Differences of Hemogram Parameters and Their Ratios among Patients with Takotsubo Syndrome, Acute Coronary Syndrome and Healthy Individuals
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
6. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Topf, A.; Mirna, M.; Paar, V.; Motloch, L.J.; Grueninger, J.; Dienhart, C.; Schulze, P.C.; Brandt, M.C.; Larbig, R.; Hoppe, U.C.; et al. The differential diagnostic value of selected cardiovascular biomarkers in Takotsubo syndrome. Clin. Res. Cardiol. 2022, 111, 197–206, Erratum in Clin. Res. Cardiol. 2022, 111, 241. [Google Scholar] [CrossRef] [PubMed]
- Topf, A.; Mirna, M.; Bacher, N.; Paar, V.; Edlinger, C.; Motloch, L.J.; Gharibeh, S.; Bannehr, M.; Hoppe, U.C.; Lichtenauer, M. The Value of Fetuin-A as a Predictor to Identify Takotsubo Patients at Risk of Cardiovascular Events. J. Cardiovasc. Dev. Dis. 2021, 8, 127. [Google Scholar] [CrossRef]
- Topf, A.; Mirna, M.; Bacher, N.; Paar, V.; Motloch, L.J.; Ohnewein, B.; Larbig, R.; Grueninger, J.; Hoppe, U.C.; Lichtenauer, M.; et al. Analysis of Selected Cardiovascular Biomarkers in Takotsubo Cardiomyopathy and the Most Frequent Cardiomyopathies. Front. Cardiovasc. Med. 2021, 8, 700169. [Google Scholar] [CrossRef] [PubMed]
- Rawish, E.; Stiermaier, T.; Santoro, F.; Brunetti, N.D.; Eitel, I. Current Knowledge and Future Challenges in Takotsubo Syndrome: Part 1-Pathophysiology and Diagnosis. J. Clin. Med. 2021, 10, 479. [Google Scholar] [CrossRef] [PubMed]
- Bentzon, J.F.; Otsuka, F.; Virmani, R.; Falk, E. Mechanisms of plaque formation and rupture. Circ. Res. 2014, 114, 1852–1866. [Google Scholar] [CrossRef] [PubMed]
- Ghadri, J.-R.; Wittstein, I.S.; Prasad, A.; Sharkey, S.; Dote, K.; Akashi, Y.J.; Cammann, V.L.; Crea, F.; Galiuto, L.; Desmet, W.; et al. International Expert Consensus Document on Takotsubo Syndrome (Part II): Diagnostic Workup, Outcome, and Management. Eur. Heart J. 2018, 39, 2047–2062. [Google Scholar] [CrossRef]
- Bajari, R.; Tak, S. Predictive prognostic value of neutrophil-lymphocytes ratio in acute coronary syndrome. Indian Heart J. 2017, 69 (Suppl. S1), S46–S50. [Google Scholar] [CrossRef]
- Santoro, F.; Guastafierro, F.; Zimotti, T.; Mallardi, A.; Leopizzi, A.; Cannone, M.; Di Biase, M.; Brunetti, N.D. Neutrophil/lymphocyte ratio predicts in-hospital complications in Takotsubo syndrome. Results from a prospective multi-center registry. Clin. Cardiol. 2020, 43, 1294–1300. [Google Scholar] [CrossRef]
- Madjid, M.; James, T.W. Inflammatory markers in coronary heart disease. Br. Med. Bull. 2011, 100, 23–38. [Google Scholar] [CrossRef] [Green Version]
- Niccoli, G.; Cosentino, N. Eosinophils: A new player in coronary atherosclerotic disease. Hypertens. Res. 2012, 35, 269–271. [Google Scholar] [CrossRef]
