Concerns with Male Infertility Induced by Exposure to Titanium Nanoparticles and the Supporting Impact of Pelargonium graveolens Essential Oil: Morphometric Records in Male-Wistar Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of TiO2 Nanoparticles
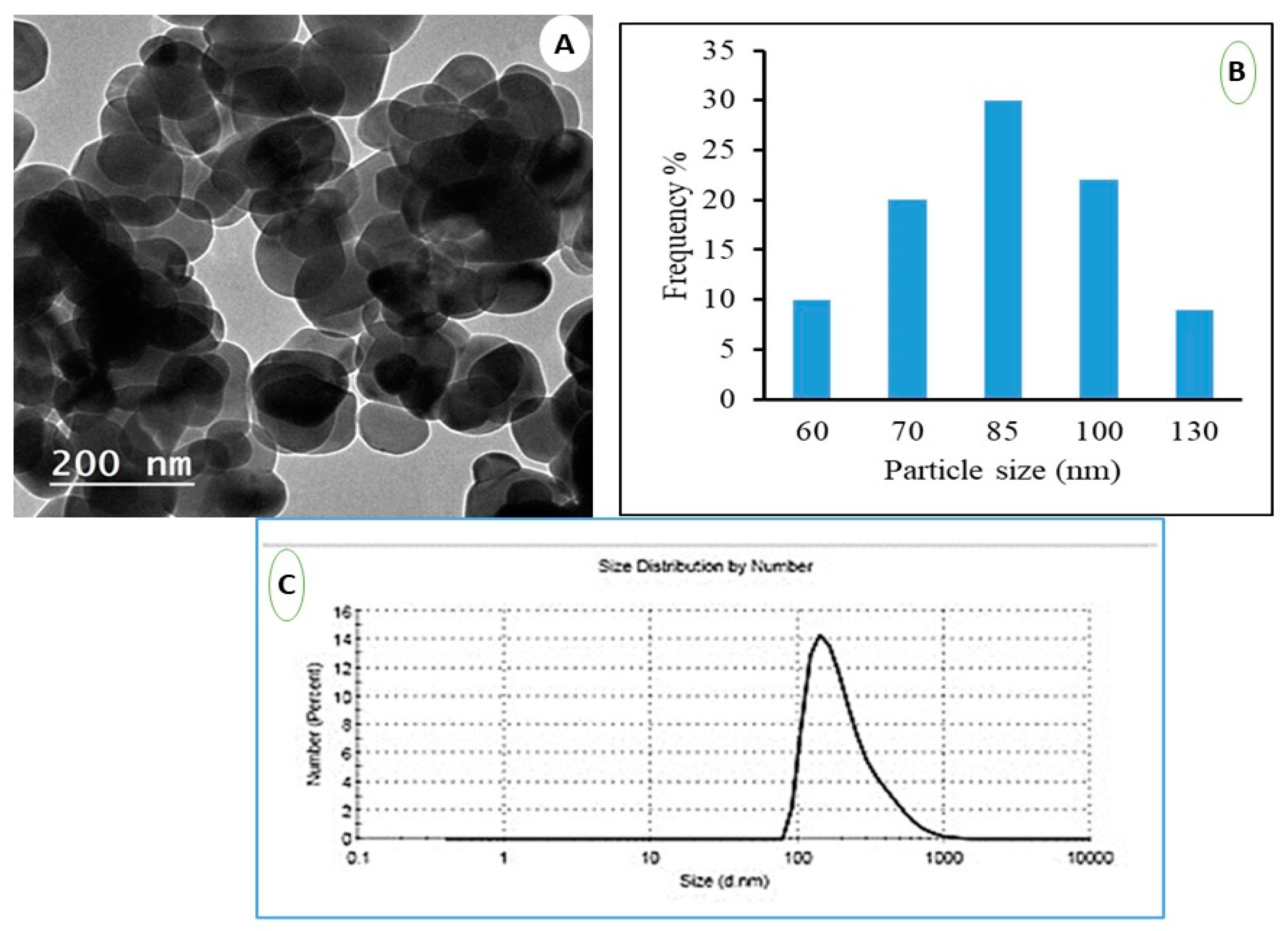
2.2. Transmission Electron Microscope (TEM)
2.3. Dynamic Light Scattering (DLS)
2.4. Plant Essential Oil Extraction and Gas Chromatography/Mass Spectrometry Analysis (GC-MS)
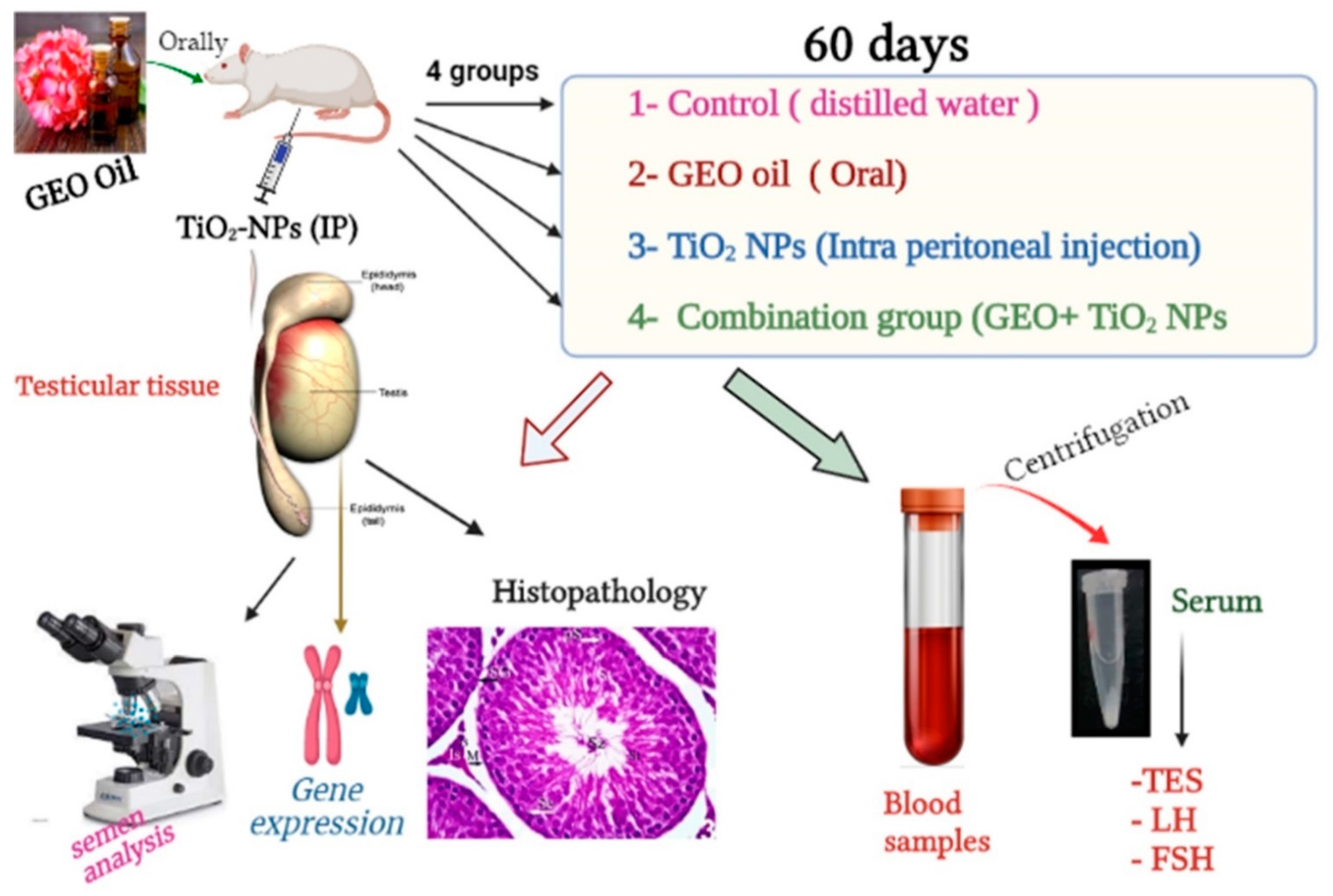
2.5. Experimental Design
2.6. Dose Selection Strategy
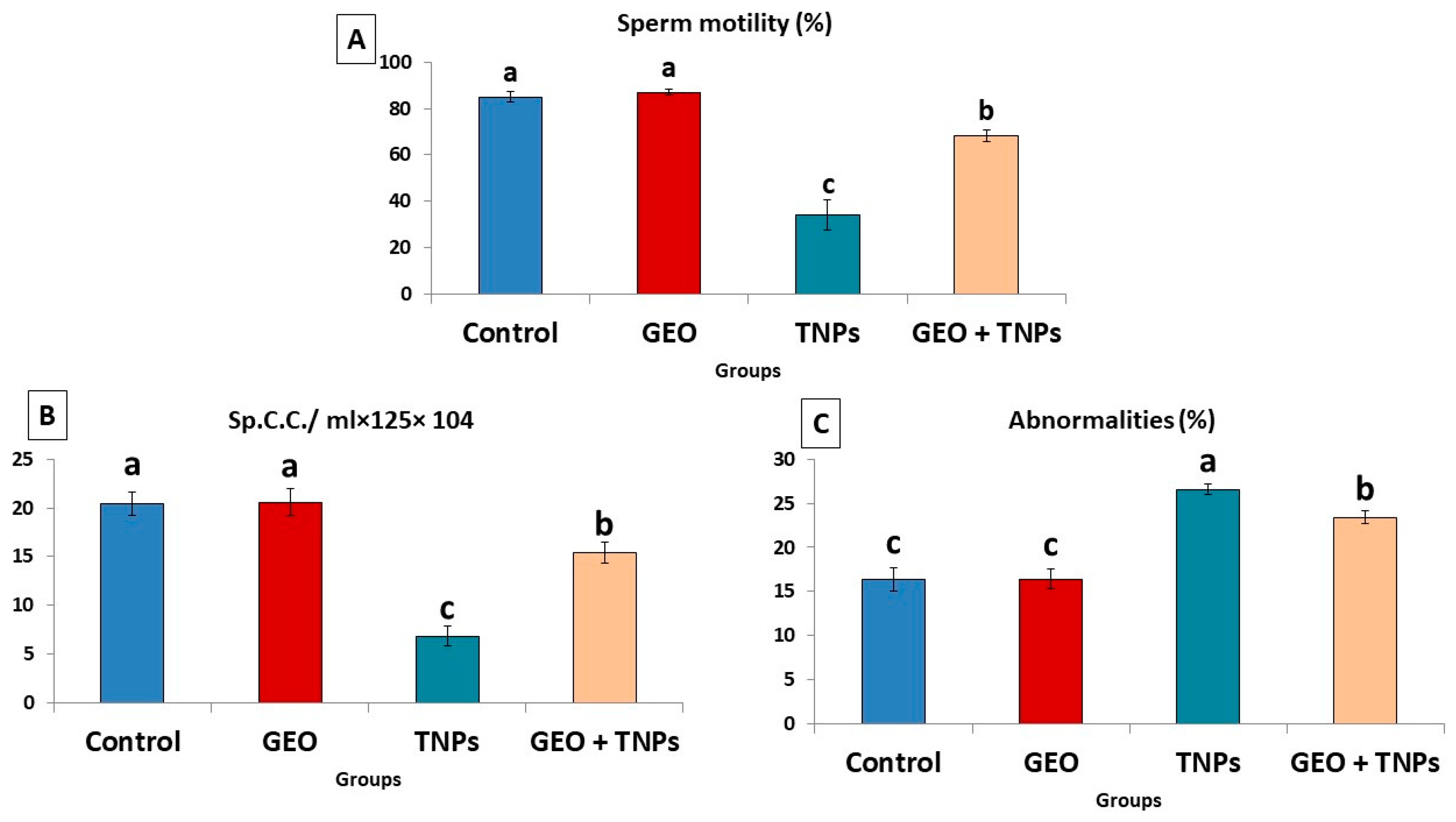
2.7. Sperm Motility Assay, Count, and Morphological Abnormalities
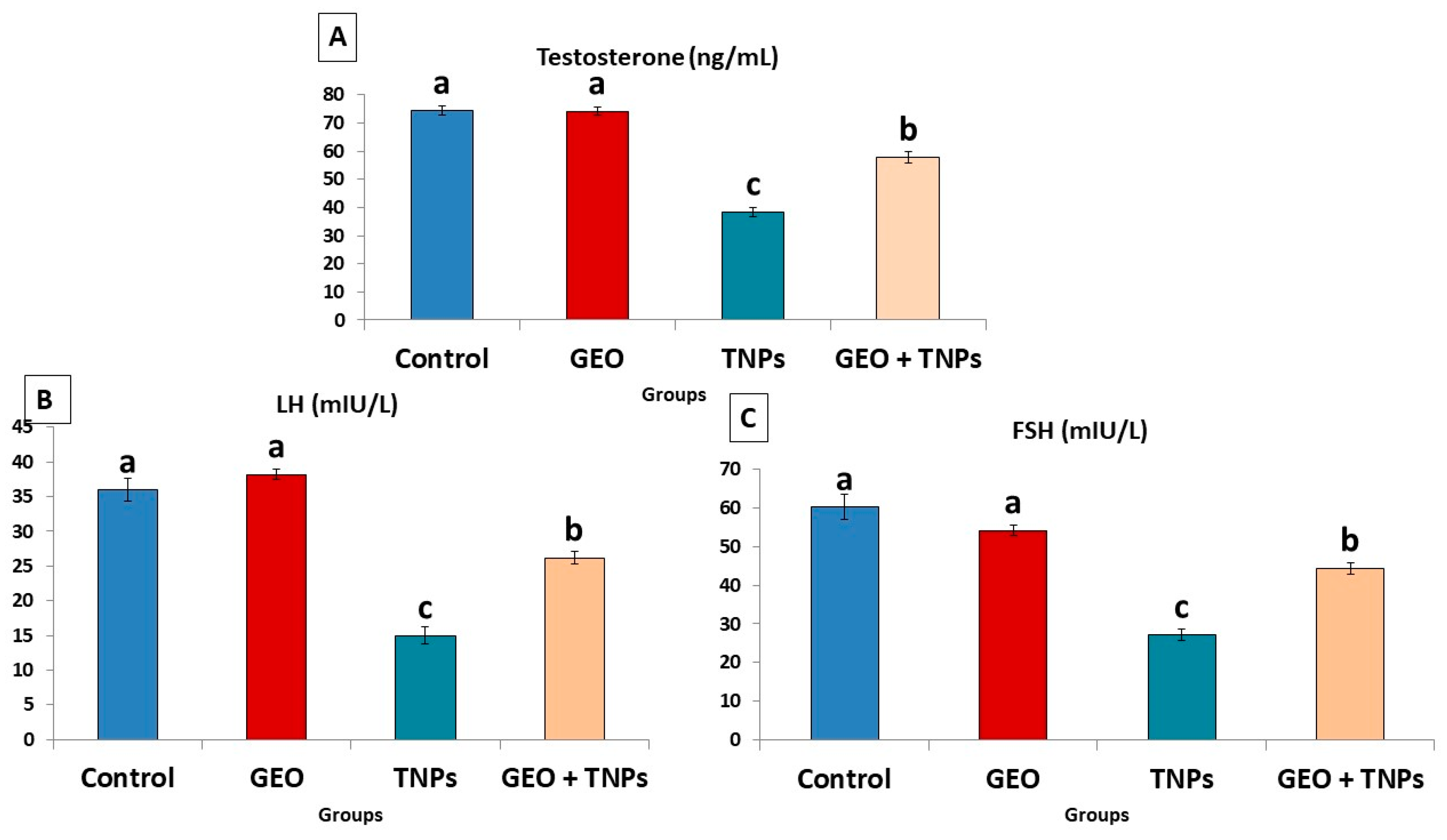
2.8. Hormonal Analysis
2.9. Testicular Oxidative/Antioxidant Status
2.10. Gene Expression
2.11. Histopathological Investigations
2.12. Data Analysis
3. Results
3.1. Characterization of Nanoparticle of TiO2
3.2. GC-MS Analysis of GEO Essential Oils
3.3. Effect of TiO2 NPs and/or GEO Essential Oil Administration on Spermiogram
3.4. Effect of TiO2 NPs and GEO Essential Oil Administration on Male Reproductive Hormones
3.5. Effect of TiO2 NPs and GEO Essential Oil Administration on Testicular Lipid Peroxidation Level and Antioxidant Enzymes
3.6. Effects of TiO2 NPs and/or GEO Essential Oil Administration on the Testicular Gene Expression
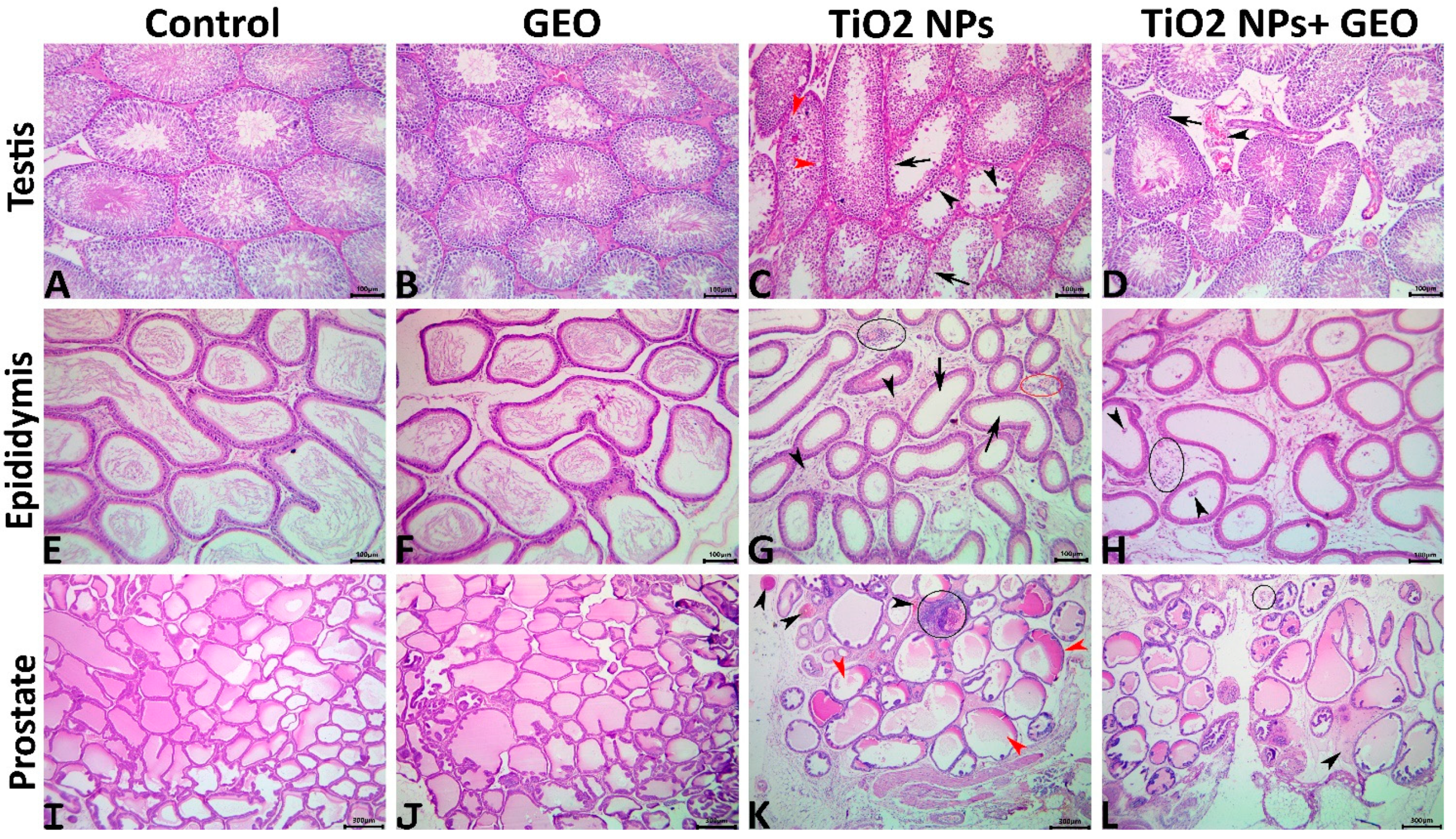
3.7. Histopathological Findings
3.7.1. Testes
3.7.2. Epididymis
3.7.3. Prostate
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Yang, J.; Luo, M.; Tan, Z.; Dai, M.; Xie, M.; Lin, J.; Hua, H.; Ma, Q.; Zhao, J.; Liu, A. Oral administration of nano-titanium dioxide particle disrupts hepatic metabolic functions in a mouse model. Environ. Toxicol. Pharmacol. 2017, 49, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Medina, C.; Santos-Martinez, M.; Radomski, A.; Corrigan, O.; Radomski, M. Nanoparticles: Pharmacological and toxicological significance. Br. J. Pharmacol. 2007, 150, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Robertson, T.A.; Sanchez, W.Y.; Roberts, M.S. Are commercially available nanoparticles safe when applied to the skin? J. Biomed. Nanotechnol. 2010, 6, 452–468. [Google Scholar] [CrossRef] [PubMed]
- Saud Alarifi, D.A.; Al-Doaiss, A.A.; Ali, B.A.; Ahmed, M.; Al-Khedhairy, A.A. Histologic and apoptotic changes induced by titanium dioxide nanoparticles in the livers of rats. Int. J. Nanomed. 2013, 8, 3937. [Google Scholar]
- Morgan, A.; Galal, M.K.; Ogaly, H.A.; Ibrahim, M.A.; Abd-Elsalam, R.M.; Noshy, P. Tiron ameliorates oxidative stress and inflammation in titanium dioxide nanoparticles induced nephrotoxicity of male rats. Biomed. Pharmacother. 2017, 93, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Fabian, E.; Landsiedel, R.; Ma-Hock, L.; Wiench, K.; Wohlleben, W.; Van Ravenzwaay, B. Tissue distribution and toxicity of intravenously administered titanium dioxide nanoparticles in rats. Arch. Toxicol. 2008, 82, 151–157. [Google Scholar] [CrossRef]
