Spleen: Reparative Regeneration and Influence on Liver
Abstract
1. Introduction
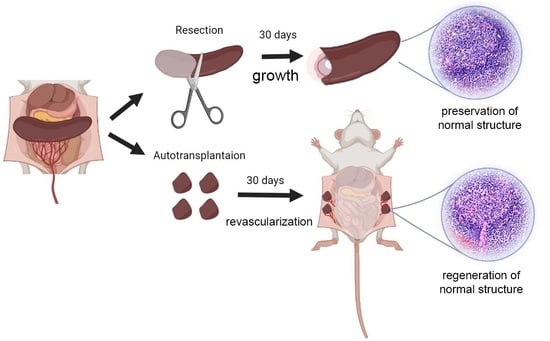
2. Splenic Regeneration after Resections
| Authors | Volume of Resection | Regeneration Period | Antibody Production | T Cell Activity | Other | |
|---|---|---|---|---|---|---|
| 1. | L. D. Liozner and Kharlova [24] | 50% | 30 days | Decreased production of antibodies | Decreased T cell activity | The observed functional decline was explained by the enrichment with immature, functionally compromised lymphocytes in the course of regeneration |
| 2. | Cameron and Rhee [23] | 50% | 30 days | |||
| 3. | Macka and Scott Polland [25] | 50% | 30 days | An increase in the density of lymphoid follicles and their relative area | ||
| 4. | Kharlova [22] | 90% | 38 days | Slower recovery of T cells compared to B lymphocytes | ||
| 5. | Pouché et al. [26] | 50% | 90 days | The results of histologic study demonstrate a readjustment of the vascular net and the lymphoid tissue of the white pulp |
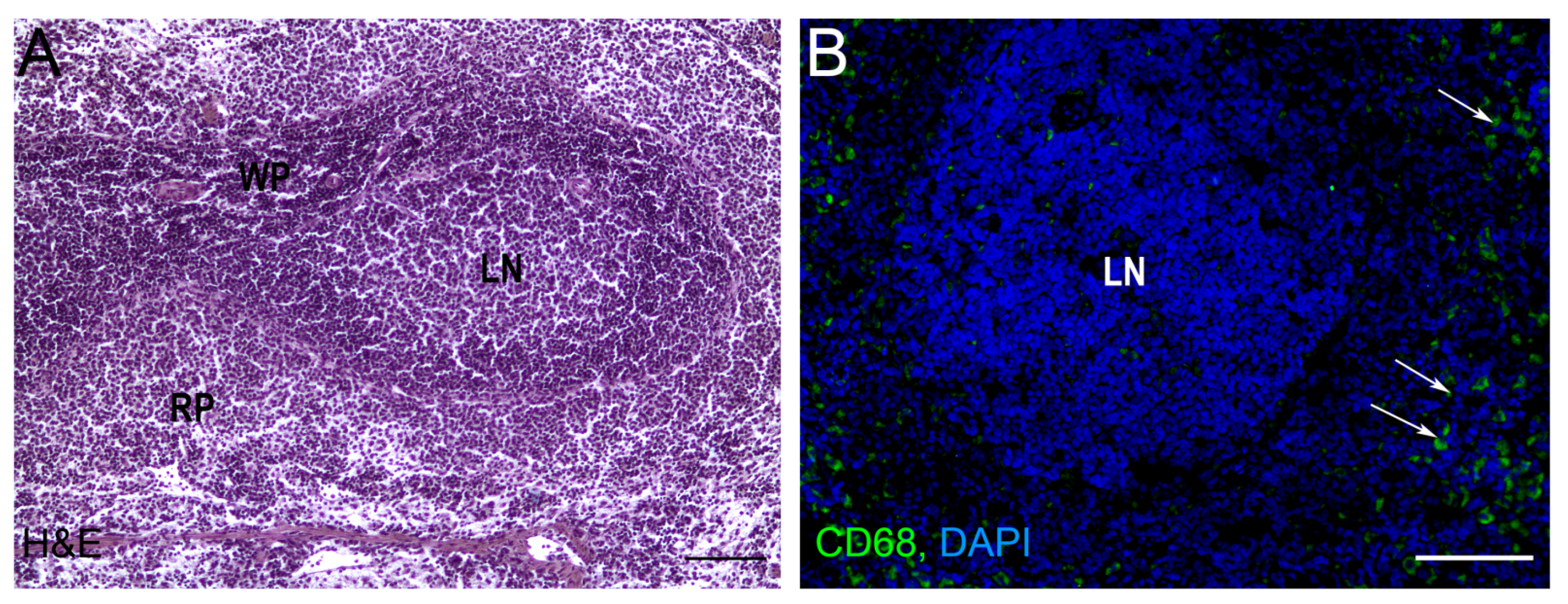
3. Autotransplantations of the Spleen
| Authors | Animal, Autograft Localization | Regeneration Period | The Effect of Autotransplantation | |
|---|---|---|---|---|
| 1. | Manley and Marine [34,35] | Rabbit, subcutaneous | 80 days | |
| 2. | Perla [36] | Rat, abdomen wall | 12–21 days | |
| 3. | Calder and Scholar [33] | Rat, mouse, omentum | 30 days | |
| 4. | Cameron and Rhee [23] | Rat, mouse, omentum | 60 days | |
| 5. | Braga et al. [37] | Rat, mesenterium | 60 days | |
| 6. | Han et al. [47] | Rat, liver lobe | 35 days | |
| 7. | Han et al. [47] | Rat, mesenterium | 84 days | |
| 8. | Miko et al. [52] | Mouse, omentum | 42 days | Clearance of senescent erythrocytes from the blood, decreased platelet count and fibrinogen levels, recovery of IgM levels, a numbers of the circulating CD3+ T and CD19+ B cells remained reduced |
| 9. | Sipka et al. [53] | Mouse, omentum | Clearance of senescent erythrocytes from the blood, decreased platelet count and fibrinogen levels | |
| 10. | Patel et al. [57] | Rat, omentum | Anti-pneumococcal defense | |
| 11. | Leemans et al. [40] | Rat, omentum | Spleen autotransplants improve humoral response to pneumococcal capsular polysaccharides | |
| 12. | Marques et al. [38,63] | Rat, omentum | Efficient clearance of E. coli and pneumococci | |
| 13. | Teixeira [64] | Mouse, retroperitoneum | Production of high titers of S. aureus-specific IgM and IgG1 |
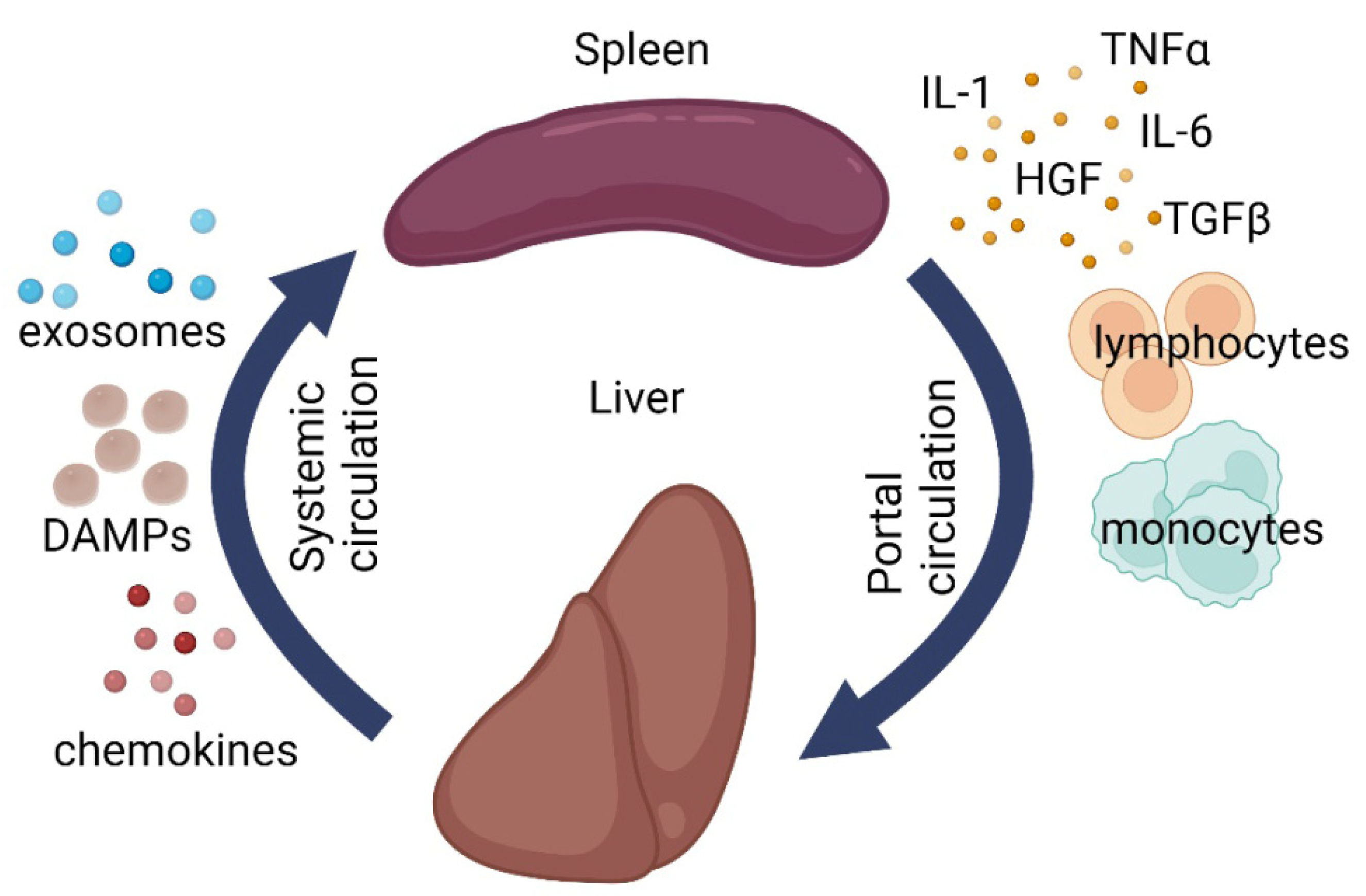
4. Splenic Influence on Repair Processes in the Liver
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lewis, S.M.; Williams, A.; Eisenbarth, S.C. Structure and function of the immune system in the spleen. Sci. Immunol. 2019, 4, eaau6085. [Google Scholar] [CrossRef] [PubMed]
- Delves, J.P. (Ed.) Encyclopedia of Immunology, 2nd ed; Academic Press: Cambridge, MA, USA, 1998; ISBN 978-0-12-226765-9. [Google Scholar]
- Cesta, M.F. Normal Structure, Function, and Histology of the Spleen. Toxicol. Pathol. 2006, 34, 455–465. [Google Scholar] [CrossRef]
- Mebius, R.E.; Kraal, G. Structure and function of the spleen. Nat. Rev. Immunol. 2005, 5, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Buzelé, R.; Barbier, L.; Sauvanet, A.; Fantin, B. Medical complications following splenectomy. J. Visc. Surg. 2016, 153, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Kristinsson, S.Y.; Gridley, G.; Hoover, R.N.; Check, D.; Landgren, O. Long-term risks after splenectomy among 8,149 cancer-free American veterans: A Cohort Study with up to 27 Years Follow-up. Haematologica 2014, 99, 392. [Google Scholar] [CrossRef]