- Mirna, M.; Schmutzler, L.; Topf, A.; Hoppe, U.C.; Lichtenauer, M. Neutrophil-to-lymphocyte ratio and monocyte-to-lymphocyte ratio predict length of hospital stay in myocarditis. Sci. Rep. 2021, 11, 18101. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Cong, B.L.; Wang, M.; Abdullah, M.; Wang, X.L.; Zhang, Y.H.; Xu, S.J.; Cui, L. Neutrophil to lymphocyte ratio as a predictor of myocardial damage and cardiac dysfunction in acute coronary syndrome patients. Integr. Med. Res. 2018, 7, 192–199. [Google Scholar] [CrossRef]
- Toor, I.S.; Jaumdally, R.; Lip, G.Y.; Millane, T.; Varma, C. Eosinophil count predicts mortality following percutaneous coronary intervention. Thromb. Res. 2012, 130, 607–611. [Google Scholar] [CrossRef] [PubMed]
- Min, B.; Brown, M.A.; Legros, G. Understanding the roles of basophils: Breaking dawn. Immunology 2012, 135, 192–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Çekici, Y.; Yılmaz, M.; Seçen, Ö. New inflammatory indicators: Association of high eosinophil-to-lymphocyte ratio and low lymphocyte-to-monocyte ratio with smoking. J. Int. Med. Res. 2019, 47, 4292–4303. [Google Scholar] [CrossRef]
- López-Verdugo, F.; Furuzawa-Carballeda, J.; Romero-Hernández, F.; Coss-Adame, E.; Valdovinos, M.A.; Priego-Ranero, A.; Olvera-Prado, H.; Narváez-Chavez, S.; Peralta-Figueroa, J.; Torres-Villalobos, G. Hematological indices as indicators of silent inflammation in achalasia patients: A cross-sectional study. Medicine 2020, 99, e19326. [Google Scholar] [CrossRef] [PubMed]
- Shantsila, E.; Lip, G.Y. Monocytes in acute coronary syndromes. Arter. Thromb. Vasc. Biol. 2009, 29, 1433–1438. [Google Scholar] [CrossRef]
- Scantlebury, D.C.; Prasad, A. Diagnosis of Takotsubo cardiomyopathy. Circ. J. 2014, 78, 2129–2139. [Google Scholar] [CrossRef] [Green Version]
- Collet, J.P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European Society of Cardiology. Eur. Heart J. 2021, 42, 1289–1367. [Google Scholar] [CrossRef]
- Galderisi, M.; Cosyns, B.; Edvardsen, T.; Cardim, N.; Delgado, V.; Di Salvo, G. Standardization of adult transthoracic echocardiography reporting in agreement with recent chamber quantification, diastolic function, and heart valve disease recommendations: An expert consensus document of the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 1301–1310. [Google Scholar] [CrossRef] [Green Version]
- Hanley, J.A.; McNeil, B.J. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology 1983, 148, 839–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agewall, S.; Giannitsis, E.; Jernberg, T.; Katus, H. Troponin elevation in coronary vs. non-coronary disease. Eur. Heart J. 2011, 32, 404–411. [Google Scholar] [CrossRef]
- Sattler, S.; Couch, L.S.; Harding, S.E. Takotsubo Syndrome: Latest Addition to the Expanding Family of Immune-Mediated Diseases? JACC Basic Transl. Sci. 2018, 3, 779–781. [Google Scholar] [CrossRef] [PubMed]
