The Beneficial Effects of Physical Activity in Lung Cancer Prevention and/or Treatment
Abstract
1. Introduction
2. Lung Cancer: Principal Information
2.1. Causes
2.2. Diagnosis
2.3. Treatment
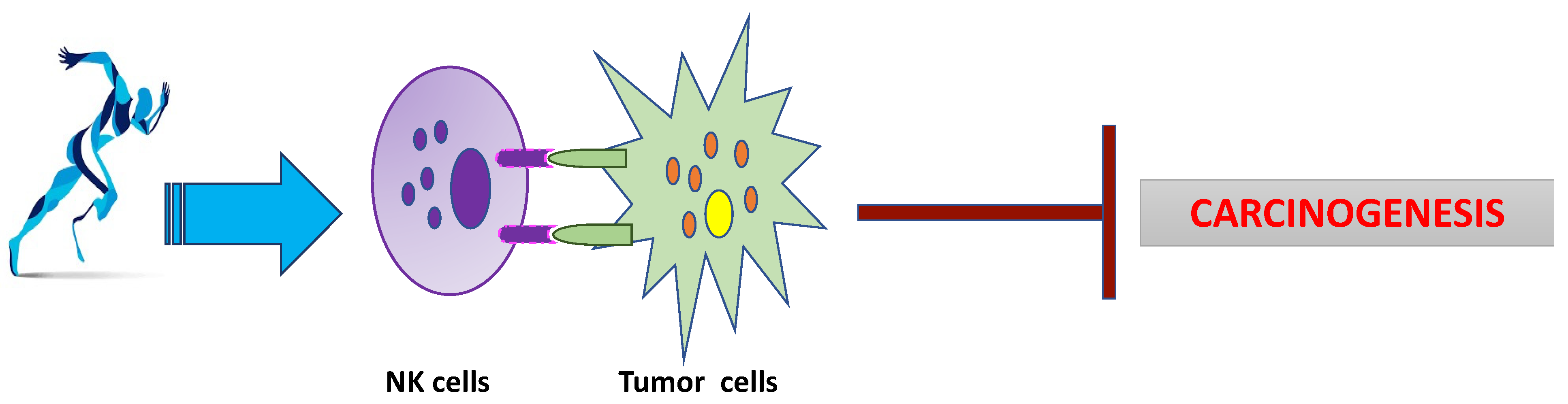
3. Physical Activity: Summary Beneficial Effects
- ○
- Occupational
- ○
- Household
- ○
- Transport
- ○
- Recreational or leisure-time
4. Physical Activity in Lung Cancer Prevention
5. Physical Activity in Lung Cancer Treatment and Rehabilitation
5.1. Physical Activity in Lung Cancer Treatment
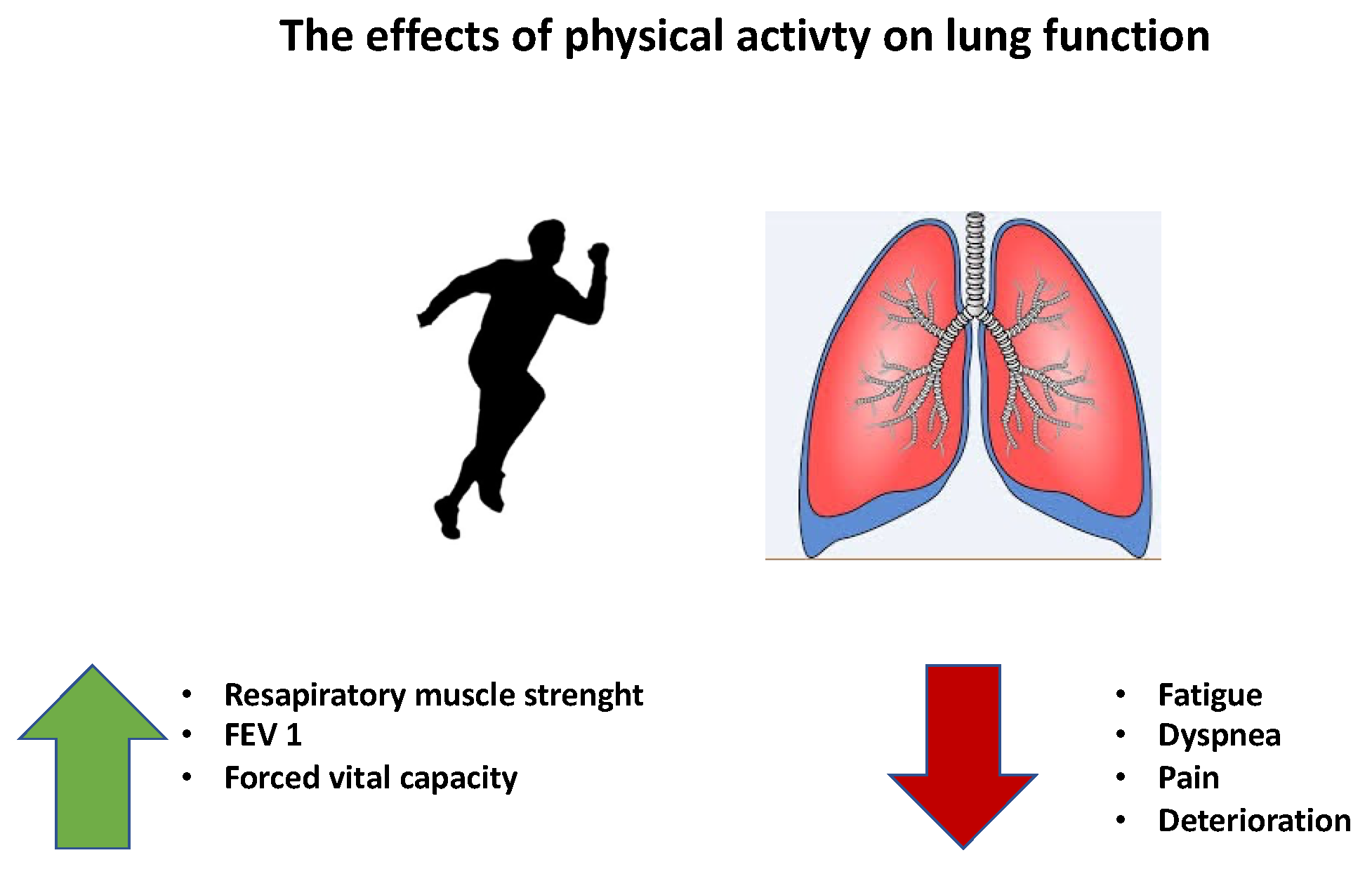
5.2. Physical Activity in Lung Cancer Rehabilitation
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Fiorelli, A.; Messina, G.; Capaccio, D.; Santini, M. Recurrent spontaneous pneumomediastinum: A rare but possible event! J. Thorac. Dis. 2012, 4, 431–433. [Google Scholar] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Dyba, T.; Randi, G.; Bettio, M.; Gavin, A.; Visser, O.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur. J. Cancer 2018, 103, 356–387. [Google Scholar] [CrossRef]
- Duggan, M.A.; Anderson, W.F.; Altekruse, S.; Penberthy, L.; Sherman, M.E. The Surveillance, Epidemiology, and End Results (SEER) Program and Pathology: Toward Strengthening the Critical Relationship. Am. J. Surgical. Pathol. 2016, 40, e94–e102. [Google Scholar] [CrossRef] [PubMed]
- Schabath, M.B.; Cress, D.; Munoz-Antonia, T. Racial and Ethnic Differences in the Epidemiology and Genomics of Lung Cancer. Cancer Control. 2016, 23, 338–346. [Google Scholar] [CrossRef]
- Van Laar, M.; van Amsterdam, W.A.C.; van Lindert, A.S.R.; de Jong, P.A.; Verhoeff, J.J.C. Prognostic factors for overall survival of stage III non-small cell lung cancer patients on computed tomography: A systematic review and meta-analysis. Radiother Oncol. 2020, 151, 152–175. [Google Scholar] [CrossRef]
- Van Meerbeeck, J.P.; Fennell, D.A.; De Ruysscher, D.K. Small-cell lung cancer. Lancet 2011, 378, 1741–1755. [Google Scholar] [CrossRef]
- Santini, M.; Fiorelli, A.; Messina, G.; Mazzella, A.; Accardo, M. The Feasibility of LigaSure to Create Intestinal Anastomosis: Results of Ex Vivo Study. Surg. Innov. 2015, 22, 266–273. [Google Scholar] [CrossRef]
- Ebell, M.H.; Bentivegna, M.; Hulme, C. Cancer-Specific Mortality, All-Cause Mortality, and Overdiagnosis in Lung Cancer Screening Trials: A Meta-Analysis. Ann. Fam. Med. 2020, 18, 545–552. [Google Scholar] [CrossRef]