- Fouquet, G.; Larroche, C.; Carpentier, B.; Terriou, L.; Urbanski, G.; Lacout, C.; Lazaro, E.; Salmon Gandonnière, C.; Perlat, A.; Lifermann, F.; et al. Splenectomy for haemophagocytic lymphohistiocytosis of unknown origin: Risks and Benefits in 21 Patients. Br. J. Haematol. 2021, 194, 638–642. [Google Scholar] [CrossRef]
- Bonnet-Gajdos, M.; Berger, J.P.; Gerota, I.; Vergoz, D.; Ferrer, M.; Gruner, M.L. Asplenia: A Study of 21 Subjects Splenectomized During Childhood for Spleen Injury (author’s transl). La Nouv. Press. Médicale 1981, 10, 313–316. [Google Scholar]
- Sinwar, P.D. Overwhelming post splenectomy infection syndrome—Review study. Int. J. Surg. 2014, 12, 1314–1316. [Google Scholar] [CrossRef]
- King, H.; Shumacker, H.B. Splenic studies. I. Susceptibility to infection after splenectomy performed in infancy. Ann. Surg. 1952, 136, 239–242. [Google Scholar] [CrossRef]
- Bisharat, N.; Omari, H.; Lavi, I.; Raz, R. Risk of infection and death among post-splenectomy patients. J. Infect. 2001, 43, 182–186. [Google Scholar] [CrossRef]
- Morozov, D.A.; Klyuev, S.A. Hyposlenism After Splenectomy. Ann. Russ. Acad. Med. Sci. 2015, 70, 413–418. [Google Scholar] [CrossRef]
- Luu, S.; Spelman, D.; Woolley, I.J. Post-splenectomy sepsis: Preventative Strategies, Challenges, and Solutions. Infect. Drug Resist. 2019, 12, 2839–2851. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.G.; Cheong, J.H.; Hyung, W.J.; Kim, J.; Choi, S.H.; Noh, S.H. Adverse effect of splenectomy on recurrence in total gastrectomy cancer patients with perioperative transfusion. Am. J. Surg. 2006, 192, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Mellemkjøer, L.; Olsen, J.R.H.; Linef, M.S.; Gridley, G.; McLaughlin, J.K. Cancer Risk after Splenectomy. Cancer 1995, 75, 577–583. [Google Scholar] [CrossRef]
- Arlashkina, O.M.; Struchko, G.Y.; Merkulova, L.M.; Mikhailova, L.P. Changes in the white pulp of the spleen in the offspring of splenectomy rats of different age periods after the administration of 1,2-dimethylhydrazine. Clin. Exp. Morphol. 2019, 8, 48–59. [Google Scholar] [CrossRef]
- Spangler, W.L.; Kass, P.H. Pathologic Factors Affecting Postsplenectomy Survival in Dogs. J. Vet. Intern. Med. 1997, 11, 166–171. [Google Scholar] [CrossRef]
- Knowles, D.P.; Sears, K.P.; Knowles, D.P.; Fry, L.M. Clinical Progression of Theileria haneyi in Splenectomized Horses Reveals Decreased Virulence Compared to Theileria equi. Pathogens 2022, 11, 254. [Google Scholar] [CrossRef]
- Thompson, J.R.; Kersting, K.W.; Wass, W.M.; Davis, I.A. Splenectomy in cattle via transthoracic approach. Am. J. Vet. Res. 1992, 53, 143–144. [Google Scholar]
- Boyce, W.L.; Wellde, B.T.; Reardon, M.J.; Bhogal, M.S.; Chumo, D.A. Effects of splenectomy on Trypanosoma congolense infection in cattle. Ann. Trop. Med. Parasitol. 1989, 83, 195–200. [Google Scholar] [CrossRef]
- Liozner, L. Organ Regeneration: A Study of Developmental Biology in Mammals (Studies in Soviet Science), 1st ed.; Liozner, L.D., Ed.; Springer: New York, NY, USA, 1974; ISBN 978-1-4684-8458-8. [Google Scholar]
- Kharlova, G.V. Regeneration of Lymphoid Organs in Mammals; Meditsina: Moscow, Russia, 1975. [Google Scholar]
- Cameron, G.R.; Rhee, K.-S. Compensatory hypertrophy of the spleen: A Study of Splenic Growth. J. Pathol. Bacteriol. 1959, 78, 335–349. [Google Scholar] [CrossRef]
- Liozner, L.D.; Kharlova, G.V. Regeneration of the spleen in mice after removal of a large part of the organ. Biull. Eksp. Biol. Med. 1960, 49, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Macka, E.M.; Scott Polland, W. Comensatory Hypertrophy of the spleen. J. Exp. Med. 1931, 53, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Pouche, A.; Chiodera, P.; Bosio, P.; Allevi, G.; Tiberio, G. Experimental splenic regeneration. Surg. Gynecol. Obstet. 1986, 162, 25–28. [Google Scholar] [PubMed]