- Wilson, H.M.; Cheyne, L.; Brown, P.A.J.; Kerr, K.; Hannah, A.; Srinivasan, J.; Duniak, N.; Horgan, G.; Dawson, D.K. Characterization of the Myocardial Inflammatory Response in Acute Stress-Induced (Takotsubo) Cardiomyopathy. JACC Basic Transl. Sci. 2018, 3, 766–778. [Google Scholar] [CrossRef] [PubMed]
- Vultaggio, A.; Matucci, A.; Del Pace, S.; Simonetti, I.; Parronchi, P.; Rossi, O.; Maggi, E.; Gensini, G.; Romagnani, S. Tako-Tsubo-like syndrome during anaphylactic reaction. Eur. J. Heart Fail. 2007, 9, 209–211. [Google Scholar] [CrossRef] [PubMed]
- Kraus, J.; Schuler, J.; Hoppe, U.C.; Hawranek, T.; Altenberger, J. Der allergische Myokardinfarkt-Kounis-Syndrom. J. Kardiol.—Austrian J. Cardiol. 2012, 19, 118–122. [Google Scholar]
- Rouzaud Laborde, C.; Delmas, C.; Mialet-Perez, J.; Pizzinat, N.; Biendel-Picquet, C.; Boudou, N.; Dumonteil, N.; Spreux-Varoquaux, O.; Carrié, D.; Galinier, M.; et al. First evidence of increased plasma serotonin levels in Tako-Tsubo cardiomyopathy. BioMed Res. Int. 2013, 2013, 847069. [Google Scholar] [CrossRef]
- Conrad, S.K.; Catalano, M.C.; Catalano, G. The Use of Fluoxetine in a Patient with Takotsubo Cardiomyopathy. J. Psychiatr. Pract. 2016, 22, 234–238. [Google Scholar] [CrossRef]
- Visser, A.K.; van Waarde, A.; Willemsen, A.T.; Bosker, F.J.; Luiten, P.G.M.; den Boer, J.A.; Kema, I.P.; Dierckx, R.A.J.O. Measuring serotonin synthesis: From conventional methods to PET tracers and their (pre)clinical implications. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 576–591. [Google Scholar] [CrossRef] [Green Version]
- Scally, C.; Abbas, H.; Ahearn, T.; Srinivasan, J.; Mezincescu, A.; Rudd, A.; Spath, N.; Yucel-Finn, A.; Yuecel, R.; Oldroyd, K. Myocardial and Systemic Inflammation in Acute Stress-Induced (Takotsubo) Cardiomyopathy. Circulation 2019, 139, 1581–1592. [Google Scholar] [CrossRef]
| TTC (n = 40) | ACS (n = 63) | Control (n = 68) | p-Value | ||||
|---|---|---|---|---|---|---|---|
| Median | IQR | Median | IQR | Median | IQR | ||
| Age (years) | 72.0 | 62.3–79.8 | 64.0 | 56.0–72.0 | 65.0 | 54.0–71.8 | 0.002 |
| BMI (kg/m2) | 26.7 | 22.1–29.3 | 27.8 | 25.9–31.3 | 27.7 | 23.8–30.7 | 0.034 |
| EF (%) | 40.0 | 35.0–45.8 | 50.0 | 45.0–66.8 | 67.0 | 62.8–74.0 | <0.0001 |
| Creatinine (µmol/L) | 69.1 | 60.3–81.0 | 77.0 | 65.3–94.5 | 70.0 | 64.0–85.8 | 0.036 |
| LDL cholesterol (mg/dL) | 88.0 | 72.0–120.0 | 123.5 | 88.1–151.8 | 125.2 | 105.2–159.8 | <0.0001 |
| CRP (mg/L) | 0.5 | 0.1–0.5 | 3.5 | 0.0–9.2 | 0.4 | 0.3–0.6 | 0.019 |
| Smoking | 11/40 (27.5%) | 18/63 (28.6%) | 28/68 (41.2%) | 0.033 | |||
| Hypertension | 31/40 (77.5%) | 53/63 (84.1%) | 59/68 (86.8%) | 0.450 | |||
| Diabetes | 4/40 (10.0%) | 12/63 (19.0%) | 19/68 (27.9%) | 0.031 | |||
| Sex (female) | 36/40 (90.0%) | 41/63 (65.1%) | 12/68 (17.6%) | <0.0001 |
| TTC (n = 40) | ACS (n = 63) | Control (n = 68) | p-Value | ||||