- Cozzolino, I.; Ronchi, A.; Messina, G.; Montella, M.; Morgillo, F.; Vicidomini, G.; Tirino, V.; Grimaldi, A.; Zito Marino, F.; Santini, M.; et al. Adequacy of Cytologic Samples by Ultrasound-Guided Percutaneous Transthoracic Fine-Needle Aspiration Cytology of Peripheral Pulmonary Nodules for Morphologic Diagnosis and Molecular Evaluations: Comparison with Computed Tomography–Guided Percutaneous Transthoracic Fine-Needle Aspiration Cytology. Arch. Pathol. Lab. Med. 2020, 144, 361–369. [Google Scholar]
- Fiorelli, A.; Accardo, M.; Carelli, E.; Del Prete, A.; Messina, G.; Reginelli, A.; Berritto, D.; Papale, F.; Armenia, E.; Chiodini, P.; et al. Harmonic tecnology versus neodymium-doped yttrium aluminium garnet laser and electrocautery for lung metastasectomy: An experimental study. Interact. Cardiovasc. Thorac. Surg. 2016, 23, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Barta, J.A.; Powell, C.A.; Wisnivesky, J.P. Global Epidemiology of Lung Cancer. Ann. Glob. Health 2019, 85, 8. [Google Scholar] [CrossRef] [PubMed]
- Santini, M.; Fiorelli, A.; Messina, G.; Laperuta, P.; Mazzella, A.; Accardo, M. Use of the LigaSure device and the Stapler for closure of the small bowel: A comparative ex vivo study. Surg. Today 2013, 43, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Baracos, V.E.; Arribas, L. Sarcopenic obesity: Hidden muscle wasting and its impact for survival and complications of cancer therapy. Ann. Oncol. 2018, 29 (Suppl. 2), ii1–ii9. [Google Scholar] [CrossRef] [PubMed]
- Baldessari, C.; Guaitoli, G.; Valoriani, F.; Bonacini, R.; Marcheselli, R.; Reverberi, L.; Pecchi, A.; Menozzi, R.; Torricelli, P.; Bertolini, F.; et al. Impact of body composition, nutritional and inflammatory status on outcome of non-small cell lung cancer patients treated with immunotherapy. Clin. Nutr. ESPEN 2021, 43, 64–75. [Google Scholar] [CrossRef]
- Mengheri, E.; Nobili, F.; Crocchioni, G.; Lewis, J.A. Protein starvation impairs the ability of activated lymphocytes to produce interferon-gamma. J. Interferon Res. 1992, 12, 17–21. [Google Scholar] [CrossRef]
- Osborn, O.; Olefsky, J.M. The cellular and signaling networks linking the immune system and metabolism in disease. Nat. Med. 2012, 18, 363–374. [Google Scholar] [CrossRef]
- Yang, H.; Youm, Y.H.; Vandanmagsar, B.; Ravussin, A.; Gimble, J.M.; Greenway, F.; Stephens, J.M.; Mynatt, R.L.; Dixit, V.D. Obesity increases the production of proinflammatory mediators from adipose tissue T cells and compromises TCR repertoire diversity: Implications for systemic inflammation and insulin resistance. J. Immunol. 2010, 185, 1836–1845. [Google Scholar] [CrossRef]