- Jaeger, A.; Weiss, D.G.; Jonas, L.; Kriehuber, R. Oxidative stress-induced cytotoxic and genotoxic effects of nano-sized titanium dioxide particles in human HaCaT keratinocytes. Toxicology 2012, 296, 27–36. [Google Scholar] [CrossRef]
- Johnston, H.J.; Hutchison, G.R.; Christensen, F.M.; Peters, S.; Hankin, S.; Stone, V. Identification of the mechanisms that drive the toxicity of TiO2 particulates: The contribution of physicochemical characteristics. Part. Fibre Toxicol. 2009, 6, 1–27. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, F.; Jin, C.; Liang, H.; Zhong, X.; Yang, Y. Mitochondrial injury induced by nanosized titanium dioxide in A549 cells and rats. Environ. Toxicol. Pharmacol. 2013, 36, 66–72. [Google Scholar] [CrossRef]
- Mohamed, A.A.-R.; Abdellatief, S.A.; Khater, S.I.; Ali, H.; Al-Gabri, N.A. Fenpropathrin induces testicular damage, apoptosis, and genomic DNA damage in adult rats: Protective role of camel milk. Ecotoxicol. Environ. Saf. 2019, 181, 548–558. [Google Scholar] [CrossRef]
- Eleiwa, N.Z.; Galal, A.A.; Abd El-Aziz, R.M.; Hussin, E.M. Antioxidant activity of Spirulina platensis alleviates doxorubicin-induced oxidative stress and reprotoxicity in male rats. Orient. Pharm. Exp. Med. 2018, 18, 87–95. [Google Scholar] [CrossRef]
- Osama, E.; Galal, A.A.; Abdalla, H.; El-Sheikh, S.M. Chlorella vulgaris ameliorates testicular toxicity induced by deltamethrin in male rats via modulating oxidative stress. Andrologia 2019, 51, e13214. [Google Scholar] [CrossRef] [PubMed]
- Tyler, C.; Jobling, S.; Sumpter, J. Endocrine disruption in wildlife: A critical review of the evidence. Crit. Rev. Toxicol. 1998, 28, 319–361. [Google Scholar] [CrossRef] [PubMed]
- Hassanein, K.M.; El-Amir, Y.O. Ameliorative effects of thymoquinone on titanium dioxide nanoparticles induced acute toxicity in rats. Int. J. Vet. Sci. Med. 2018, 6, 16–21. [Google Scholar] [CrossRef]
- El-Kirdasy, A.F.; Nassan, M.A.; Baiomy, A.A.A.; Ismail, T.A.; Soliman, M.M.; Attia, H.F. Potential ameliorative role of n-acetylcysteine against testicular dysfunction induced by titanium dioxide in male albino rats. Am. J. Pharmacol. Toxicol. 2014, 9, 29. [Google Scholar] [CrossRef][Green Version]
- Hong, F.; Zhao, X.; Chen, M.; Zhou, Y.; Ze, Y.; Wang, L.; Wang, Y.; Ge, Y.; Zhang, Q.; Ye, L. TiO2 nanoparticles-induced apoptosis of primary cultured Sertoli cells of mice. J. Biomed. Mater. Res. Part A 2016, 104, 124–135. [Google Scholar] [CrossRef]