|---|---|---|---|---|---|---|---|
| Median | IQR | Median | IQR | Median | IQR | ||
| Leucocyte count (G/L) | 9.305 | 7.378–12.248 | 8.900 | 7.400–11.000 | 6.900 | 6.000–7.900 | <0.0001 |
| Lymphocyte count (G/L) | 1.500 | 1.025–2.155 | 1.600 | 1.300–2.400 | 1.920 | 1.563–2.395 | 0.015 |
| Neutrophil count (G/L) | 5.230 | 3.840–10.480 | 6.400 | 4.680–8.300 | 4.000 | 3.300–4.700 | 0.002 |
| Monocyte count (G/L) | 0.610 | 0.485–0.720 | 0.800 | 0.590–0.850 | 0.500 | 0.400–0.693 | <0.0001 |
| Basophil count (G/L) | 0.030 | 0.010–0.050 | 0.000 | 0.000–0.013 | 0.000 | 0.000–0.030 | <0.0001 |
| Eosinophil count (G/L) | 0.070 | 0.010–0.125 | 0.055 | 0.000–0.200 | 0.140 | 0.100–0.208 | 0.140 |
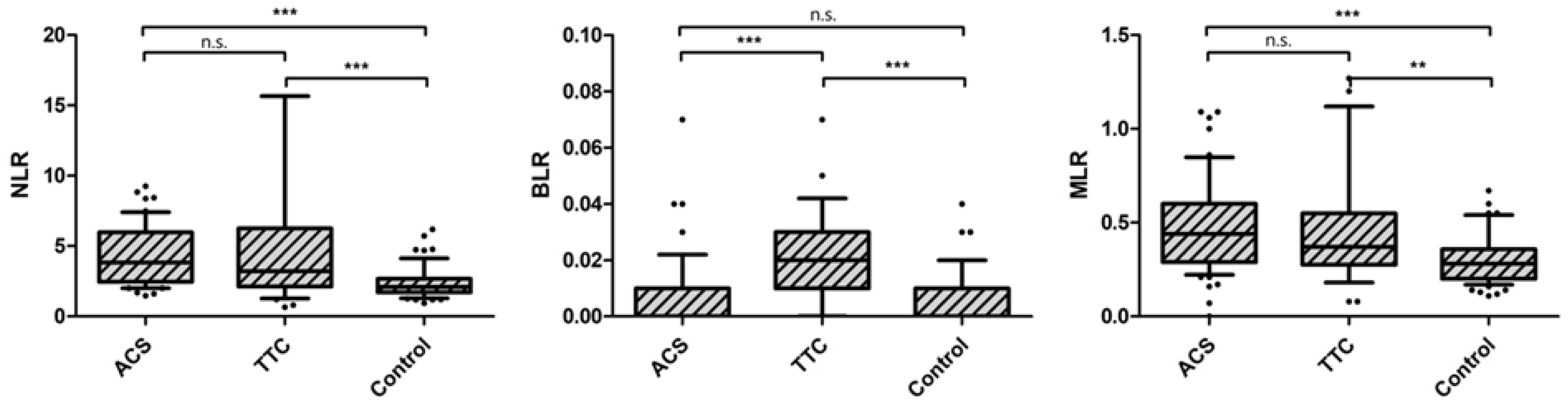
| Neutrophil/Lymphocyte ratio | 3.204 | 2.125–6.266 | 3.822 | 2.296–5.929 | 2.095 | 1.706–2.667 | <0.0001 |
| Basophil/Lymphocyte ratio | 0.019 | 0.011–0.029 | 0.000 | 0.000–0.008 | 0.000 | 0.000–0.014 | <0.0001 |
| Monocyte/Lymphocyte ratio | 0.369 | 0.274–0.548 | 0.444 | 0.286–0.595 | 0.280- | 0.198–0.361 | <0.0001 |
| BMI (kg/m2) | EF (%) | Age (Years) | CRP (mg/dL) | Creatinine (µmol/L) | ||
|---|---|---|---|---|---|---|
| NLR | rs | −0.002 | −0.332 | 0.133 | 0.006 | 0.036 |
| p-value | 0.980 | 0.001 | 0.158 | 0.947 | 0.676 | |
| BLR | rs | −0.143 | −0.142 | −0.123 | −0.042 | −0.127 |
| p-value | 0.067 | 0.119 | 0.168 | 0.594 | 0.115 | |
| MLR | rs | 0.109 | −0.208 | 0.192 | 0.175 | 0.223 |
| p-value | 0.164 | 0.021 | 0.030 | 0.024 | 0.005 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Topf, A.; Mirna, M.; Bacher, N.; Schmutzler, L.; Jirak, P.; Ohnewein, B.; Hoppe, U.C.; Lichtenauer, M. Differences of Hemogram Parameters and Their Ratios among Patients with Takotsubo Syndrome, Acute Coronary Syndrome and Healthy Individuals. Life 2022, 12, 788. https://doi.org/10.3390/life12060788
Topf A, Mirna M, Bacher N, Schmutzler L, Jirak P, Ohnewein B, Hoppe UC, Lichtenauer M. Differences of Hemogram Parameters and Their Ratios among Patients with Takotsubo Syndrome, Acute Coronary Syndrome and Healthy Individuals. Life. 2022; 12(6):788. https://doi.org/10.3390/life12060788
Chicago/Turabian StyleTopf, Albert, Moritz Mirna, Nina Bacher, Lukas Schmutzler, Peter Jirak, Bernhard Ohnewein, Uta C. Hoppe, and Michael Lichtenauer. 2022. "Differences of Hemogram Parameters and Their Ratios among Patients with Takotsubo Syndrome, Acute Coronary Syndrome and Healthy Individuals" Life 12, no. 6: 788. https://doi.org/10.3390/life12060788
APA StyleTopf, A., Mirna, M., Bacher, N., Schmutzler, L., Jirak, P., Ohnewein, B., Hoppe, U. C., & Lichtenauer, M. (2022). Differences of Hemogram Parameters and Their Ratios among Patients with Takotsubo Syndrome, Acute Coronary Syndrome and Healthy Individuals. Life, 12(6), 788. https://doi.org/10.3390/life12060788