- Soeters, P.B.; Wolfe, R.R.; Shenkin, A. Hypoalbuminemia: Pathogenesis and clinical significance. JPEN J. Parenter. Enter. Nutr. 2019, 43, 181–193. [Google Scholar] [CrossRef]
- Masri, F. Role of nitric oxide and its metabolites as potential markers in lungcancer. Ann. Thorac. Med. 2010, 5, 123–127. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J. Free Radicals in Biology and Medicine, 4th ed.; Oxford University: Oxford, UK, 2007. [Google Scholar]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, K.; Loridas, S. Pulmonary oxidative stress, inflammation and cancer: Respirable particulate matter, fibrous dusts and ozone as major causes of lung carcinogenesis through reactive oxygen species mechanisms. Int. J. Environ. Res. Public Health 2013, 10, 3886–3907. [Google Scholar] [CrossRef]
- Afonso, V.; Champy, R.; Mitrovic, D.; Collin, P.; Lomri, A. Reactive oxygen species andsuperoxide dismutases: Role in joint diseases. J. Bone Spine 2007, 74, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Filaire, E.; Dupuis, C.; Galvaing, G.; Aubreton, S.; Laurent, H.; Richard, R.; Filaire, M. Lung cancer: What are the links with oxidative stress, physical activity and nutrition. Lung Cancer 2013, 82, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Crohns, M. Antioxidants, Cytokines and Markers of Oxidative Stress in Lungcancer: Associations with Adverse Events, Response and Survival, 1st ed.; Lambert Academic Publishing: Saarbrücken, Germany, 2010. [Google Scholar]
- Finaud, J.; Lac, G.; Filaire, E. Oxidative stress: Relationship with exercise and training. Sports Med. 2006, 36, 327–358. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Ju, M.K.; Jeon, H.M.; Jeong, E.K.; Lee, Y.J.; Kim, C.H.; Park, H.G.; Han, S.I.; Kang, H.S. Regulation of Tumor Progression by Programmed Necrosis. Oxid. Med. Cell. Longev. 2018, 2018, 3537471. [Google Scholar] [CrossRef]
- Ashcraft, K.A.; Peace, R.M.; Betof, A.S.; Dewhirst, M.W.; Jones, L.W. Efficacy and mechanisms of aerobic exercise on cancer initiation, progression, and metastasis: A critical systematic review of in vivo preclinical data. Cancer Res. 2016, 76, 4032–4050. [Google Scholar] [CrossRef]
- Bower, J.E.; Bak, K.; Berger, A.; Breitbart, W.; Escalante, C.P.; Ganz, P.A.; Schnipper, H.H.; Lacchetti, C.; Ligibel, J.A.; Lyman, G.H.; et al. Screening, assessment, and management of fatigue in adult survivors of cancer: An American Society of Clinical Oncology clinical practice guideline adaptation. J. Clin. Oncol. 2014, 32, 1840–1850. [Google Scholar] [CrossRef]
