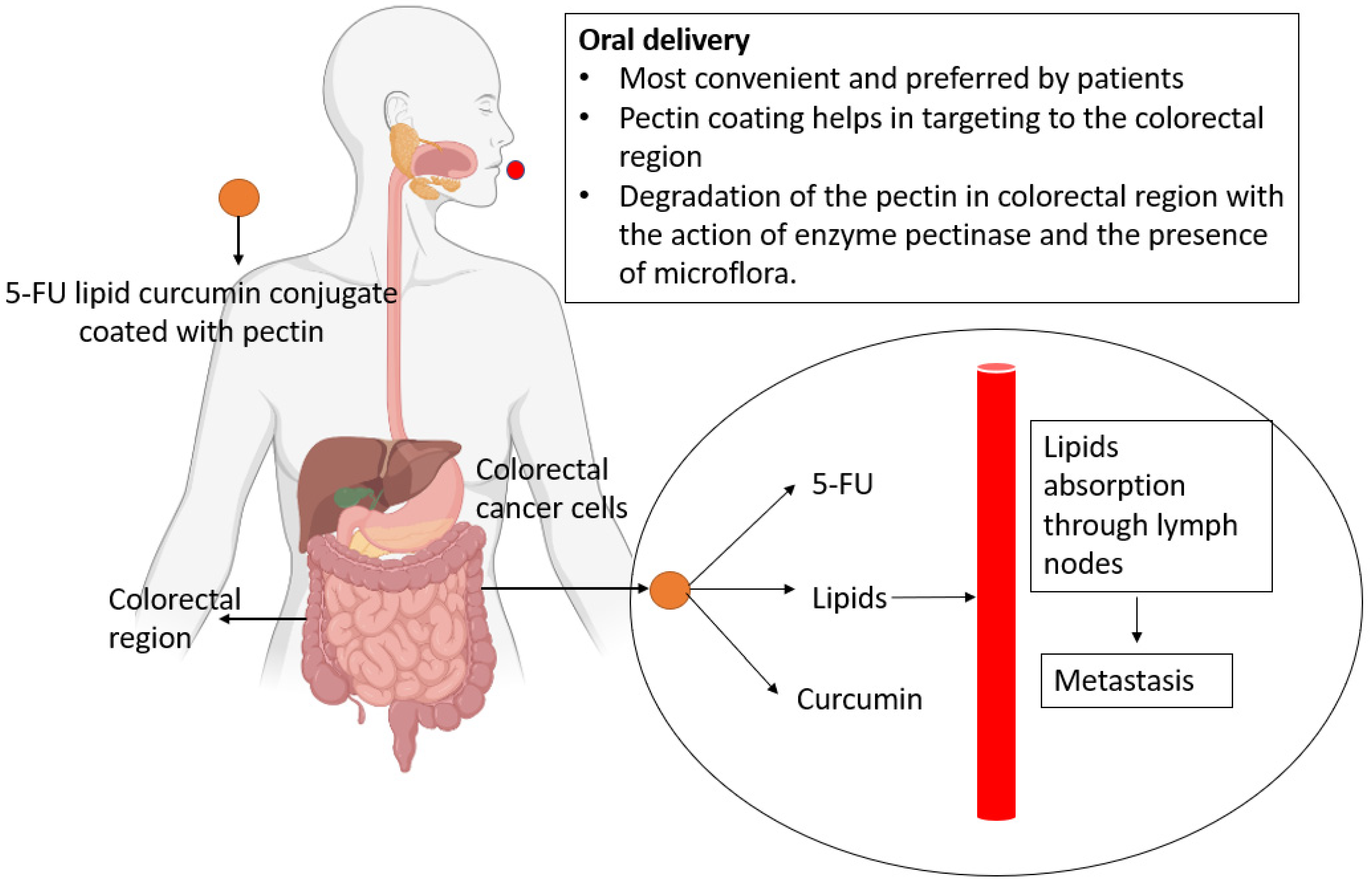
3.1. Outline and the Hypothetic Concept
3.2. Hypothetical Approaches
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, D.W.; Lee, S.; Kwon, S.; Nam, W.; Cha, I.-H.; Kim, H.J. Deep Learning-Based Survival Prediction of Oral Cancer Patients. Sci. Rep. 2019, 9, 6994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Alfonso, P.; Muñoz Martín, A.J.; Ortega Morán, L.; Soto Alsar, J.; Torres Perez-Solero, G.; Blanco Codesido, M.; Calvo Ferrandiz, P.A.; Grasso Cicala, S. Oral Drugs in the Treatment of Metastatic Colorectal Cancer. Ther. Adv. Med. Oncol. 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Williamson, B.; Bonn, B.; Lindmark, B.; Colclough, N.; Harlfinger, S.; Llinas, A.; Hilgendorf, C. Metabolism and Pharmacokinetic Optimization Strategies in Drug Discovery. In Drug Discovery and Development E-Book: Technology in Transition; Elsevier: Amsterdam, The Netherlands, 2021; Volume 134, pp. 134–158. [Google Scholar]
- Jelski, W.; Mroczko, B. Biochemical Markers of Colorectal Cancer–Present and Future. Cancer Manag. Res. 2020, 12, 4789. [Google Scholar] [CrossRef] [PubMed]
- Pilleron, S.; Sarfati, D.; Janssen-Heijnen, M.; Vignat, J.; Ferlay, J.; Bray, F.; Soerjomataram, I. Global Cancer Incidence in Older Adults, 2012 and 2035: A Population-based Study. Int. J. Cancer 2019, 144, 49–58. [Google Scholar] [CrossRef]
- Sawicki, T.; Ruszkowska, M.; Danielewicz, A.; Niedźwiedzka, E.; Arłukowicz, T.; Przybyłowicz, K.E. A Review of Colorectal Cancer in Terms of Epidemiology, Risk Factors, Development, Symptoms and Diagnosis. Cancers 2021, 13, 2025. [Google Scholar] [CrossRef]
- Asano, H.; Fukano, H.; Takagi, M.; Takayama, T. Risk Factors for the Recurrence of Stage II Perforated Colorectal Cancer: A Retrospective Observational Study. Asian J. Surg. 2022; in press. [Google Scholar]
- Dong, Y.; Tu, R.; Liu, H.; Qing, G. Regulation of Cancer Cell Metabolism: Oncogenic MYC in the Driver’s Seat. Signal Transduct. Target. Ther. 2020, 5, 124. [Google Scholar] [CrossRef]
- Simon, S.; Cavalu, S.; Eniu, D.; Simon, V. Surface Properties of Collagen-Functionalized Aluminosilicate Particles Embedding Iron and Dysprosium Designed for Cancer Therapy. J. Mol. Struct. 2021, 1236, 130341. [Google Scholar] [CrossRef]
- Michaelis, M.; Wass, M.N.; Cinatl, J. Drug-Adapted Cancer Cell Lines as Preclinical Models of Acquired Resistance. Cancer Drug Resist. 2019, 2, 447–456. [Google Scholar] [CrossRef] [Green Version]
- Aniogo, E.C.; Plackal Adimuriyil George, B.; Abrahamse, H. The Role of Photodynamic Therapy on Multidrug Resistant Breast Cancer. Cancer Cell Int. 2019, 19, 91. [Google Scholar] [CrossRef]
- Ishida, T.; Takahashi, T.; Kurokawa, Y.; Nishida, T.; Hirota, S.; Serada, S.; Fujimoto, M.; Naka, T.; Teranishi, R.; Saito, T. Targeted Therapy for Drug-Tolerant Persister Cells after Imatinib Treatment for Gastrointestinal Stromal Tumours. Br. J. Cancer 2021, 125, 1511–1522. [Google Scholar] [CrossRef]
