Proteomic Analysis of Endometrial Cancer Tissues from Patients with Type 2 Diabetes Mellitus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Selection
2.2. Tissue Protein Extraction
2.3. Protein Labeling with Cyanine Dyes, 2D-DIGE, and Image Scanning
2.4. Statistical Analysis
2.5. Protein Digestion and MALDI Analysis
2.6. Bioinformatics Analysis
3. Results
3.1. Clinical and Biochemical Data
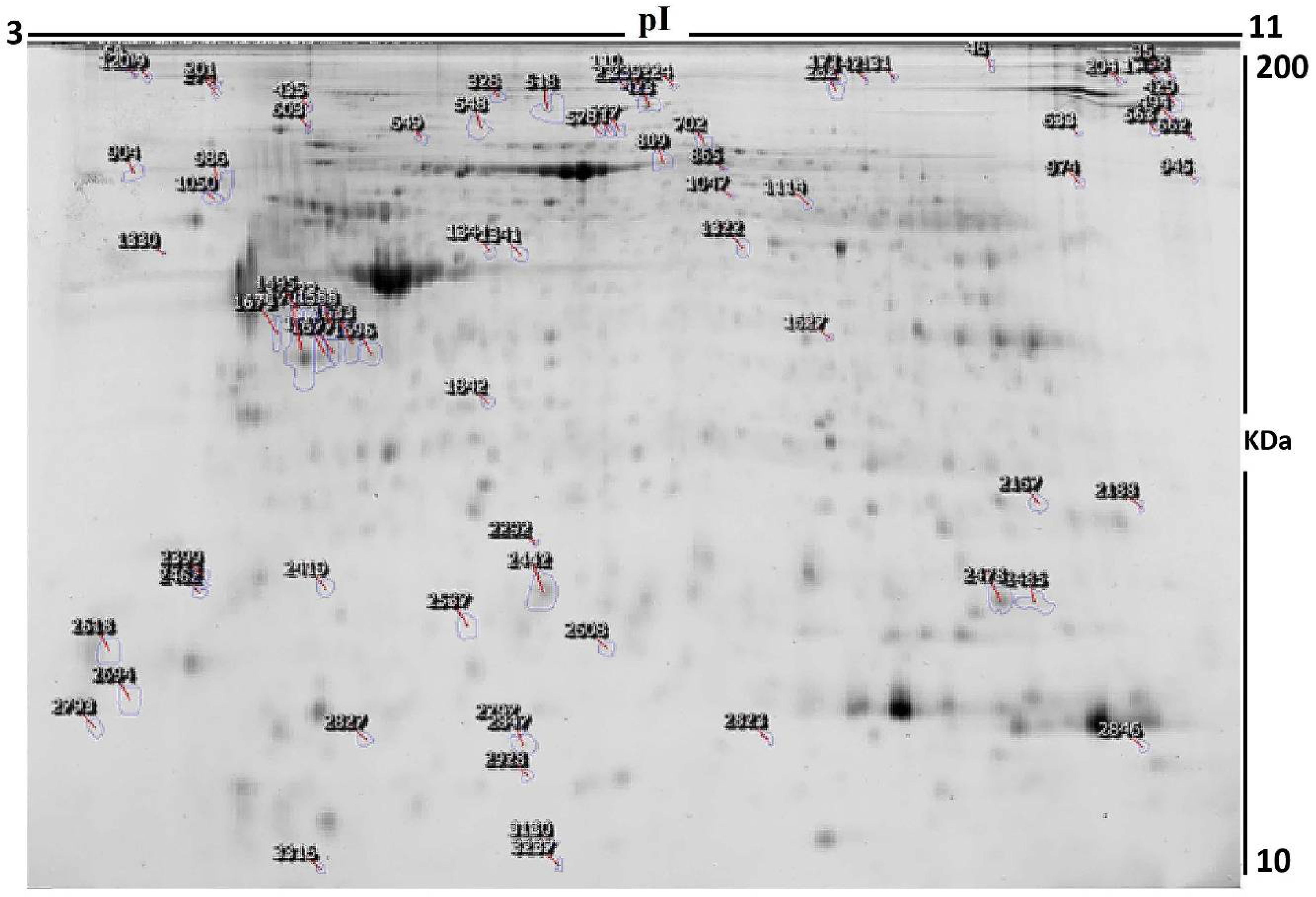