- Sha, F.E.I.; Zhuang, S.; Zhou, L.I.; Zhang, L.; Yang, Y.; Zhang, S.; Jiang, Y.I.; Qiu, G.; Chen, C.; Zheng, J.; et al. Biomarkers for cancer-related fatigue and adverse reactions to chemotherapy in lung cancer patients. Mol. Clin. Oncol. 2015, 3, 163–166. [Google Scholar] [CrossRef]
- Hung, R.; Krebs, P.; Coups, E.J. Fatigue and functional impairment in early-stage non-small cell lung cancer survivors. J. Pain Symptom Manag. 2011, 41, 426–435. [Google Scholar] [CrossRef]
- Mustian, K.M.; Alfano, C.M.; Heckler, C.; Kleckner, A.S.; Kleckner, I.R.; Leach, C.R.; Mohr, D.; Palesh, O.G.; Peppone, L.J.; Piper, B.F.; et al. Comparison of pharmaceutical, psychological, and exercise treatments for cancer-related fatigue: A meta-analysis. JAMA Oncol. 2017, 3, 961–968. [Google Scholar] [CrossRef]
- D’Silva, A.; Gardiner, P.A.; Boyle, T.; Bebb, D.G.; Johnson, S.T.; Vallance, J.K. Associations of objectively assessed physical activity and sedentary time with health-related quality of life among lung cancer survivors: A quantile regression approach. Lung Cancer 2018, 119, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Janssen, S.M.; Abbink, J.J.; Lindeboom, R.; Vlieland, T.P.V. Outcomes of pulmonary rehabilitation after treatment for non-small cell lung cancer stages I to IIIa: An observational study. J. Cardiopulm. Rehabil. Prev. 2017, 37, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.Y.; Rau, K.M.; Lin, C.C. Longitudinal study on the impact of physical activity on the symptoms of lung cancer survivors. Support. Care Cancer 2015, 23, 3545–3553. [Google Scholar] [CrossRef] [PubMed]
- Scheffer, D.; Latini, A. Exercise-induced immune system response: Anti-inflammatory status on peripheral and central organs. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 10. [Google Scholar] [CrossRef] [PubMed]
- Koelwyn, G.J.; Wennerberg, E.; Demaria, S.; Jones, L.W. Exercise in Regulation of Inflammation-Immune Axis Function in Cancer Initiation and Progression. Oncology 2015, 29, 908–922. [Google Scholar] [PubMed]
- Xie, S.; Wu, Z.; Qi, Y.; Wu, B.; Zhu, X. The metastasizing mechanisms of lung cancer: Recent advances and therapeutic challenges. Biomed. Pharm. 2021, 138, 111450. [Google Scholar] [CrossRef]
- Zhang, L.L.; Wang, S.Z.; Chen, H.L.; Yuan, A.Z. Tai chi exercise for cancer-related fatigue in patients with lung cancer undergoing chemotherapy: A randomized controlled trial. J. Pain Symptom Manag. 2016, 51, 504–511. [Google Scholar] [CrossRef]
- Gerritsen, J.K.; Vincent, A.J. Exercise improves quality of life in patients with cancer: A systematic review and meta-analysis of randomized controlled trials. Br. J. Sports Med. 2016, 50, 796–803. [Google Scholar] [CrossRef]