- Sukhova, G.K.; Podrabinek, T.R.; Kharlova, G.V. The influence of the regenerating hemopoietic organs on the number and type of splenic colonies. Byulleten Eksp. Biol. I Meditsiny 1978, 85, 219–221. [Google Scholar]
- Kharlova, G.V. Antibody formation processes in the regenerated spleen of intact and thymectomy mice. Byulleten Eksp. Biol. I Meditsiny 1971, 72, 52–54. [Google Scholar]
- Mezhlumian, A.A. On stimulation of spleen regeneration in rabbits. Biull. Eksp. Biol. Med. 1964, 57, 103–106. [Google Scholar]
- Fremont, R.D.; Rice, T.W. Splenosis: A Review. South. Med. J. 2007, 100, 589–593. [Google Scholar] [CrossRef]
- Smoot, T.; Revels, J.; Soliman, M.; Liu, P.; Menias, C.O.; Hussain, H.H.; Savas, H.; Gaballah, A.H. Abdominal and pelvic splenosis: Atypical Findings, Pitfalls, and Mimics. Abdom. Radiol. 2022, 47, 923–947. [Google Scholar] [CrossRef]
- Dijkstra, C.D.; Langevoort, H.L. Regeneration of splenic tissue after autologous subcutaneous implantation: Development of Non-lymphoid Cells in the White Pulp of the Rat Spleen. Cell Tissue Res. 1982, 222, 69–79. [Google Scholar] [CrossRef]
- Calder, R.M.; Scholar, G. Autoplastic splenic grafts: Their Use in the Study of the Growth of Splenic Tissue. J. Pathol. Bacteriol. 1939, 49, 351–362. [Google Scholar] [CrossRef]
- Manley, O.T.; Marine, D. The Transplantation of splenic tissue into the subcutaneous fascia of the abdomen in rabbits. J. Exp. Med. 1917, 25, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Marine, D.; Manley, O.T. Homeotransplantation and Autotransplantation of the Spleen in Rabbits: III. Further Data on Growth, Permanence, Effect of Age, and Partial or Complete Removal of the Spleen J. Exp. Med. 1920, 32, 113–133. [Google Scholar] [CrossRef] [PubMed]
- Perla, D. The Regeneration of Autoplastic Splenic Transplants. Am. J. Pathol. 1936, 12, 665. [Google Scholar] [PubMed]
- Braga, A.A.; Malagó, R.; Anacleto, T.P.; da Silva, C.R.N.; Andreollo, N.A.; Fernandes, F.L.F. Histological aspects of autologous transplantation of different fragments of the spleen in rats. Acta Cir. Bras. 2012, 27, 880–884. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Marques, R.G.; Petroianu, A.; Coelho, J.M.C.O.; Portela, M.C. Regeneration of splenic autotransplants. Ann. Hematol. 2002, 81, 622–626. [Google Scholar] [CrossRef]
- Westermann, J.; Peschel, P.; Pabst, R. Immunoarchitecture of regenerated splenic transplants: Influence of Donor and Host Age on the Regeneration of Splenic Compartments. Cell Tissue Res. 1988, 254, 403–413. [Google Scholar] [CrossRef]
- Leemans, R.; Harms, G.; Rijkers, G.T.; Timens, W. Spleen autotransplantation provides restoration of functional splenic lymphoid compartments and improves the humoral immune response to pneumococcal polysaccharide vaccine. Clin. Exp. Immunol. 1999, 117, 596–604. [Google Scholar] [CrossRef]
- Westermann, J.; Michel, S.; Lopez-Kostka, S.; Bode, U.; Rothkötter, H.J.; Bette, M.; Weihe, E.; Straub, R.H.; Pabst, R. Regeneration of implanted splenic tissue in the rat: Reinnervation is Host Age-Dependent and Necessary for Tissue Development. J. Neuroimmunol. 1998, 88, 67–76. [Google Scholar] [CrossRef]
- Westermann, J.; Willführ, K.U.; Pabst, R. Influence of donor and host age on the regeneration and blood flow of splenic transplants. J. Pediatr. Surg. 1988, 23, 835–838. [Google Scholar] [CrossRef]
- Metcalf, D. Spleen graft growth in splenectomised mice. Aust. J. Exp. Biol. Med. Sci. 1963, 41, 51–60. [Google Scholar] [CrossRef]