- Slima, A.B.; Ali, M.B.; Barkallah, M.; Traore, A.I.; Boudawara, T.; Allouche, N.; Gdoura, R. Antioxidant properties of Pelargonium graveolens L’Her essential oil on the reproductive damage induced by deltamethrin in mice as compared to alpha-tocopherol. Lipids Health Dis. 2013, 12, 1–9. [Google Scholar]
- Mainardi, T.; Kapoor, S.; Bielory, L. Complementary and alternative medicine: Herbs, phytochemicals and vitamins and their immunologic effects. J. Allergy Clin. Immunol. 2009, 123, 283–294.e10. [Google Scholar] [CrossRef]
- Amabeoku, G. Antidiarrhoeal activity of Geranium incanum Burm. f.(Geraniaceae) leaf aqueous extract in mice. J. Ethnopharmacol. 2009, 123, 190–193. [Google Scholar] [CrossRef]
- Elmann, A.; Mordechay, S.; Rindner, M.; Ravid, U. Anti-neuroinflammatory effects of geranium oil in microglial cells. J. Funct. Foods 2010, 2, 17–22. [Google Scholar] [CrossRef]
- Mezni, A.; Alghool, S.; Sellami, B.; Ben Saber, N.; Altalhi, T. Titanium dioxide nanoparticles: Synthesis, characterisations and aquatic ecotoxicity effects. Chem. Ecol. 2018, 34, 288–299. [Google Scholar] [CrossRef]
- Xiaoming, F. Synthesis and optical absorpition properies of anatase tio2 nanoparticles via a hydrothermal hydrolysis method. Rare Met. Mater. Eng. 2015, 44, 1067–1070. [Google Scholar] [CrossRef]
- El-Sayed, A.A.; Amr, A.; Kamel, O.M.; El-Saidi, M.M.; Abdelhamid, A.E. Eco-friendly fabric modification based on AgNPs@ Moringa for mosquito repellent applications. Cellulose 2020, 27, 8429–8442. [Google Scholar] [CrossRef]
- Fan, W.; Yan, W.; Xu, Z.; Ni, H. Colloids and Surfaces B: Biointerfaces Formation mechanism of monodisperse, low molecular weight chitosan nanoparticles by ionic gelation technique. Colloids Surf. B Biointerfaces 2012, 90, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Brum, A.; Pereira, S.A.; Owatari, M.S.; Chagas, E.C.; Chaves, F.C.M.; Mouriño, J.L.P.; Martins, M.L. Effect of dietary essential oils of clove basil and ginger on Nile tilapia (Oreochromis niloticus) following challenge with Streptococcus agalactiae. Aquaculture 2017, 468, 235–243. [Google Scholar] [CrossRef]
- Al-Sagheer, A.; Mahmoud, H.; Reda, F.; Mahgoub, S.; Ayyat, M. Supplementation of diets for Oreochromis niloticus with essential oil extracts from lemongrass (Cymbopogon citratus) and geranium (Pelargonium graveolens) and effects on growth, intestinal microbiota, antioxidant and immune activities. Aquac. Nutr. 2018, 24, 1006–1014. [Google Scholar] [CrossRef]
- Boukhris, M.; Bouaziz, M.; Feki, I.; Jemai, H.; El Feki, A.; Sayadi, S. Hypoglycemic and antioxidant effects of leaf essential oil of Pelargonium graveolens L’Hér. in alloxan induced diabetic rats. Lipids Health Dis. 2012, 11, 81. [Google Scholar] [CrossRef]
- Doudi, M.; Setorki, M. Influence of Titanium Dioxide Nanoparticles on Oxidative Stress and Pulmonary Dysfunction. Zahedan J. Res. Med. Sci. 2015, 17, e1062. [Google Scholar] [CrossRef]