- Messaggi-Sartor, M.; Marco, E.; Martínez-Téllez, E.; Rodriguez-Fuster, A.; Palomares, C.; Chiarella, S.; Muniesa, J.M.; Orozco-Levi, M.; Barreiro, E.; Güell, M.R. Combined aerobic exercise and high-intensity respiratory muscle training in patients surgically treated for non-small cell lung cancer: A pilot randomized clinical trial. Eur. J. Phys. Rehabil. Med. 2019, 55, 113–122. [Google Scholar] [CrossRef]
- Cavalheri, V.; Jenkins, S.; Cecins, N.; Gain, K.; Phillips, M.J.; Sanders, L.H.; Hill, K. Exercise training for people following curative intent treatment for non-small cell lung cancer: A randomized controlled trial. Braz. J. Phys. Ther. 2017, 21, 58–68. [Google Scholar] [CrossRef]
- Quist, M.; Adamsen, L.; Rørth, M.; Laursen, J.H.; Christensen, K.B.; Langer, S.W. The impact of a multidimensional exercise intervention on physical and functional capacity, anxiety, and depression in patients with advanced-stage lung cancer undergoing chemotherapy. Integr. Cancer Ther. 2015, 14, 341–349. [Google Scholar] [CrossRef]
- Granger, C.L.; Chao, C.; McDonald, C.F.; Berney, S.; Denehy, L. Safety and feasibility of an exercise intervention for patients following lung resection: A pilot randomized controlled trial. Integr. Cancer Ther. 2013, 12, 213–224. [Google Scholar] [CrossRef]
- Moscatelli, F.; Messina, G.; Valenzano, A.; Cibelli, G.; Monda, M. Relationship between RPE and blood lactate after fatiguing handgrip exercise in taekwondo and sedentary subjects. Biol. Med. 2015, 7, S3008. [Google Scholar]
- Monda, V.; Valenzano, A.; Moscatelli, F.; Salerno, M.; Sessa, F.; Triggiani, A.I.; Viggiano, A.; Capranica, L.; Marsala, G.; De Luca, V.; et al. Primary Motor Cortex Excitability in Karate Athletes: A Transcranial Magnetic Stimulation Study. Front. Physiol. 2017, 8, 695. [Google Scholar] [CrossRef]
- De Fusco, C.; Messina, A.; Monda, V.; Viggiano, E.; Moscatelli, F.; Valenzano, A.; Esposito, T.; Sergio, C.; Cibelli, G.; Monda, M.; et al. Osteopontin: Relation between Adipose Tissue and Bone Homeostasis. Stem Cells Int. 2017, 2017, 4045238. [Google Scholar] [CrossRef]
- Kurgan, N.; Tsakiridis, E.; Kouvelioti, R.; Moore, J.; Klentrou, P.; Tsiani, E. Inhibition of Human Lung Cancer Cell Proliferation and Survival by Post-Exercise Serum Is Associated with the Inhibition of Akt, mTOR, p70 S6K, and Erk1/2. Cancers 2017, 9, 46. [Google Scholar] [CrossRef]
- Hwang, C.L.; Yu, C.J.; Shih, J.Y.; Yang, P.C.; Wu, Y.T. Effects of exercise training on exercise capacity in patients with non-small cell lung cancer receiving targeted therapy. Support. Care Cancer 2012, 20, 3169–3177. [Google Scholar] [CrossRef]