- McMahon, C.M.; Ferng, T.; Canaani, J.; Wang, E.S.; Morrissette, J.J.D.; Eastburn, D.J.; Pellegrino, M.; Durruthy-Durruthy, R.; Watt, C.D.; Asthana, S. Clonal Selection with RAS Pathway Activation Mediates Secondary Clinical Resistance to Selective FLT3 Inhibition in Acute Myeloid Leukemia. Cancer Discov. 2019, 9, 1050–1063. [Google Scholar] [CrossRef] [PubMed]
- Sindhu, R.K.; Najda, A.; Kaur, P.; Shah, M.; Singh, H.; Kaur, P.; Cavalu, S.; Jaroszuk-Sierocińska, M.; Rahman, M.H. Potentiality of Nanoenzymes for Cancer Treatment and Other Diseases: Current Status and Future Challenges. Materials 2021, 14, 5965. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.R.; Islam, F.; Nafady, M.H.; Akter, M.; Mitra, S.; Das, R.; Urmee, H.; Shohag, S.; Akter, A.; Chidambaram, K.; et al. Natural Small Molecules in Breast Cancer Treatment: Understandings from a Therapeutic Viewpoint. Molecules 2022, 27, 2165. [Google Scholar] [CrossRef] [PubMed]
- Parish, T. Steps to Address Anti-Microbial Drug Resistance in Today’s Drug Discovery. Expert Opin. Drug Discov. 2019, 14, 91–94. [Google Scholar] [CrossRef] [Green Version]
- Merz, V.; Gaule, M.; Zecchetto, C.; Cavaliere, A.; Casalino, S.; Pesoni, C.; Contarelli, S.; Sabbadini, F.; Bertolini, M.; Mangiameli, D. Targeting KRAS: The Elephant in the Room of Epithelial Cancers. Front. Oncol. 2021, 11, 361. [Google Scholar] [CrossRef] [PubMed]
- Patton, E.E.; Zon, L.I.; Langenau, D.M. Zebrafish Disease Models in Drug Discovery: From Preclinical Modelling to Clinical Trials. Nat. Rev. Drug Discov. 2021, 20, 611–628. [Google Scholar] [CrossRef]
- Das, S. Pectin Based Multi-Particulate Carriers for Colon-Specific Delivery of Therapeutic Agents. Int. J. Pharm. 2021, 605, 120814. [Google Scholar] [CrossRef]
- Cavalu, S.; Banica, F.; Gruian, C.; Vanea, E.; Goller, G.; Simon, V. Microscopic and spectroscopic investigation of bioactive glasses for antibiotic controlled release. J. Mol. Struct. 2013, 1040, 47–52. [Google Scholar] [CrossRef]
- Eliaa, S.G.; Al-Karmalawy, A.A.; Saleh, R.M.; Elshal, M.F. Empagliflozin and Doxorubicin Synergistically Inhibit the Survival of Triple-Negative Breast Cancer Cells via Interfering with the MTOR Pathway and Inhibition of Calmodulin: In Vitro and Molecular Docking Studies. ACS Pharmacol. Transl. Sci. 2020, 3, 1330–1338. [Google Scholar] [CrossRef]
- Pizzagalli, M.D.; Bensimon, A.; Superti-Furga, G. A Guide to Plasma Membrane Solute Carrier Proteins. FEBS J. 2021, 288, 2784–2835. [Google Scholar] [CrossRef]
- Rommasi, F.; Esfandiari, N. Liposomal Nanomedicine: Applications for Drug Delivery in Cancer Therapy. Nanoscale Res. Lett. 2021, 16, 95. [Google Scholar] [CrossRef] [PubMed]
- Kisiel, J.B.; Papadopoulos, N.; Liu, M.C.; Crosby, D.; Srivastava, S.; Hawk, E.T. Multicancer Early Detection Test: Preclinical, Translational, and Clinical Evidence–Generation Plan and Provocative Questions. Cancer 2022, 128, 861–874. [Google Scholar] [CrossRef] [PubMed]