- Malagó, R.; Reis, N.S.; Araújo, M.R.; Andreollo, N.A. Late histological aspects of spleen autologous transplantation in rats. Acta Cir. Bras. 2008, 23, 274–281. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Miko, I.; Brath, E.; Furka, I.; Kovacs, J.; Kelvin, D.; Zhong, R. Spleen autotransplantation in mice: A Novel Experimental Model for immunology study. Microsurgery 2001, 21, 140–142. [Google Scholar] [CrossRef] [PubMed]
- Di Carlo, I.; Pulvirenti, E.; Toro, A. A new technique for spleen autotransplantation. Surg. Innov. 2012, 19, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Meng, B.; Cui, G.; Wu, Z.; Yu, L.; Zhu, H.; Ma, H.; Shi, J.; Lv, Y. Regeneration of Splenic Autotransplants Attached on Liver by a Tissue Adhesive. Transplant. Proc. 2010, 42, 1944–1948. [Google Scholar] [CrossRef] [PubMed]
- Alves, H.J.; Viana, G.; Magalha, N.M.; Arantes, R.M.E.; Coelho, P.M.Z.; Cunha-melo, R. Kinetics of neovascularisation of splenic autotransplants in mice. J. Anat. 1999, 195, 387–392. [Google Scholar] [CrossRef]
- Holdsworth, R.J. Regeneration of the spleen and splenic autotransplantation. Br. J. Surg. 1991, 78, 270–278. [Google Scholar] [CrossRef]
- Iinuma, H.; Okinaga, K.; Sato, S.; Tomioka, M.; Matsumoto, K. Optimal site and amount of splenic tissue for autotransplantation. J. Surg. Res. 1992, 53, 109–116. [Google Scholar] [CrossRef]
- Jacob, H.S.; Macdonald, R.A.; Jandl, J.H. Regulation OF Spleen Growth and Sequestering Function. J. Clin. Investig. 1963, 42, 1476. [Google Scholar] [CrossRef]
- Miko, I.; Brath, E.; Nemeth, N.; Toth, F.F.; Sipka, S.; Kovacs, J.; Sipka, S.; Fachet, J.; Furka, A.; Furka, I.; et al. Hematological, hemorheological, immunological, and morphological studies of spleen autotransplantation in mice: Preliminary Results. Microsurgery 2003, 23, 483–488. [Google Scholar] [CrossRef]
- Sipka, S.; Brath, E.; Toth, F.F.; Fabian, A.; Krizsan, C.; Barath, S.; Sipka, S.; Nemeth, N.; Balint, A.; Furka, I.; et al. Distribution of peripheral blood cells in mice after splenectomy or autotransplantation. Microsurgery 2006, 26, 43–49. [Google Scholar] [CrossRef]
- Pabst, R. Regeneration of autotransplanted splenic fragments: Basic Immunological and Clinical Relevance. Clin. Exp. Immunol. 1999, 117, 423. [Google Scholar] [CrossRef] [PubMed]
- Pabst, R.; Westermann, J.; RothköTter, H.J. Immunoarchitecture of regenerated splenic and lymph node transplants. Int. Rev. Cytol. 1991, 128, 215–260. [Google Scholar] [CrossRef] [PubMed]
- Riera, M.; Buczacki, S.; Khan, Z.A.J. Splenic regeneration following splenectomy and impact on sepsis: A Clinical Review. J. R. Soc. Med. 2009, 102, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.; Williams, J.S.; Naim, J.O.; Hinshaw, J.R. Protection against pneumococcal sepsis in splenectomized rats by implantation of splenic tissue into an omental pouch. Curr. Surg. 1981, 38, 323–325. [Google Scholar]
- Patel, J.; Williams, J.S.; Naim, J.O.; Hinshaw, J.R. Protection against pneumococcal sepsis in splenectomized rats by implantation of splenic tissue into an omental pouch. Surgery 1982, 91, 638–641. [Google Scholar] [CrossRef]
- Steely, W.M.; Satava, R.M.; Harris, R.W.; Quispe, G. Comparison of omental splenic autotransplant to partial splenectomy. Protective effect against septic death. Am. Surg. 1987, 53, 702–705. [Google Scholar]
- Likhite, V.V. Protection against fulminant sepsis in splenectomized mice by implantation of autochthonous splenic tissue. Exp. Hematol. 1978, 6, 433–439. [Google Scholar]
- Sipka, S.; Bráth, E.; Tóth, F.F.; Aleksza, M.; Kulcsár, A.; Fábián, Á.; Baráth, S.; Balogh, P.; Sipka, S.; Furka, I.; et al. Cellular and serological changes in the peripheral blood of splenectomized and spleen autotransplanted mice. Transpl. Immunol. 2006, 16, 99–104. [Google Scholar] [CrossRef]