3.2. Proteomic Analysis and Identification of Differentially Expressed Proteins
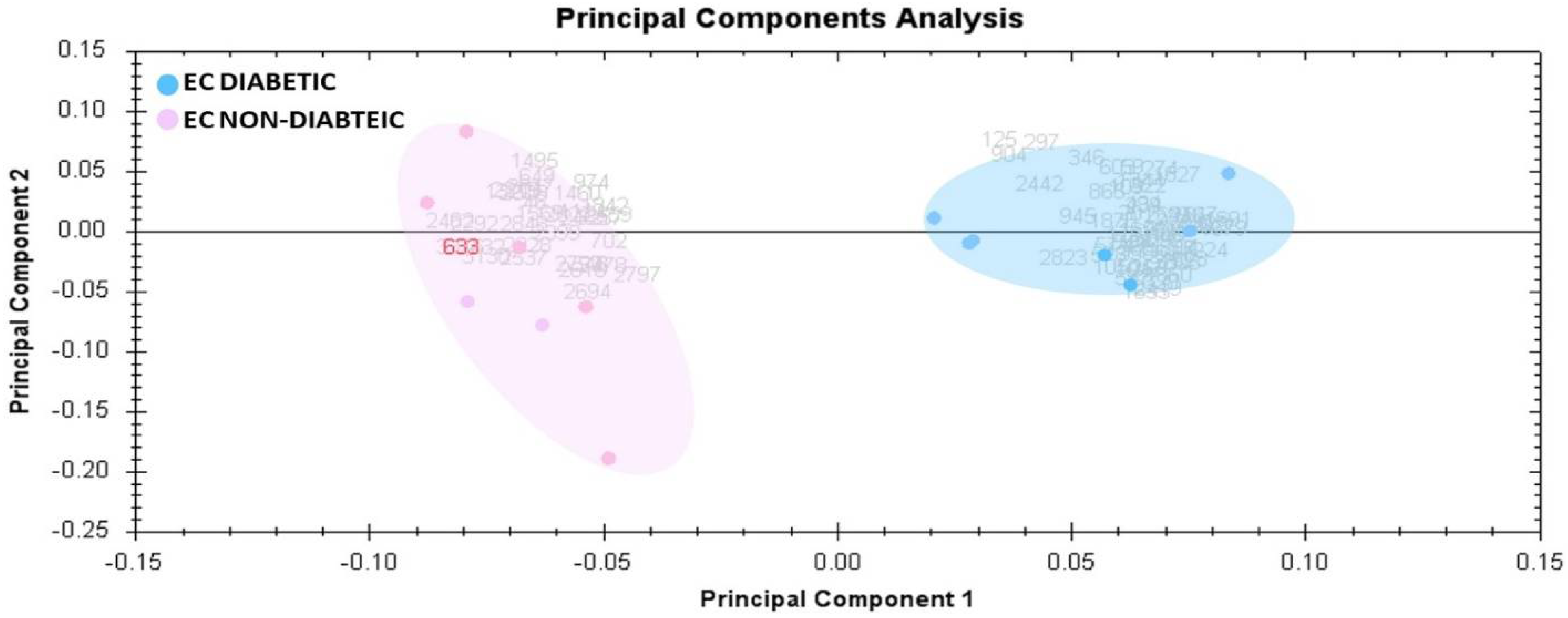
3.3. Principal Component Analysis
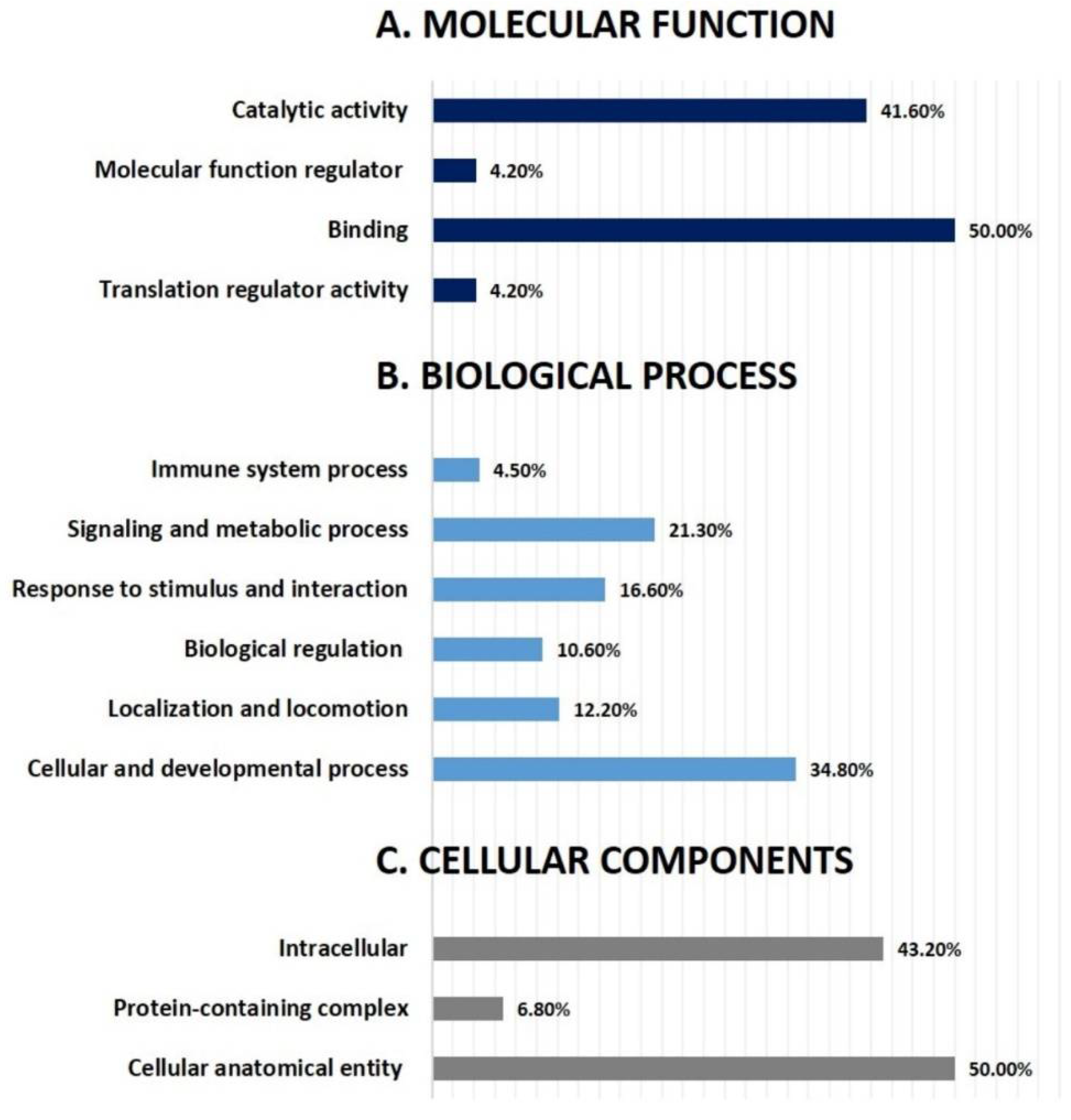
3.4. Network Pathway Analysis and Functional Classification of Proteins
4. Discussion
4.1. Proteins Associated with Cancer
4.2. Proteins Associated with Cancer Metastasis
4.3. Proteins with the Possible Interplay between Diabetes and EC
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Felix, A.S.; Brinton, L.A. Cancer Progress and Priorities: Uterine Cancer. Cancer Epidemiol. Biomark. Prev. 2018, 27, 985–994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murali, R.; Soslow, R.A.; Weigelt, B. Classification of endometrial carcinoma: More than two types. Lancet Oncol. 2014, 15, e268–e278. [Google Scholar] [CrossRef]
- American Cancer Society Endometrial Cancer—What is Endometrial Cancer; American Cancer Society: Atlanta, GA, USA, 2021; pp. 1–11.