- Baillie, G.S.; Tejeda, G.S.; Kelly, M.P. Therapeutic Targeting of 3′, 5′-Cyclic Nucleotide Phosphodiesterases: Inhibition and Beyond. Nat. Rev. Drug Discov. 2019, 18, 770–796. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Chen, M.; Zhang, J.; Maincent, P.; Xia, X.; Wu, W. Updated Progress of Nanocarrier-Based Intranasal Drug Delivery Systems for Treatment of Brain Diseases. Crit. Rev. Ther. Drug Carr. Syst. 2018, 35, 433–467. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.R.; Qian, L.; Zhou, X. Developmental Origins and Oncogenic Pathways in Malignant Brain Tumors. Wiley Interdiscip. Rev. Dev. Biol. 2019, 8, e342. [Google Scholar] [CrossRef]
- Assaraf, Y.G.; Brozovic, A.; Gonçalves, A.C.; Jurkovicova, D.; Linē, A.; Machuqueiro, M.; Saponara, S.; Sarmento-Ribeiro, A.B.; Xavier, C.P.R.; Vasconcelos, M.H. The Multi-Factorial Nature of Clinical Multidrug Resistance in Cancer. Drug Resist. Updat. 2019, 46, 100645. [Google Scholar] [CrossRef]
- Pasello, M.; Giudice, A.M.; Scotlandi, K. The ABC Subfamily A Transporters: Multifaceted Players with Incipient Potentialities in Cancer. Semin. Cancer Biol. 2020, 60, 57–71. [Google Scholar] [CrossRef]
- Wang, X.; An, X.; Wang, X.; Bao, C.; Li, J.; Yang, D.; Bai, C. Curcumin Ameliorated Ventilator-Induced Lung Injury in Rats. Biomed. Pharmacother. 2018, 98, 754–761. [Google Scholar] [CrossRef]
- Abdelfatah, S.; Böckers, M.; Asensio, M.; Kadioglu, O.; Klinger, A.; Fleischer, E.; Efferth, T. Isopetasin and S-Isopetasin as Novel P-Glycoprotein Inhibitors against Multidrug-Resistant Cancer Cells. Phytomedicine 2021, 86, 153196. [Google Scholar] [CrossRef]
- El-Far, A.H.; Tantawy, M.A.; Al Jaouni, S.K.; Mousa, S.A. Thymoquinone-Chemotherapeutic Combinations: New Regimen to Combat Cancer and Cancer Stem Cells. Naunyn Schmiedeberg’s Arch. Pharmacol. 2020, 393, 1581–1598. [Google Scholar] [CrossRef]
- Eid, S.Y.; Althubiti, M.A.; Abdallah, M.E.; Wink, M.; El-Readi, M.Z. The Carotenoid Fucoxanthin Can Sensitize Multidrug Resistant Cancer Cells to Doxorubicin via Induction of Apoptosis, Inhibition of Multidrug Resistance Proteins and Metabolic Enzymes. Phytomedicine 2020, 77, 153280. [Google Scholar] [CrossRef] [PubMed]
- Majidinia, M.; Mirza-Aghazadeh-Attari, M.; Rahimi, M.; Mihanfar, A.; Karimian, A.; Safa, A.; Yousefi, B. Overcoming Multidrug Resistance in Cancer: Recent Progress in Nanotechnology and New Horizons. IUBMB Life 2020, 72, 855–871. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Y.; Luqman, S.; Meena, A. Research Progress in Flavonoids as Potential Anticancer Drug Including Synergy with Other Approaches. Curr. Top. Med. Chem. 2020, 20, 1791–1809. [Google Scholar] [CrossRef] [PubMed]
- Figueira-Mansur, J.; Schrago, C.G.; Salles, T.S.; Alvarenga, E.S.L.; Vasconcellos, B.M.; Melo, A.C.A.; Moreira, M.F. Phylogenetic Analysis of the ATP-Binding Cassette Proteins Suggests a New ABC Protein Subfamily J in Aedes Aegypti (Diptera: Culicidae). BMC Genom. 2020, 21, 463. [Google Scholar] [CrossRef]
- Hanssen, K.M.; Haber, M.; Fletcher, J.I. Targeting Multidrug Resistance-Associated Protein 1 (MRP1)-Expressing Cancers: Beyond Pharmacological Inhibition. Drug Resist. Updat. 2021, 59, 100795. [Google Scholar] [CrossRef]
- Elfadadny, A.; El-Husseiny, H.M.; Abugomaa, A.; Ragab, R.F.; Mady, E.A.; Aboubakr, M.; Samir, H.; Mandour, A.S.; El-Mleeh, A.; El-Far, A.H. Role of Multidrug Resistance-Associated Proteins in Cancer Therapeutics: Past, Present, and Future Perspectives. Environ. Sci. Pollut. Res. 2021, 28, 49447–49466. [Google Scholar] [CrossRef]