- Miko, I.; Brath, E.; Nemeth, N.; Furka, A.; Sipka, S.; Peto, K.; Serfozo, J.; Kovacs, J.; Imre, S.; Benko, I.; et al. Spleen autotransplantation. Morphological and functional follow-up after spleen autotransplantation in mice: A Research Summary. Microsurgery 2007, 27, 312–316. [Google Scholar] [CrossRef]
- Marques, R.G.; Caetano, C.E.R.; Diestel, C.F.; Lima, E.; Portela, M.C.; Oliveira, A.V.; Oliveira, M.B.N.; Bernardo-Filho, M. Critical mass of splenic autotransplant needed for the development of phagocytic activity in rats. Clin. Exp. Immunol. 2012, 170, 77–85. [Google Scholar] [CrossRef]
- Teixeira, F.M.; Fernandes, B.F.; Rezende, A.B.; Machado, R.R.P.; Alves, C.C.S.; Perobelli, S.M.; Nunes, S.I.; Farias, R.E.; Rodrigues, M.F.; Ferreira, A.P.; et al. Staphylococcus aureus infection after splenectomy and splenic autotransplantation in BALB/c mice. Clin. Exp. Immunol. 2008, 154, 255–263. [Google Scholar] [CrossRef]
- Surendran, A.; Smith, M.; Houli, N.; Usatoff, V.; Spelman, D.; Choi, J. Splenic autotransplantation: A Systematic Review. ANZ J. Surg. 2020, 90, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.K.H.; Watanabe, T. Determinants of postnatal spleen tissue regeneration and organogenesis. NPJ Regen. Med. 2018, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Brendolan, A.; Rosado, M.M.; Carsetti, R.; Selleri, L.; Dear, T.N. Development and function of the mammalian spleen. BioEssays 2007, 29, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Short, C.; Lim, H.K.; Tan, J.; O’Neill, H.C. Targeting the Spleen as an Alternative Site for Hematopoiesis. Bioessays 2019, 41, 1800234. [Google Scholar] [CrossRef]
- Cao, Y.A.; Wagers, A.J.; Beilhack, A.; Dusich, J.; Bachmann, M.H.; Negrin, R.S.; Weissman, I.L.; Contag, C.H. Shifting foci of hematopoiesis during reconstitution from single stem cells. Proc. Natl. Acad. Sci. USA 2004, 101, 221. [Google Scholar] [CrossRef]
- Kiel, M.J.; Yilmaz, Ö.H.; Iwashita, T.; Yilmaz, O.H.; Terhorst, C.; Morrison, S.J. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell 2005, 121, 1109–1121. [Google Scholar] [CrossRef]
- Sugiyama, T.; Kohara, H.; Noda, M.; Nagasawa, T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity 2006, 25, 977–988. [Google Scholar] [CrossRef]
- Inra, C.N.; Zhou, B.O.; Acar, M.; Murphy, M.M.; Richardson, J.; Zhao, Z.; Morrison, S.J. A perisinusoidal niche for extramedullary haematopoiesis in the spleen. Nature 2015, 527, 466–471. [Google Scholar] [CrossRef]
- O’Neill, H.C.; Lim, H.K.; Periasamy, P.; Kumarappan, L.; Tan, J.K.H.; O’Neill, T.J. Transplanted spleen stromal cells with osteogenic potential support ectopic myelopoiesis. PLoS ONE 2019, 14, e0223416. [Google Scholar] [CrossRef]
- Tan, J.K.H.; Watanabe, T. Murine Spleen Tissue Regeneration from Neonatal Spleen Capsule Requires Lymphotoxin Priming of Stromal Cells. J. Immunol. 2014, 193, 1194–1203. [Google Scholar] [CrossRef] [PubMed]
- Glanville, S.H.; Bekiaris, V.; Jenkinson, E.J.; Lane, P.J.L.; Anderson, G.; Withers, D.R. Transplantation of embryonic spleen tissue reveals a role for adult non-lymphoid cells in initiating lymphoid tissue organization. Eur. J. Immunol. 2009, 39, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.K.H.; Watanabe, T. Stromal Cell Subsets Directing Neonatal Spleen Regeneration. Sci. Rep. 2017, 7, 40401. [Google Scholar] [CrossRef]
- Li, L.; Duan, M.; Chen, W.; Jiang, A.; Li, X.; Yang, J.; Li, Z. The spleen in liver cirrhosis: Revisiting an Old Enemy with Novel Targets. J. Transl. Med. 2017, 15, 111. [Google Scholar] [CrossRef]
- Tarantino, G.; Scalera, A.; Finelli, C. Liver-spleen axis: Intersection between Immunity, Infections and Metabolism. World J. Gastroenterol. 2013, 19, 3534. [Google Scholar] [CrossRef]