- Dhillon, H.M.; Bell, M.L.; van der Ploeg, H.P.; Turner, J.D.; Kabourakis, M.; Spencer, L.; Lewis, C.; Hui, R.; Blinman, P.; Clarke, S.J.; et al. Impact of physical activity on fatigue and quality of life in people with advanced lung cancer: A randomized controlled trial. Ann. Oncol. 2017, 28, 1889–1897. [Google Scholar] [CrossRef]
- Chen, H.M.; Tsai, C.M.; Wu, Y.C.; Lin, K.C.; Lin, C.C. Randomised controlled trial on the effectiveness of home-based walking exercise on anxiety, depression and cancer-related symptoms in patients with lung cancer. Br. J. Cancer 2015, 112, 438–445. [Google Scholar] [CrossRef]
- Kuehr, L.; Wiskemann, J.; Abel, U.; Ulrich, C.M.; Hummler, S.; Thomas, M. Exercise in patients with non-small cell lung cancer. Med. Sci. Sports Exerc. 2014, 46, 656–663. [Google Scholar] [CrossRef]
- Cheville, A.L.; Kollasch, J.; Vandenberg, J.; Shen, T.; Grothey, A.; Gamble, G.; Basford, J.R. A home-based exercise program to improve function, fatigue, and sleep quality in patients with stage IV lung and colorectal cancer: A randomized controlled trial. J. Pain Symptom Manag. 2013, 45, 811–821. [Google Scholar] [CrossRef]
- Lemonnier, I.; Guillemin, F.; Arveux, P.; Clément-Duchêne, C.; Velten, M.; Woronoff-Lemsi, M.C.; Jolly, D.; Baumann, C. Quality of life after the initial treatments of non-small cell lung cancer: A persistent predictor for patients’ survival. Health Qual. Life Outcomes 2014, 12, 73. [Google Scholar] [CrossRef]
- Tarumi, S.; Yokomise, H.; Gotoh, M.; Kasai, Y.; Matsuura, N.; Chang, S.S.; Go, T. Pulmonary rehabilitation during induction chemoradiotherapy for lung cancer improves pulmonary function. J. Thorac. Cardiovasc. Surg. 2015, 149, 569–573. [Google Scholar] [CrossRef]
- Licker, M.; Triponez, F.; Diaper, J.; Karenovics, W.; Bridevaux, P.O. Preoperative evaluation of lung cancer patients. Curr. Anesth. Rep. 2014, 4, 124–134. [Google Scholar] [CrossRef]
- Jones, L.W.; Watson, D.; Herndon, J.E. Peak oxygen consumption and long-term all-cause mortality in nonsmall cell lung cancer. Cancer 2010, 116, 4825–4832. [Google Scholar] [CrossRef]
- Kasymjanova, G.; Correa, J.A.; Kreisman, H.; Dajczman, E.; Pepe, C.; Dobson, S.; Lajeunesse, L.; Sharma, R.; Small, D. Prognostic value of the six-minute walk in advanced non-small cell lung cancer. J. Thorac. Oncol. 2009, 4, 602–607. [Google Scholar] [CrossRef]