- Gao, S.; Yang, X.; Xu, J.; Qiu, N.; Zhai, G. Nanotechnology for Boosting Cancer Immunotherapy and Remodeling Tumor Microenvironment: The Horizons in Cancer Treatment. ACS Nano 2021, 15, 12567–12603. [Google Scholar] [CrossRef]
- Mohammad, I.S.; He, W.; Yin, L. Understanding of Human ATP Binding Cassette Superfamily and Novel Multidrug Resistance Modulators to Overcome MDR. Biomed. Pharmacother. 2018, 100, 335–348. [Google Scholar] [CrossRef]
- Sivák, L. Overcoming Cancer Resistance to Chemotherapy through HPMA Copolymer Conjugates. Ph.D. Thesis, Univerzita Karlova-Přírodovědecká Fakulta, Prague, Czech Republic, 16 September 2019. [Google Scholar]
- Rather, R.A.; Bhagat, M. Quercetin as an Innovative Therapeutic Tool for Cancer Chemoprevention: Molecular Mechanisms and Implications in Human Health. Cancer Med. 2020, 9, 9181–9192. [Google Scholar] [CrossRef]
- Arvanitis, C.D.; Ferraro, G.B.; Jain, R.K. The Blood–Brain Barrier and Blood–Tumour Barrier in Brain Tumours and Metastases. Nat. Rev. Cancer 2020, 20, 26–41. [Google Scholar] [CrossRef]
- Razzaq, S.; Rauf, A.; Raza, A.; Akhtar, S.; Tabish, T.A.; Sandhu, M.A.; Zaman, M.; Ibrahim, I.M.; Shahnaz, G.; Rahdar, A. A Multifunctional Polymeric Micelle for Targeted Delivery of Paclitaxel by the Inhibition of the P-Glycoprotein Transporters. Nanomaterials 2021, 11, 2858. [Google Scholar] [CrossRef] [PubMed]
- Karthika, C.; Sureshkumar, R. Incorporation of Natural Assumption to Deal with Cancer. Environ. Sci. Pollut. Res. 2021, 28, 4902–4917. [Google Scholar] [CrossRef] [PubMed]
- Amawi, H.; Sim, H.-M.; Tiwari, A.K.; Ambudkar, S.V.; Shukla, S. ABC Transporter-Mediated Multidrug-Resistant Cancer. Drug Transp. Drug Dispos. Eff. Toxic. 2019, 1141, 549–580. [Google Scholar]
- Wang, J.-Q.; Yang, Y.; Cai, C.-Y.; Teng, Q.-X.; Cui, Q.; Lin, J.; Assaraf, Y.G.; Chen, Z.-S. Multidrug Resistance Proteins (MRPs): Structure, Function and the Overcoming of Cancer Multidrug Resistance. Drug Resist. Updat. 2021, 54, 100743. [Google Scholar] [CrossRef] [PubMed]
- Sosnik, A.; Bendayan, R. Drug Efflux Pumps in Cancer Resistance Pathways: From Molecular Recognition and Characterization to Possible Inhibition Strategies in Chemotherapy; Academic Press: Cambridge, MA, USA, 2019; ISBN 0128141417. [Google Scholar]
- Goebel, J.; Chmielewski, J.; Hrycyna, C.A. The Roles of the Human ATP-Binding Cassette Transporters P-Glycoprotein and ABCG2 in Multidrug Resistance in Cancer and at Endogenous Sites: Future Opportunities for Structure-Based Drug Design of Inhibitors. Cancer Drug Resist. 2021, 4, 784–804. [Google Scholar] [CrossRef] [PubMed]
- Power, J.F. The Role of Extracellular Vesicles in the Transfer of Multidrug Resistance in Human Ovarian Cancer Cells. Master’s Thesis, Queen’s University, Kingston, ON, Canada, 2018. [Google Scholar]