- Babaeva, A.G. Cellular and humoral immunity factors as regulators of regenerative morphogenesis. Ontogenez 1989, 20, 453–460. [Google Scholar] [PubMed]
- Babaeva, A.G. Lymphocytes as regulators of cell proliferation and differentiation of non-lymphoid organs. Vestn. Akad. Med. Nauk SSSR 1990, 43–45, 2356655. [Google Scholar]
- Babaeva, A.G.; Druzhkova, T.A.; Yudina, N.V.; Gimmelpharb, E.I.; Medvedev, A. Lymphoid cell-derived humoral factors as possible mediators in regeneration information transfer. Monogr. Dev. Biol. 1992, 23, 223–229. [Google Scholar]
- Yamada, S.; Morine, Y.; Imura, S.; Ikemoto, T.; Arakawa, Y.; Iwahashi, S.; Saito, Y.; Yoshikawa, M.; Teraoku, H.; Shimada, M. Liver regeneration after splenectomy in patients with liver cirrhosis. Hepatol. Res. 2016, 46, 443–449. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Jin, Y.; Zhao, L.; Zhao, F.; Feng, J.; Li, A.; Wei, Y. Splenectomy Leads to Amelioration of Altered Gut Microbiota and Metabolome in Liver Cirrhosis Patients. Front. Microbiol. 2018, 9, 963. [Google Scholar] [CrossRef]
- Romanelli, R.G.; Stasi, C. Recent Advancements in Diagnosis and Therapy of Liver Cirrhosis. Curr. Drug Targets 2016, 17, 1804–1817. [Google Scholar] [CrossRef]
- Higashi, T.; Friedman, S.L.; Hoshida, Y. Hepatic stellate cells as key target in liver fibrosis. Adv. Drug Deliv. Rev. 2017, 121, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Akahoshi, T.; Hashizume, M.; Tanoue, K.; Shimabukuro, R.; Gotoh, N.; Tomikawa, M.; Sugimachi, K. Role of the spleen in liver fibrosis in rats may be mediated by transforming growth factor beta-1. J. Gastroenterol. Hepatol. 2002, 17, 59–65. [Google Scholar] [CrossRef]
- Asanoma, M.; Ikemoto, T.; Mori, H.; Utsunomiya, T.; Imura, S.; Morine, Y.; Iwahashi, S.; Saito, Y.; Yamada, S.; Shimada, M. Cytokine expression in spleen affects progression of liver cirrhosis through liver-spleen cross-talk. Hepatol. Res. 2014, 44, 1217–1223. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Guo, X.; Jiao, G.; Luo, L.; Zhou, L.; Zhang, J.; Wang, B. Splenectomy Promotes Macrophage Polarization in a Mouse Model of Concanavalin A- (ConA-) Induced Liver Fibrosis. BioMed Res. Int. 2019, 2019, 5756189. [Google Scholar] [CrossRef] [PubMed]
- Yada, A.; Iimuro, Y.; Uyama, N.; Uda, Y.; Okada, T.; Fujimoto, J. Splenectomy attenuates murine liver fibrosis with hypersplenism stimulating hepatic accumulation of Ly-6Clo macrophages. J. Hepatol. 2015, 63, 905–916. [Google Scholar] [CrossRef]
- Murata, K.; Shiraki, K.; Sugimoto, K.; Takase, K.; Nakano, T.; Furusaka, A.; Tameda, Y. Splenectomy enhances liver regeneration through tumor necrosis factor (TNF)-alpha following dimethylnitrosamine-induced cirrhotic rat model. Hepatogastroenterology 2001, 48, 1022–1027. [Google Scholar]
- Elchaninov, A.V.; Fatkhudinov, T.K.; Vishnyakova, P.A.; Nikitina, M.P.; Lokhonina, A.V.; Makarov, A.V.; Arutyunyan, I.V.; Kananykhina, E.Y.; Poltavets, A.S.; Butov, K.R.; et al. Molecular mechanisms of splenectomy-induced hepatocyte proliferation. PLoS ONE 2020, 15, e0233767. [Google Scholar] [CrossRef]
- Swirski, F.K.; Nahrendorf, M.; Etzrodt, M.; Wildgruber, M.; Cortez-Retamozo, V.; Panizzi, P.; Figueiredo, J.-L.; Kohler, R.H.; Chudnovskiy, A.; Waterman, P.; et al. Identification of Splenic Reservoir Monocytes and Their Deployment to Inflammatory Sites. Science 2009, 325, 612–616. [Google Scholar] [CrossRef]
- Kim, E.; Yang, J.; Beltran, C.D.; Cho, S. Role of spleen-derived monocytes/macrophages in acute ischemic brain injury. J. Cereb. Blood Flow Metab. 2014, 34, 1411–1419. [Google Scholar] [CrossRef]