- Hunter, D.A.; Bartel, L.K.; Byrd, I.; Bogan, B.; Yau, W.; Wu, J.; Gato, W.E. Short-Term Effects of Titanium Dioxide Nanofiber on the Renal Function of Male Sprague Dawley Rats. J. Environ. Pathol. Toxicol. Oncol. 2018, 37, 127–138. [Google Scholar] [CrossRef]
- Mohamed, A.A.-R.; Mohamed, W.A.; Khater, S.I. Imidacloprid induces various toxicological effects related to the expression of 3β-HSD, NR5A1, and OGG1 genes in mature and immature rats. Environ. Pollut. 2017, 221, 15–25. [Google Scholar] [CrossRef]
- Slott, V.L.; Suarez, J.D.; Perreault, S.D. Rat sperm motility analysis: Methodologic considerations. Reprod. Toxicol. 1991, 5, 449–458. [Google Scholar] [CrossRef]
- Beutler, E.; Duron, O.; Kelly, B.M. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 1963, 61, 882–888. [Google Scholar] [PubMed]
- Filler, R. Methods for Evaluation of Rat Epididymal Sperm Morphology. In Methods in Toxicology Vol. 3 (Part A). Male Reproductive Toxicology; Chapin, R.E., Heindel, J.J., Eds.; Academic Press Limited: London, UK, 1993. [Google Scholar]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Mihara, M.; Uchiyama, M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal. Biochem. 1978, 86, 271–278. [Google Scholar] [PubMed]
- Yuan, J.S.; Reed, A.; Chen, F.; Stewart, C.N. Statistical analysis of real-time PCR data. BMC Bioinform. 2006, 7, 1–12. [Google Scholar] [CrossRef]
- Kittel, B.; Ruehl-Fehlert, C.; Morawietz, G.; Klapwijk, J.; Elwell, M.R.; Lenz, B.; O’Sullivan, M.G.; Roth, D.R.; Wadsworth, P.F. Revised guides for organ sampling and trimming in rats and mice–Part 2: A joint publication of the RITA) and NACAD) groups. Exp. Toxicol. Pathol. 2004, 55, 413–431. [Google Scholar] [CrossRef] [PubMed]
- Suvarna, S.K.; Layton, C.; Bancroft, J.D. Bancroft’s Theory and Practice of Histological Techniques, 8th ed.; Churchill Livingstone Elsevier: Oxford, UK, 2018. [Google Scholar]
- Chen, X.; Mao, S.S. Titanium dioxide nanomaterials: Synthesis, properties, modifications, and applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef]
- Long, T.C.; Tajuba, J.; Sama, P.; Saleh, N.; Swartz, C.; Parker, J.; Hester, S.; Lowry, G.V.; Veronesi, B. Nanosize Titanium Dioxide Stimulates Reactive Oxygen Species in Brain Microglia and Damages Neurons in Vitro. Environ. Health Perspect. 2007, 115, 1631–1637. [Google Scholar] [CrossRef]
- Hong, F.; Yu, X.; Wu, N.; Zhang, Y.-Q. Progress of in vivo studies on the systemic toxicities induced by titanium dioxide nanoparticles. Toxicol. Res. 2017, 6, 115–133. [Google Scholar] [CrossRef]
- Nur, Ç.E.B.İ. Chemical Fingerprinting of the Geranium (Pelargonium graveolens) Essential Oil by Using FTIR, Raman and GC-MS Techniques. Avrupa Bilim Ve Teknol. Derg. 2021, 25, 810–814. [Google Scholar]