- Nanayakkara, A.K.; Follit, C.A.; Chen, G.; Williams, N.S.; Vogel, P.D.; Wise, J.G. Targeted Inhibitors of P-Glycoprotein Increase Chemotherapeutic-Induced Mortality of Multidrug Resistant Tumor Cells. Sci. Rep. 2018, 8, 967. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.; He, C.; Wang, M.; Ma, X.; Mo, F.; Yang, S.; Han, J.; Wei, X. Targeting Epigenetic Regulators for Cancer Therapy: Mechanisms and Advances in Clinical Trials. Signal Transduct. Target. Ther. 2019, 4, 62. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, A.C.; Richiardone, E.; Jorge, J.; Polónia, B.; Xavier, C.P.R.; Salaroglio, I.C.; Riganti, C.; Vasconcelos, M.H.; Corbet, C.; Sarmento-Ribeiro, A.B. Impact of Cancer Metabolism on Therapy Resistance-Clinical Implications. Drug Resist. Updat. 2021, 59, 100797. [Google Scholar] [CrossRef]
- Kumar, A.; Rathi, E.; Hariharapura, R.C.; Kini, S.G. Is Viral E6 Oncoprotein a Viable Target? A Critical Analysis in the Context of Cervical Cancer. Med. Res. Rev. 2020, 40, 2019–2048. [Google Scholar] [CrossRef]
- Ross, D.D.; Yang, W.; Abruzzo, L.V.; Dalton, W.S.; Schneider, E.; Lage, H.; Dietel, M.; Greenberger, L.; Cole, S.P.C.; Doyle, L.A. Atypical Multidrug Resistance: Breast Cancer Resistance Protein Messenger RNA Expression in Mitoxantrone-Selected Cell Lines. J. Natl. Cancer Inst. 1999, 91, 429–433. [Google Scholar] [CrossRef] [Green Version]
- Nakanishi, T.; Ross, D.D. Breast Cancer Resistance Protein (BCRP/ABCG2): Its Role in Multidrug Resistance and Regulation of Its Gene Expression. Chin. J. Cancer 2012, 31, 73. [Google Scholar] [CrossRef] [Green Version]
- Folger, A.; Wang, Y. The Cytotoxicity and Clearance of Mutant Huntingtin and Other Misfolded Proteins. Cells 2021, 10, 2835. [Google Scholar] [CrossRef]
- Brangi, M.; Litman, T.; Ciotti, M.; Nishiyama, K.; Kohlhagen, G.; Takimoto, C.; Robey, R.; Pommier, Y.; Fojo, T.; Bates, S.E. Camptothecin Resistance: Role of the ATP-Binding Cassette (ABC), Mitoxantrone-Resistance Half-Transporter (MXR), and Potential for Glucuronidation in MXR-Expressing Cells. Cancer Res. 1999, 59, 5938–5946. [Google Scholar]
- Lindner, S.; Halwachs, S.; Wassermann, L.; Honscha, W. Expression and Subcellular Localization of Efflux Transporter ABCG 2/BCRP in Important Tissue Barriers of Lactating Dairy Cows, Sheep and Goats. J. Vet. Pharmacol. Ther. 2013, 36, 562–570. [Google Scholar] [CrossRef]
- Abdallah, E.A.; Fanelli, M.F.; e Silva, S.V.; Machado Netto, M.C.; Gasparini, J.L., Jr.; Araújo, D.V.; Ocea, L.M.M.; Buim, M.E.C.; Tariki, M.S.; da Silva, A.V.; et al. MRP1 Expression in CTCs Confers Resistance to Irinotecan-based Chemotherapy in Metastatic Colorectal Cancer. Int. J. Cancer 2016, 139, 890–898. [Google Scholar] [CrossRef]
- Fu, D. Where Is It and How Does It Get There–Intracellular Localization and Traffic of P-Glycoprotein. Front. Oncol. 2013, 3, 321. [Google Scholar] [CrossRef] [Green Version]
- Akter, R.; Rahman, M.; Kaushik, D.; Mittal, V.; Uivarosan, D.; Nechifor, A.C.; Behl, T.; Karthika, C.; Stoicescu, M.; Munteanu, M.A. Chemo-Preventive Action of Resveratrol: Suppression of P53—A Molecular Targeting Approach. Molecules 2021, 26, 5325. [Google Scholar] [CrossRef]