- Chlebowski, R.T.; Anderson, G.L.; Sarto, G.E.; Haque, R.; Runowicz, C.D.; Aragaki, A.K.; Thomson, C.A.; Howard, B.V.; Wactawski-Wende, J.; Chen, C.; et al. Continuous Combined Estrogen Plus Progestin and Endometrial Cancer: The Women’s Health Initiative Randomized Trial. J. Natl. Cancer Inst. 2016, 108, djv350. [Google Scholar] [CrossRef] [Green Version]
- Giovannucci, E.; Harlan, D.M.; Archer, M.C.; Bergenstal, R.M.; Gapstur, S.M.; Habel, L.A.; Pollak, M.; Regensteiner, J.G.; Yee, D. Diabetes and cancer: A consensus report. Diabetes Care 2010, 33, 1674–1685. [Google Scholar] [CrossRef] [Green Version]
- Constantine, G.D.; Kessler, G.; Graham, S.; Goldstein, S.R. Increased Incidence of Endometrial Cancer Following the Women’s Health Initiative: An Assessment of Risk Factors. J. Womens Health 2019, 28, 237–243. [Google Scholar] [CrossRef]
- Byrne, F.L.; Martin, A.R.; Kosasih, M.; Caruana, B.T.; Farrell, R. The Role of Hyperglycemia in Endometrial Cancer Pathogenesis. Cancers 2020, 12, 1191. [Google Scholar] [CrossRef]
- Byrne, F.L.; Poon, I.K.H.; Modesitt, S.C.; Tomsig, J.L.; Chow, J.D.Y.; Healy, M.E.; Baker, W.D.; Atkins, K.A.; Lancaster, J.M.; Marchion, D.C.; et al. Metabolic vulnerabilities in endometrial cancer. Cancer Res. 2014, 74, 5832–5845. [Google Scholar] [CrossRef] [Green Version]
- Meireles, C.G.; Pereira, S.A.; Valadares, L.P.; Rêgo, D.F.; Simeoni, L.A.; Guerra, E.N.S.; Lofrano-Porto, A. Effects of metformin on endometrial cancer: Systematic review and meta-analysis. Gynecol. Oncol. 2017, 147, 167–180. [Google Scholar] [CrossRef]
- Zhou, X.; Xu, Y.; Yin, D.; Zhao, F.; Hao, Z.; Zhong, Y.; Zhang, J.; Zhang, B.; Yin, X. Type 2 diabetes mellitus facilitates endometrial hyperplasia progression by activating the proliferative function of mucin O-glycosylating enzyme GALNT2. Biomed. Pharmacother. 2020, 131, 110764. [Google Scholar] [CrossRef] [PubMed]
- Alfadda, A.A.; Benabdelkamel, H.; Masood, A.; Moustafa, A.; Sallam, R.; Bassas, A.; Duncan, M. Proteomic analysis of mature adipocytes from obese patients in relation to aging. Exp. Gerontol. 2013, 48, 1196–1203. [Google Scholar] [CrossRef] [PubMed]
- Olson, M.F.; Sahai, E. The actin cytoskeleton in cancer cell motility. Clin. Exp. Metastasis 2008, 26, 273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, F.; Hou, G.; Liu, H.; Zhang, M. Peroxiredoxin 1 promotes tumorigenesis through regulating the activity of mTOR/p70S6K pathway in esophageal squamous cell carcinoma. Med. Oncol. 2015, 32, 25. [Google Scholar] [CrossRef]
- Ding, C.; Fan, X.; Wu, G. Peroxiredoxin 1—An antioxidant enzyme in cancer. J. Cell. Mol. Med. 2017, 21, 193–202. [Google Scholar] [CrossRef]
- Gao, L.; Meng, J.; Yue, C.; Wu, X.; Su, Q.; Wu, H.; Zhang, Z.; Yu, Q.; Gao, S.; Fan, S.; et al. Integrative analysis the characterization of peroxiredoxins in pan-cancer. Cancer Cell Int. 2021, 21, 366. [Google Scholar] [CrossRef]