- Diez, V.; Traikov, S.; Schmeisser, K.; Adhikari, A.K.D.; Kurzchalia, T.V. Glycolate combats massive oxidative stress by restoring redox potential in Caenorhabditis elegans. Commun. Biol. 2021, 4, 151. [Google Scholar] [CrossRef] [PubMed]
- Younus, H. Therapeutic potentials of superoxide dismutase. Int. J. Health Sci. (Qassim) 2018, 12, 88–93. [Google Scholar]
- Asadi, N.; Bahmani, M.; Kheradmand, A.; Rafieian-Kopaei, M. The Impact of Oxidative Stress on Testicular Function and the Role of Antioxidants in Improving it: A Review. J. Clin. Diagn. Res. 2017, 11, IE01–IE05. [Google Scholar] [CrossRef] [PubMed]
- Grunewald, S.; Said, T.; Paasch, U.; Glander, H.J.; Agarwal, A. Relationship between sperm apoptosis signalling and oocyte penetration capacity. Int. J. Androl. 2008, 31, 325–330. [Google Scholar] [CrossRef]
- Abd El-Hakim, Y.M.; Abdel-Rahman Mohamed, A.; Khater, S.I.; Hamed Arisha, A.; Metwally, M.M.M.; Nassan, M.A.; Hassan, M.E. Chitosan-Stabilized Selenium Nanoparticles and Metformin Synergistically Rescue Testicular Oxidative Damage and Steroidogenesis-Related Genes Dysregulation in High-Fat Diet/Streptozotocin-Induced Diabetic Rats. Antioxidants 2020, 10, 17. [Google Scholar] [CrossRef]
- Gao, G.; Ze, Y.; Zhao, X.; Sang, X.; Zheng, L.; Ze, X.; Gui, S.; Sheng, L.; Sun, Q.; Hong, J.; et al. Titanium dioxide nanoparticle-induced testicular damage, spermatogenesis suppression, and gene expression alterations in male mice. J. Hazard. Mater. 2013, 258–259, 133–143. [Google Scholar] [CrossRef]
- Younis, N.S.; Abduldaium, M.S.; Mohamed, M.E. Protective effect of geraniol on oxidative, inflammatory and apoptotic alterations in isoproterenol-induced cardiotoxicity: Role of the keap1/nrf2/HO-1 and PI3K/Akt/mTOR pathways. Antioxidants 2020, 9, 977. [Google Scholar] [CrossRef]
- Farhath, M.S.; Vijaya, P.; Vimal, M. Antioxidant activity of geraniol, geranial acetate, gingerol and eugenol. Res. Pharm. 2015, 3. Available online: https://updatepublishing.com/journal/index.php/rip/article/view/222 (accessed on 14 February 2022).
- Nivitabishekam, S.N.; Asad, M.; Prasad, V.S. Pharmacodynamic interaction of Momordica charantia with rosiglitazone in rats. Chem.-Biol. Interact. 2009, 177, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Stocco, D. The role of the StAR protein in steroidogenesis: Challenges for the future. J. Endocrinol. 2000, 164, 247–253. [Google Scholar] [CrossRef]
- Hammar, M.; Petersson, F. Testosterone production in vitro in human testicular tissue. Andrologia 1986, 18, 196–200. [Google Scholar] [CrossRef]