- Blomster, L.; Brennan, F.; Lao, H.; Harle, D.; Harvey, A.; Ruitenberg, M. Mobilisation of the splenic monocyte reservoir and peripheral CX3CR1 deficiency adversely affects recovery from spinal cord injury. Exp. Neurol. 2013, 247, 226–240. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, G.; Maggio, R.D.; Benedetti, A.; Morroni, J.; Bouche, M.; Lozanoska-Ochser, B. Splenic Ly6Chi monocytes are critical players in dystrophic muscle injury and repair. JCI Insight 2020, 5, e130807. [Google Scholar] [CrossRef] [PubMed]
- Shand, F.; Ueha, S.; Otsuji, M.; Koid, S.; Shichino, S.; Tsukui, T.; Kosugi-Kanaya, M.; Abe, J.; Tomura, M.; Ziogas, J.; et al. Tracking of intertissue migration reveals the origins of tumor-infiltrating monocytes. Proc. Natl. Acad. Sci. USA. 2014, 111, 7771–7776. [Google Scholar] [CrossRef] [PubMed]
- Zigmond, E.; Samia-Grinberg, S.; Pasmanik-Chor, M.; Brazowski, E.; Shibolet, O.; Halpern, Z.; Varol, C. Infiltrating Monocyte-Derived Macrophages and Resident Kupffer Cells Display Different Ontogeny and Functions in Acute Liver Injury. J. Immunol. 2014, 193, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Elchaninov, A.; Nikitina, M.; Vishnyakova, P.; Lokhonina, A.; Makarov, A.; Sukhikh, G.; Fatkhudinov, T. Macro- and microtranscriptomic evidence of the monocyte recruitment to regenerating liver after partial hepatectomy in mouse model. Biomed. Pharmacother. 2021, 138, 111516. [Google Scholar] [CrossRef]
- Arutyunyan, I.; Elchaninov, A.; Fatkhudinov, T.; Makarov, A.; Kananykhina, E.; Usman, N.; Bolshakova, G.; Glinkina, V.; Goldshtein, D.; Sukhikh, G. Elimination of allogeneic multipotent stromal cells by host macrophages in different models of regeneration. Int. J. Clin. Exp. Pathol. 2015, 8, 4469–4480. [Google Scholar]
- Tanabe, K.; Taura, K.; Koyama, Y.; Yamamoto, G.; Nishio, T.; Okuda, Y.; Nakamura, K.; Toriguchi, K.; Takemoto, K.; Yamanaka, K.; et al. Migration of splenic lymphocytes promotes liver fibrosis through modification of T helper cytokine balance in mice. J. Gastroenterol. 2015, 50, 1054–1068. [Google Scholar] [CrossRef]
- Marquez-Medina, D.; Salla-Fortuny, J.; Salud-Salvia, A. Role of gamma-delta T-cells in cancer: Another Opening Door to Immunotherapy. Clin. Transl. Oncol. 2012, 14, 891–895. [Google Scholar] [CrossRef]
- Bi, J.; Zheng, X.; Chen, Y.; Wei, H.; Sun, R.; Tian, Z. TIGIT safeguards liver regeneration through regulating natural killer cell-hepatocyte crosstalk. Hepatology 2014, 60, 1389–1398. [Google Scholar] [CrossRef]
- Huang, W.; Dong, Z.; Wei, H.; Ding, C.; Sun, R.; Tian, Z. Selective elimination of hepatic natural killer T cells with concanavalin A improves liver regeneration in mice. Liver Int. 2006, 26, 339–345. [Google Scholar] [CrossRef]
- Godfrey, D.I.; Stankovic, S.; Baxter, A.G. Raising the NKT cell family. Nat. Immunol. 2010, 11, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Hua, J. Immune cells in liver regeneration. Oncotarget 2017, 8, 3628–3639. [Google Scholar] [CrossRef]
- Ito, K.; Ozasa, H.; Horikawa, S. Effects of prior splenectomy on remnant liver after partial hepatectomy with Pringle maneuver in rats. Liver Int. 2005, 25, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, Y.; Shimada, M.; Utsunomya, T.; Imura, S.; Morine, Y.; Ikemoto, T.; Takasu, C. Effects of splenectomy on hepatic gene expression profiles after massive hepatectomy in rats. J. Gastroenterol. Hepatol. 2013, 28, 1669–1677. [Google Scholar] [CrossRef]
- Kim, J.; Kim, C.-J.; Ko, I.-G.; Joo, S.H.; Ahn, H.J. Splenectomy affects the balance between hepatic growth factor and transforming growth factor-β and its effect on liver regeneration is dependent on the amount of liver resection in rats. J. Korean Surg. Soc. 2012, 82, 238. [Google Scholar] [CrossRef] [PubMed]