- Waku, T.; Nakamura, N.; Koji, M.; Watanabe, H.; Katoh, H.; Tatsumi, C.; Tamura, N.; Hatanaka, A.; Hirose, S.; Katayama, H. NRF3-POMP-20S Proteasome Assembly Axis Promotes Cancer Development via Ubiquitin-Independent Proteolysis of P53 and Retinoblastoma Protein. Mol. Cell. Biol. 2020, 40, e00597–e00619. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Zhan, Y.; Xu, J.; Wang, Y.; Sun, M.; Chen, J.; Liang, T.; Wu, L.; Xu, K. β-Sitosterol Reverses Multidrug Resistance via BCRP Suppression by Inhibiting the P53–MDM2 Interaction in Colorectal Cancer. J. Agric. Food Chem. 2020, 68, 3850–3858. [Google Scholar] [CrossRef]
- Karthika, C.; Hari, B.; Rahman, M.H.; Akter, R.; Najda, A.; Albadrani, G.M.; Sayed, A.A.; Akhtar, M.F.; Abdel-Daim, M.M. Multiple Strategies with the Synergistic Approach for Addressing Colorectal Cancer. Biomed. Pharmacother. 2021, 140, 111704. [Google Scholar] [CrossRef]
- Eng, C.; Jácome, A.A.; Agarwal, R.; Hayat, M.H.; Byndloss, M.X.; Holowatyj, A.N.; Bailey, C.; Lieu, C.H. A Comprehensive Framework for Early-Onset Colorectal Cancer Research. Lancet Oncol. 2022, 23, e116–e128. [Google Scholar] [CrossRef]
- Mirmozaffari, M. Presenting an Expert System for Early Diagnosis of Gastrointestinal Diseases. Int. J. Gastroenterol. Sci. 2020, 1, 21–27. [Google Scholar]
- Paškevičiūtė, M.; Petrikaitė, V. Overcoming Transporter-Mediated Multidrug Resistance in Cancer: Failures and Achievements of the Last Decades. Drug Deliv. Transl. Res. 2019, 9, 379–393. [Google Scholar] [CrossRef]
- Recasens, A.; Munoz, L. Targeting Cancer Cell Dormancy. Trends Pharmacol. Sci. 2019, 40, 128–141. [Google Scholar] [CrossRef]
- Karthika, C.; Sureshkumar, R. Can Curcumin along with Chemotherapeutic Drug and Lipid Provide an Effective Treatment of Metastatic Colon Cancer and Alter Multidrug Resistance? Med. Hypotheses 2019, 132, 109325. [Google Scholar] [CrossRef]
- Karthika, C.; Hari, B.; Mano, V.; Radhakrishnan, A.; Janani, S.K.; Akter, R.; Kaushik, D.; Rahman, M.H. Curcumin as a Great Contributor for the Treatment and Mitigation of Colorectal Cancer. Exp. Gerontol. 2021, 152, 111438. [Google Scholar] [CrossRef]
- Cayero-Otero, M.D.; Espinosa-Oliva, A.M.; Herrera, A.J.; Garcia-Dominguez, I.; Fernandez-Arevalo, M.; Martin-Banderas, L.; de Pablos, R.M. Potential Use of Nanomedicine for the Anti-Inflammatory Treatment of Neurodegenerative Diseases. Curr. Pharm. Des. 2018, 24, 1589–1616. [Google Scholar] [CrossRef]
- Miere, F.; Vicas, S.I.; Timar, A.V.; Ganea, M.; Zdrinca, M.; Cavalu, S.; Fritea, L.; Vicas, L.; Muresan, M.; Pallag, A. Preparation and Characterization of Two Different Liposomal Formulations with Bioactive Natural Extract for Multiple Applications. Processes 2021, 9, 432. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering Precision Nanoparticles for Drug Delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Cavalu, S.; Bisboaca, S.; Mates, I.M.; Pasca, P.M.; Laslo, V.; Costea, T.; Fritea, L.; Vicas, S. Novel Formulation Based on Chitosan-Arabic Gum Nanoparticles Entrapping Propolis Extract. Rev. Chim. 2018, 69, 3756–3760. [Google Scholar] [CrossRef]
- Bukowski, K.; Kciuk, M.; Kontek, R. Mechanisms of Multidrug Resistance in Cancer Chemotherapy. Int. J. Mol. Sci. 2020, 21, 3233. [Google Scholar] [CrossRef]