- Rey, R.; Campo, S.; Ayuso, S.; Nagle, C.; Chemes, H. Testicular steroidogenesis in the Cebus monkey throughout postnatal development. Biol. Reprod. 1995, 52, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Sahin, E.; Colla, S.; Liesa, M.; Moslehi, J.; Müller, F.L.; Guo, M.; Cooper, M.; Kotton, D.; Fabian, A.J.; Walkey, C. Telomere dysfunction induces metabolic and mitochondrial compromise. Nature 2011, 470, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Oh, S. Disturbance in testosterone production in leydig cells by polycyclic aromatic hydevrepocarbons. Dev. Reprod. 2014, 18, 187–195. [Google Scholar] [CrossRef]
- Elewa, Y.H.A.; Mohamed, A.A.-R.; Galal, A.A.A.; El-naseery, N.I.; Ichii, O.; Kon, Y. Food Yellow4 reprotoxicity in relation to localization of DMC1 and apoptosis in rat testes: Roles of royal jelly and cod liver oil. Ecotoxicol. Environ. Saf. 2019, 169, 696–706. [Google Scholar] [CrossRef] [PubMed]
- Mar, P.D.; El Khalfi, B.; Soukri, A. Protective effect of oregano and sage essentials oils against the effect of extracellular H2O2 and SNP in Tetrahymena thermophila and Tetrahymena pyriformis. J. King Saud Univ.-Sci. 2020, 32, 279–287. [Google Scholar] [CrossRef]
- Mbaye, M.M.; El Khalfi, B.; Addoum, B.; Mar, P.D.; Saadani, B.; Louanjli, N.; Soukri, A. The Effect of Supplementation with Some Essential Oils on the Mobility and the Vitality of Human Sperm. Sci. World J. 2019, 2019, 4878912. [Google Scholar] [CrossRef]

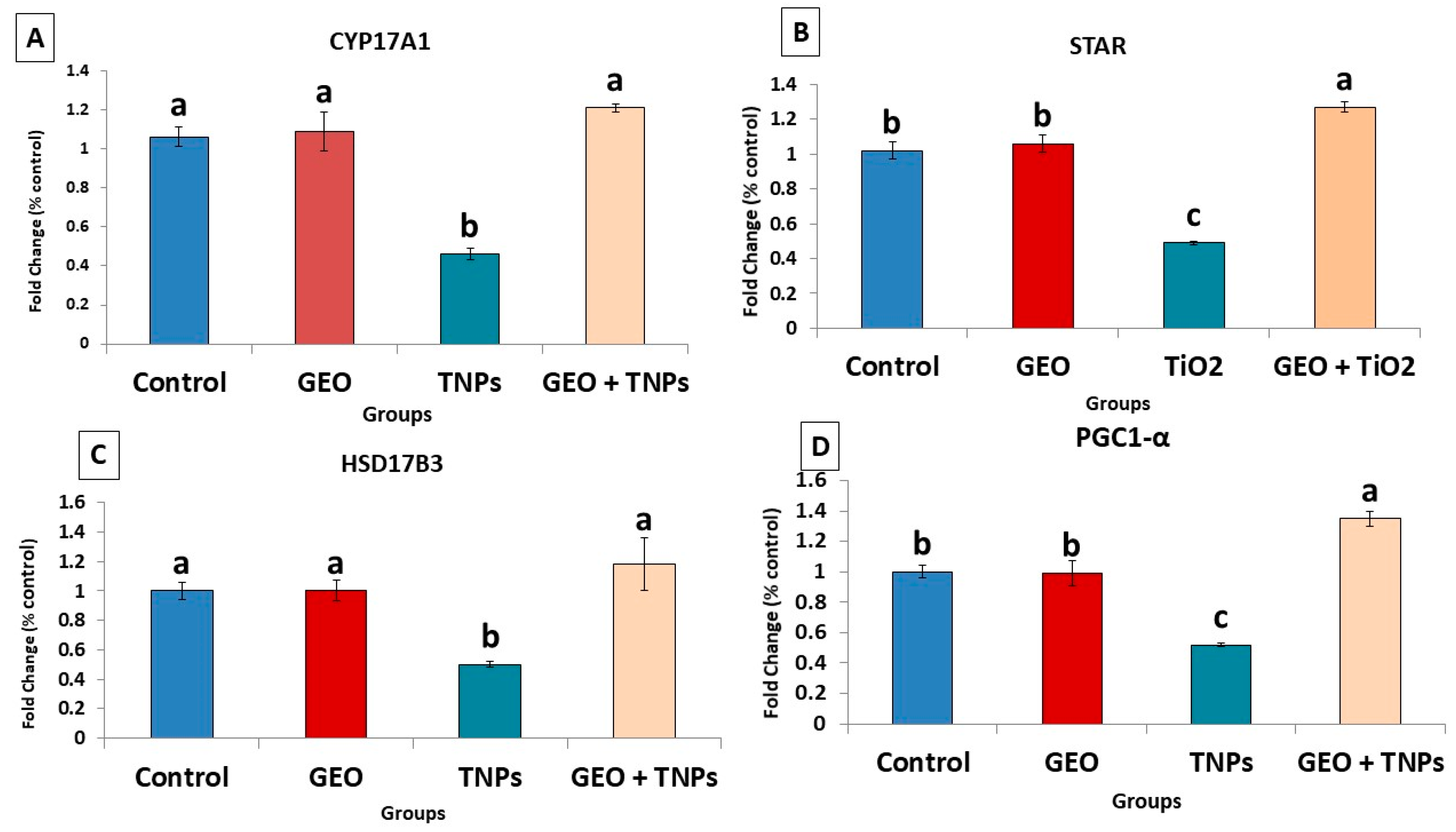
| Gene | Forward Primer (5′–3′) | Reverse Primer (5′–3′) | Accession No | Product Size |
|---|---|---|---|---|
| StAr | CCCAAATGTCAAGGAAATCA | AGGCATCTCCCCAAAGTG | NM_031558.3 | 187 |
| CYP17A1 | TGGCTTTCCTGGTGCACAATC | TGAAAGTTGGTGTTCGGCTGAAG | NM_012753.2 | 90 |
| HSD17B3 | AGTGTGTGAGGTTCTCCCGGTACCT | TACAACATTGAGTCCATGTCTGGCCAG | NM_054007.1 | 161 |
| PGC1-α | ATGTGTCGCCTTCTTGCTCT | ATCTACTGCCTGGGGACCTT | NM_031347.1 | 180 |
| GAPDH | GGCACAGTCAAGGCTGAGAATG | ATGGTGGTGAAGACGCCAGTA | NM_017008.4 | 143 |
| Peak | RT | Name | Formula | Area | Area Sum % |
|---|---|---|---|---|---|
| 1. | 24.299 | Citronellol | C10H20O | 55,776,528.03 | 37.24 |
| 2. | 26.301 | Geraniol | C10H18O | 18,461,011.12 | 12.32 |
| 3. | 19.967 | Citronellyl ester | C11H20O2 | 14,007,012.3 | 9.35 |
| 4. | 15.887 | D-isomenthone | C10H18O | 9,245,683.65 | 6.17 |
| 5. | 3.476 | Alpha.-Pinene | C10H16 | 1,391,254.48 | 0.93 |
| 6. | 17.941 | L-linalool | C10H18O | 8,055,441.23 | 5.38 |
| 7. | 11.807 | CIS-ROSE OXIDE | C10H18O | 1,926,217.6 | 1.29 |
| 8. | 15.029 | l-Menthone | C10H18O | 3,170,974.01 | 2.12 |
| 9. | 16.259 | Alfa.-Copaene | C15H24 | 1,234,444.25 | 0.82 |
| 10. | 17.014 | Beta-Bourbonene | C15H24 | 2,648,338.41 | 1.77 |
| 11. | 18.382 | Isopulegol | C10H18O | 699,337.32 | 0.47 |
| 12. | 19.206 | Caryophyllene | C15H24 | 2,651,769.58 | 1.77 |
| 13. | 33.483 | (epi-γ-Eudesmol) | C15H26O | 10,293,442.99 | 6.87 |