- Ueda, S.; Yamanoi, A.; Hishikawa, Y.; Dhar, D.K.; Tachibana, M.; Nagasue, N. Transforming growth factor-beta1 released from the spleen exerts a growth inhibitory effect on liver regeneration in rats. Lab. Investig. 2003, 83, 1595–1603. [Google Scholar] [CrossRef][Green Version]
- Morinaga, A.; Ogata, T.; Kage, M.; Kinoshita, H.; Aoyagi, S. Comparison of liver regeneration after a splenectomy and splenic artery ligation in a dimethylnitrosamine-induced cirrhotic rat model. HPB 2010, 12, 22–30. [Google Scholar] [CrossRef]
- Lee, S.C.; Jeong, H.J.; Choi, B.-J.; Kim, S.-J. Role of the spleen in liver regeneration in relation to transforming growth factor-β1 and hepatocyte growth factor. J. Surg. Res. 2015, 196, 270–277. [Google Scholar] [CrossRef]
- Tang, W.-P.; Akahoshi, T.; Piao, J.-S.; Narahara, S.; Murata, M.; Kawano, T.; Hamano, N.; Ikeda, T.; Hashizume, M. Splenectomy enhances the therapeutic effect of adipose tissue-derived mesenchymal stem cell infusion on cirrhosis rats. Liver Int. 2016, 36, 1151–1159. [Google Scholar] [CrossRef]
- Yin, S.; Wang, H.; Park, O.; Wei, W.; Shen, J.; Gao, B. Enhanced liver regeneration in IL-10-deficient mice after partial hepatectomy via stimulating inflammatory response and activating hepatocyte STAT3. Am. J. Pathol. 2011, 178, 1614–1621. [Google Scholar] [CrossRef]
- Kuriyama, N.; Isaji, S.; Kishiwada, M.; Ohsawa, I.; Hamada, T.; Mizuno, S.; Usui, M.; Sakurai, H.; Tabata, M.; Yamada, T. Dual cytoprotective effects of splenectomy for small-for-size liver transplantation in rats. Liver Transplant 2012, 18, 1361–1370. [Google Scholar] [CrossRef] [PubMed]
- Babaeva, A.G.; Zotikov, E.A. Immunology of Processes of Adaptive Growth, Proliferation and their Disorders; Medicine: Moscow, Russia, 1987. [Google Scholar]
- Rudich, N.; Zamir, G.; Pappo, O.; Shlomai, Z.; Faroja, M.; Weiss, I.D.; Wald, H.; Galun, E.; Peled, A.; Wald, O. Focal liver necrosis appears early after partial hepatectomy and is dependent on T cells and antigen delivery from the gut. Liver Int. 2009, 29, 1273–1284. [Google Scholar] [CrossRef] [PubMed]
- Fukazawa, A.; Yokoi, Y.; Kurachi, K.; Uno, A.; Suzuki, S.; Konno, H.; Nakamura, S. Implication of B Lymphocytes in Endotoxin-Induced Hepatic Injury After Partial Hepatectomy in Rats. J. Surg. Res. 2007, 137, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Strick-Marchand, H.; Masse, G.X.; Weiss, M.C.; Di Santo, J.P. Lymphocytes support oval cell-dependent liver regeneration. J. Immunol. 2008, 181, 2764–2771. [Google Scholar] [CrossRef] [PubMed]
- Babaeva, A.G. Immunological Mechanisms of Regulation of Recovery Processes; Medicine: Moscow, Russia, 1972. [Google Scholar]
- Piotto, C.; Julier, Z.; Martino, M.M. Immune Regulation of Tissue Repair and Regeneration via miRNAs—New Therapeutic Target. Front. Bioeng. Biotechnol. 2018, 6, 98. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elchaninov, A.; Vishnyakova, P.; Sukhikh, G.; Fatkhudinov, T. Spleen: Reparative Regeneration and Influence on Liver. Life 2022, 12, 626. https://doi.org/10.3390/life12050626
Elchaninov A, Vishnyakova P, Sukhikh G, Fatkhudinov T. Spleen: Reparative Regeneration and Influence on Liver. Life. 2022; 12(5):626. https://doi.org/10.3390/life12050626
Chicago/Turabian StyleElchaninov, Andrey, Polina Vishnyakova, Gennady Sukhikh, and Timur Fatkhudinov. 2022. "Spleen: Reparative Regeneration and Influence on Liver" Life 12, no. 5: 626. https://doi.org/10.3390/life12050626
APA StyleElchaninov, A., Vishnyakova, P., Sukhikh, G., & Fatkhudinov, T. (2022). Spleen: Reparative Regeneration and Influence on Liver. Life, 12(5), 626. https://doi.org/10.3390/life12050626