- Chaudhary, M. Role of Plant Secondary Metabolites as Modulators of Multidrug Resistance in Cancer Therapy. In Plant Secondary Metabolites; Springer: Berlin/Heidelberg, Germany, 2022; pp. 415–435. [Google Scholar]
- Milewska, S.; Niemirowicz-Laskowska, K.; Siemiaszko, G.; Nowicki, P.; Wilczewska, A.Z.; Car, H. Current Trends and Challenges in Pharmacoeconomic Aspects of Nanocarriers as Drug Delivery Systems for Cancer Treatment. Int. J. Nanomed. 2021, 16, 6593. [Google Scholar] [CrossRef]
- Maderuelo, C.; Lanao, J.M.; Zarzuelo, A. Enteric Coating of Oral Solid Dosage Forms as a Tool to Improve Drug Bioavailability. Eur. J. Pharm. Sci. 2019, 138, 105019. [Google Scholar] [CrossRef]
- Kodan, A.; Futamata, R.; Kimura, Y.; Kioka, N.; Nakatsu, T.; Kato, H.; Ueda, K. ABCB1/MDR1/P-gp Employs an ATP-dependent Twist-and-squeeze Mechanism to Export Hydrophobic Drugs. FEBS Lett. 2021, 595, 707–716. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Imai, Y.; Shiseki, M.; Tanaka, J.; Motoji, T. Knockdown of the Wnt Receptor Frizzled-1 (FZD1) Reduces MDR1/P-Glycoprotein Expression in Multidrug Resistant Leukemic Cells and Inhibits Leukemic Cell Proliferation. Leuk. Res. 2018, 67, 99–108. [Google Scholar] [CrossRef]
- Ahmed, A.H. Identifying Anti-Cancer Drugs Targeting ALK Inhibitors with P-Glycoprotein and Studying It’s Molecular Mechanism of Transport. Ph.D. Thesis, University of Georgia, Athens, GA, USA, 2021. [Google Scholar]
- Duke, S.O.; Owens, D.K.; Dayan, F.E. Natural Product-Based Chemical Herbicides. In Weed Control; CRC Press: Boca Raton, FL, USA, 2018; pp. 153–165. ISBN 1315155915. [Google Scholar]
- Dallavalle, S.; Dobričić, V.; Lazzarato, L.; Gazzano, E.; Machuqueiro, M.; Pajeva, I.; Tsakovska, I.; Zidar, N.; Fruttero, R. Improvement of Conventional Anti-Cancer Drugs as New Tools against Multidrug Resistant Tumors. Drug Resist. Updat. 2020, 50, 100682. [Google Scholar] [CrossRef]
- Ganesan, M.; Kanimozhi, G.; Pradhapsingh, B.; Khan, H.A.; Alhomida, A.S.; Ekhzaimy, A.; Brindha, G.R.; Prasad, N.R. Phytochemicals Reverse P-Glycoprotein Mediated Multidrug Resistance via Signal Transduction Pathways. Biomed. Pharmacother. 2021, 139, 111632. [Google Scholar] [CrossRef]
- Zargan, S.; Salehi Barough, M.; Zargan, J.; Shayesteh, M.; Haji Noor Mohammadi, A.; Mousavi, M.; Keshavarz Alikhani, H. Evaluation of the Anti-Cancer Effect of Curcumin on MCF-7 Cells in 3D Culture Conditions to Increase the Efficacy of Breast Cancer Treatment. J. Appl. Biotechnol. Rep. 2022, 9, 547–556. [Google Scholar]
- Amodio, V.; Mauri, G.; Reilly, N.M.; Sartore-Bianchi, A.; Siena, S.; Bardelli, A.; Germano, G. Mechanisms of Immune Escape and Resistance to Checkpoint Inhibitor Therapies in Mismatch Repair Deficient Metastatic Colorectal Cancers. Cancers 2021, 13, 2638. [Google Scholar] [CrossRef]
- De Porras, V.R.; Layos, L.; Martínez-Balibrea, E. Curcumin: A Therapeutic Strategy for Colorectal Cancer? Semin. Cancer Biol. 2021, 73, 321–330. [Google Scholar] [CrossRef]
- Markovic, M.; Ben-Shabat, S.; Keinan, S.; Aponick, A.; Zimmermann, E.M.; Dahan, A. Lipidic Prodrug Approach for Improved Oral Drug Delivery and Therapy. Med. Res. Rev. 2019, 39, 579–607. [Google Scholar] [CrossRef]