- Jones, L.W.; Hornsby, W.E.; Goetzinger, A.; Forbes, L.M.; Sherrard, E.L.; Quist, M.; Lane, A.T.; West, M.; Eves, N.D.; Gradison, M.; et al. Prognostic significance of functional capacity and exercise behavior in patients with metastatic non-small cell lung cancer. Lung Cancer 2012, 76, 248–252. [Google Scholar] [CrossRef]
- Cavalheri, V.; Jenkins, S.; Cecins, N.; Gain, K.; Phillips, M.; Sanders, L.H.; Hill, K. Impairments after curative intent treatment for non-small cell lung cancer: A comparison with age and gender-matched healthy controls. Respir. Med. 2015, 109, 1332–1339. [Google Scholar] [CrossRef]
- Salhi, B.; Haenebalcke, C.; Perez-Bogerd, S.; Nguyen, M.D.; Ninane, V.; Malfait, T.L.; Vermaelen, K.Y.; Surmont, V.F.; Van Maele, G.; Colman, R.; et al. Rehabilitation in patients with radically treated respiratory cancer: A randomised con-trolled trial comparing two training modalities. Lung Cancer 2015, 89, 167–174. [Google Scholar] [CrossRef]
- Granger, C.L.; McDonald, C.F.; Irving, L.; Clark, R.A.; Gough, K.; Murnane, A.; Mileshkin, L.; Krishnasamy, M.; Denehy, L. Low physical activity levels and functional decline in individuals with lung cancer. Lung Cancer 2014, 83, 292–299. [Google Scholar] [CrossRef]
- Lakoski, S.G.; Eves, N.D.; Douglas, P.S.; Jones, L.W. Exercise rehabilitation in patients with cancer. Nat. Rev. Clin. Oncol. 2012, 9, 288–296. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Messina, G.; Tartaglia, N.; Ambrosi, A.; Porro, C.; Campanozzi, A.; Valenzano, A.; Corso, G.; Fiorelli, A.; Polito, R.; Santini, M.; et al. The Beneficial Effects of Physical Activity in Lung Cancer Prevention and/or Treatment. Life 2022, 12, 782. https://doi.org/10.3390/life12060782
Messina G, Tartaglia N, Ambrosi A, Porro C, Campanozzi A, Valenzano A, Corso G, Fiorelli A, Polito R, Santini M, et al. The Beneficial Effects of Physical Activity in Lung Cancer Prevention and/or Treatment. Life. 2022; 12(6):782. https://doi.org/10.3390/life12060782
Chicago/Turabian StyleMessina, Gaetana, Nicola Tartaglia, Antonio Ambrosi, Chiara Porro, Angelo Campanozzi, Anna Valenzano, Gaetano Corso, Alfonso Fiorelli, Rita Polito, Mario Santini, and et al. 2022. "The Beneficial Effects of Physical Activity in Lung Cancer Prevention and/or Treatment" Life 12, no. 6: 782. https://doi.org/10.3390/life12060782
APA StyleMessina, G., Tartaglia, N., Ambrosi, A., Porro, C., Campanozzi, A., Valenzano, A., Corso, G., Fiorelli, A., Polito, R., Santini, M., Monda, M., Tafuri, D., Messina, G., Messina, A., & Monda, V. (2022). The Beneficial Effects of Physical Activity in Lung Cancer Prevention and/or Treatment. Life, 12(6), 782. https://doi.org/10.3390/life12060782