| 14. | 21.209 | E,E-.alpha.-farnesene | C15H24 | 1,259,318.62 | 0.84 |
| 15. | 21.461 | Cis-Verbenol | C10H16O | 937,277.21 | 0.63 |
| 16. | 22.078 | Gamma.-Elemene | C15H24 | 941,376.63 | 0.63 |
| 17. | 22.262 | Beta.-Myrcene | C10H16 | 4,132,866.08 | 2.76 |
| 18. | 22.902 | Citral | C10H16O | 1,833,252.63 | 1.22 |
| 19. | 23.795 | Delta-Cadinene | C15H24 | 2,691,428.45 | 1.8 |
| 20. | 25.546 | Geranyl propionate | C13H22O2 | 630,509.85 | 0.42 |
| 21. | 25.638 | cis-Calamenene | C15H22 | 992,839.4 | 0.66 |
| 22. | 27.12 | α-Agarofurane | C15H24O | 737,485.25 | 0.49 |
| 23. | 27.52 | Linalyl Acetate | C12H20O2 | 679,603.64 | 0.45 |
| 24. | 31.846 | Copaene | C15H24 | 892,608.23 | 0.6 |
| 25. | 34.266 | NERYL ACETATE | C12H20O2 | 1,582,583.89 | 1.06 |
| 26. | 34.381 | Spatulenol | C15H24O | 838,394.74 | 0.56 |
| 27. | 36.784 | 2-Phenylethyl tiglate | C13H16O2 | 1,228,021.02 | 0.82 |
| 28. | 38.346 | Aromandendrene | C15H24 | 848,453.07 | 0.57 |
| Organ | Lesion | Control | GEO | TiO2 | GEO + TiO2 |
|---|---|---|---|---|---|
| Testes | Numbers of ST/10× microscopic field | 14 ± 0.42 b | 13.7 ± 0.42 b | 18.5± 0.52 a | 17.3 ± 0.37 a |
| Mean diameters of ST (µm) | 270.25 ± 7.23 a | 270.18 ± 6.3 a | 209.98 ± 5.1 c | 233 ± 9.4 b | |
| Height of seminefrous (µm) (µm)epithelium | 83.9 ± 0.85 a | 84.6 ± 0.9 a | 64 ±1.6 c | 74.7± 1.5 b | |
| Numbers of permatogonia/ST | 66.3 ± 4.9 a | 67.9 ± 4.4 a | 58.47 ± 3.06 | 63.8 ± 3.8 | |
| Numbers of spermatocytes/ST | 139.5 ± 1.7 a | 138.3 ± 2.4 a 1.7 a | 102.7 ± 3.7 c | 124.13 ± 3.4 b | |
| Numbers of spermatids/ST | 208.5 ± 2.9 a | 206.2 ± 4.8 a | 165.5 ± 8.1 c | 184.9 ± 8.1 b | |
| Numbers of Sertoli/ST | 17.3 ± 0.45 a | 17.6 ± 0.46 a | 15.3 ± 0.38 b | 16.6 ± 0.37 a | |
| Numbers of ST with vacuolated epithelium | 0 ± 0 c | 0 ± 0 c | 8.3 ± 0.29 a | 5.3 ± 0.56 b | |
| Numbers of ST with necrotic epithelium | 0 ± 0 c | 0 ± 0 c | 5.1 ± 0.53 a | 1.4 ± 0.2 b | |
| Numbers of ST with depleted epithelium | 0 ± 0 c | 0 ± 0 c | 5.4 ± 0.41 a | 1.8 ± 0.41 b | |
| Numbers of ST with multinucleated giant cells | 0 ± 0 c | 0 ± 0 c | 1.7 ± 0.16 a | 0.62 ± 0.21 b | |
| Interstitial edema | 0 ± 0 b | 0 ± 0 b | 6 ± 3 a | 2 ± 2 ab | |
| Interstitial congestion | 0 ± 0 b | 0 ± 0 b | 10± 3.3 a | 6 ± 3 ab | |
| Epididymis | Tubular irregularity | 0 ± 0 b | 0 ± 0 b | 10 ± 3.3 a | 4 ± 2.6 ab |
| Absence of luminal sperms | 0 ± 0 | 0 ± 0 | 6 ± 3 | 4 ± 2.6 | |
| Presence of luminal round spermatids and/or exfoliated material | 2 ± 2 | 2 ± 2 | 8 ± 3.2 | 4 ± 2.6 | |
| Loss or disrupted of stereocilia | 0 ± 0 b | 0 ± 0 b | 10 ± 3.3 a | 6 ± 3 ab | |
| Vacuole formation | 0 ± 0 b | 0 ± 0 b | 12 ± 3.2 a | 8 ± 3.2 a | |
| Epithelial necrosis | 0 ± 0 | 0 ± 0 | 6 ± 3.5 | 4 ± 2.6 | |