- Rajput, A.; Pingale, P.; Telange, D.; Chalikwar, S.; Borse, V. Lymphatic Transport System to Circumvent Hepatic Metabolism for Oral Delivery of Lipid-Based Nanocarriers. J. Drug Deliv. Sci. Technol. 2021, 66, 102934. [Google Scholar] [CrossRef]
- Rawal, S.; Patel, M.M. Threatening Cancer with Nanoparticle Aided Combination Oncotherapy. J. Control. Release 2019, 301, 76–109. [Google Scholar] [CrossRef]
- Degors, I.M.S.; Wang, C.; Rehman, Z.U.; Zuhorn, I.S. Carriers Break Barriers in Drug Delivery: Endocytosis and Endosomal Escape of Gene Delivery Vectors. Acc. Chem. Res. 2019, 52, 1750–1760. [Google Scholar] [CrossRef] [Green Version]
- Liu, X. ABC Family Transporters. In Drug Transporters in Drug Disposition, Effects and Toxicity; Liu, X., Pan, G., Eds.; Springer: Singapore, 2019; Volume 1141, pp. 13–100. [Google Scholar]
- Karthika, C.; Sureshkumar, R.; Sajini, D.V.; Ashraf, G.M.; Rahman, M. 5-Fluorouracil and Curcumin with Pectin Coating as a Treatment Regimen for Titanium Dioxide with Dimethylhydrazine-Induced Colon Cancer Model. Environ. Sci. Pollut. Res. 2022, 1–14. [Google Scholar] [CrossRef]
- Salehi, B.; Machin, L.; Monzote, L.; Sharifi-Rad, J.; Ezzat, S.M.; Salem, M.A.; Merghany, R.M.; El Mahdy, N.M.; Kılıç, C.S.; Sytar, O. Therapeutic Potential of Quercetin: New Insights and Perspectives for Human Health. ACS Omega 2020, 5, 11849–11872. [Google Scholar] [CrossRef]
- Rahman, M.M.; Islam, M.R.; Shohag, S.; Hossain, M.E.; Rahaman, M.S.; Islam, F.; Ahmed, M.; Mitra, S.; Khandaker, M.U.; Idris, A.M.; et al. The Multifunctional Role of Herbal Products in the Management of Diabetes and Obesity: A Comprehensive Review. Molecules 2022, 27, 1713. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karthika, C.; Sureshkumar, R.; Zehravi, M.; Akter, R.; Ali, F.; Ramproshad, S.; Mondal, B.; Kundu, M.K.; Dey, A.; Rahman, M.H.; et al. Multidrug Resistance in Cancer Cells: Focus on a Possible Strategy Plan to Address Colon Carcinoma Cells. Life 2022, 12, 811. https://doi.org/10.3390/life12060811
Karthika C, Sureshkumar R, Zehravi M, Akter R, Ali F, Ramproshad S, Mondal B, Kundu MK, Dey A, Rahman MH, et al. Multidrug Resistance in Cancer Cells: Focus on a Possible Strategy Plan to Address Colon Carcinoma Cells. Life. 2022; 12(6):811. https://doi.org/10.3390/life12060811
Chicago/Turabian StyleKarthika, Chenmala, Raman Sureshkumar, Mehrukh Zehravi, Rokeya Akter, Faraat Ali, Sarker Ramproshad, Banani Mondal, Milton Kumar Kundu, Abhijit Dey, Md. Habibur Rahman, and et al. 2022. "Multidrug Resistance in Cancer Cells: Focus on a Possible Strategy Plan to Address Colon Carcinoma Cells" Life 12, no. 6: 811. https://doi.org/10.3390/life12060811
APA StyleKarthika, C., Sureshkumar, R., Zehravi, M., Akter, R., Ali, F., Ramproshad, S., Mondal, B., Kundu, M. K., Dey, A., Rahman, M. H., Antonescu, A., & Cavalu, S. (2022). Multidrug Resistance in Cancer Cells: Focus on a Possible Strategy Plan to Address Colon Carcinoma Cells. Life, 12(6), 811. https://doi.org/10.3390/life12060811