| Hyperplastic alteration | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| Interstitial inflammatory infiltrates | 0 ± 0 b | 0 ± 0 b | 8 ± 3.2 a | 4 ± 2.6 ab | |
| Interstitial vascular congestions | 0 ± 0 b | 0 ± 0 b | 8 ± 3.2 a | 6 ± 3 ab | |
| Interstitial hemorrhages | 0 ± 0 | 0 ± 0 | 2 ± 2 | 0 ± 0 | |
| Prostate | Reduced or absence of luminal secretion | 0 ± 0 | 0 ± 0 | 6 ± 3.05 | 4 ± 2.6 |
| Acinar dilatation | 0 ± 0 | 0 ± 0 | 4 ± 2.6 | 4 ± 2.6 | |
| Absence of the intra-acinar epithelial folds | 2 ± 2 b | 2 ± 2 b | 12 ± 3.2 a | 6 ± 3.05 ab | |
| Vacuolar degeneration | 0 ± 0 | 0 ± 0 | 4 ± 2.6 | 2 ± 2 | |
| Necrosis of the epithelial lining | 0 ± 0 b | 0 ± 0 b | 8 ± 3.2 a | 4 ±2.6 ab | |
| Hyperplasia of acinar epithelium | 0 ± 0 | 0 ± 0 | 2 ± 2 | 0 ± 0 | |
| Interstitial inflammatory cell infiltrate | 0 ± 0 | 0 ± 0 | 6 ± 3.05 | 4 ± 2.6 | |
| Increased connective tissue elements | 0 ± 0 | 0 ± 0 | 6 ± 3.05 | 6 ± 3.05 | |
| Interstitial congestion | 0 ± 0 b | 0 ± 0 b | 10 ± 3.3 a | 6 ± 3 ab | |
| Interstitial edema | 0 ± 0 | 0 ± 0 | 6 ± 3.05 | 4 ± 2.6 | |
| Interstitial hemorrhage | 0 ± 0 | 0 ± 0 | 2 ± 2 | 2 ± 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Said, A.A.; Nasr, Y.; Galal, A.A.A.; Abdelhamid, A.E.; Mohamed, H.A.; Metwally, M.M.M.; Said, M.A.; Nassan, M.A.; Dahran, N.; Mohamed, A.A.-R. Concerns with Male Infertility Induced by Exposure to Titanium Nanoparticles and the Supporting Impact of Pelargonium graveolens Essential Oil: Morphometric Records in Male-Wistar Rats. Life 2022, 12, 639. https://doi.org/10.3390/life12050639
Said AA, Nasr Y, Galal AAA, Abdelhamid AE, Mohamed HA, Metwally MMM, Said MA, Nassan MA, Dahran N, Mohamed AA-R. Concerns with Male Infertility Induced by Exposure to Titanium Nanoparticles and the Supporting Impact of Pelargonium graveolens Essential Oil: Morphometric Records in Male-Wistar Rats. Life. 2022; 12(5):639. https://doi.org/10.3390/life12050639
Chicago/Turabian StyleSaid, Ahmed Abdou, Yasmin Nasr, Azza A. A. Galal, Ahmed E. Abdelhamid, Haiam A. Mohamed, Mohamed M. M. Metwally, Mahmoud A. Said, Mohamed A. Nassan, Naief Dahran, and Amany Abdel-Rahman Mohamed. 2022. "Concerns with Male Infertility Induced by Exposure to Titanium Nanoparticles and the Supporting Impact of Pelargonium graveolens Essential Oil: Morphometric Records in Male-Wistar Rats" Life 12, no. 5: 639. https://doi.org/10.3390/life12050639
APA StyleSaid, A. A., Nasr, Y., Galal, A. A. A., Abdelhamid, A. E., Mohamed, H. A., Metwally, M. M. M., Said, M. A., Nassan, M. A., Dahran, N., & Mohamed, A. A.-R. (2022). Concerns with Male Infertility Induced by Exposure to Titanium Nanoparticles and the Supporting Impact of Pelargonium graveolens Essential Oil: Morphometric Records in Male-Wistar Rats. Life, 12(5), 639. https://doi.org/10.3390/life12050639