- Mullen, L.; Hanschmann, E.-M.; Lillig, C.H.; Herzenberg, L.A.; Ghezzi, P. Cysteine Oxidation Targets Peroxiredoxins 1 and 2 for Exosomal Release through a Novel Mechanism of Redox-Dependent Secretion. Mol. Med. 2015, 21, 98–108. [Google Scholar] [CrossRef]
- Peng, B.; Guo, C.; Guan, H.; Liu, S.; Sun, M.-Z. Annexin A5 as a potential marker in tumors. Clin. Chim. Acta 2014, 427, 42–48. [Google Scholar] [CrossRef]
- Karube, A.; Shidara, Y.; Hayasaka, K.; Maki, M.; Tanaka, T. Suppression of Calphobindin I (CPB I) Production in Carcinoma of Uterine Cervix and Endometrium. Gynecol. Oncol. 1995, 58, 295–300. [Google Scholar] [CrossRef]
- Oplawski, M.; Dziobek, K.; Zmarzły, N.; Grabarek, B.O.; Kiełbasiński, R.; Kieszkowski, P.; Januszyk, P.; Talkowski, K.; Schweizer, M.; Kras, P.; et al. Variances in the Level of COX-2 and iNOS in Different Grades of Endometrial Cancer. Curr. Pharm. Biotechnol. 2019, 21, 52–59. [Google Scholar] [CrossRef]
- Li, W.; Xu, R.; Jiang, L.; Shi, J.; Long, X.; Fan, B. Expression of cyclooxygenase-2 and inducible nitric oxide synthase correlates with tumor angiogenesis in endometrial carcinoma. Med. Oncol. 2005, 22, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Chen, L.; Taylor, R.N.; Li, C.; Zhou, X. Physiological and pathological implications of retinoid action in the endometrium. J. Endocrinol. 2018, 236, R169–R188. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, R.; Kuroda, K.; Ikemoto, Y.; Ochiai, A.; Matsumoto, A.; Kumakiri, J.; Kitade, M.; Itakura, A.; Muter, J.; Brosens, J.J.; et al. Reprogramming of the retinoic acid pathway in decidualizing human endometrial stromal cells. PLoS ONE 2017, 12, e0173035. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, K.; Utsunomiya, H.; Tamura, M.; Niikura, H.; Takano, T.; Yoshinaga, K.; Nagase, S.; Suzuki, T.; Ito, K.; Matsumoto, M.; et al. Expression of retinoic acid receptors in human endometrial carcinoma. Cancer Sci. 2008, 99, 267–271. [Google Scholar] [CrossRef]
- Iqbal, S.; Naseem, I. Role of vitamin A in type 2 diabetes mellitus biology: Effects of intervention therapy in a deficient state. Nutrition 2015, 31, 901–907. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, C.; Hu, M.; Zhang, J.; Xu, P.; Li, F.; Zhong, Z.; Liu, L.; Liu, X. High-fat diet enhanced retinal dehydrogenase activity, but suppressed retinol dehydrogenase activity in liver of rats. J. Pharmacol. Sci. 2015, 127, 430–438. [Google Scholar] [CrossRef] [Green Version]
- Frey, S.K.; Vogel, S. Vitamin A metabolism and adipose tissue biology. Nutrients 2011, 3, 27–39. [Google Scholar] [CrossRef] [Green Version]
- Mah, V.; Elshimali, Y.; Chu, A.; Moatamed, N.A.; Uzzell, J.P.; Tsui, J.; Schettler, S.; Shakeri, H.; Wadehra, M. ALDH1 expression predicts progression of premalignant lesions to cancer in Type I endometrial carcinomas. Sci. Rep. 2021, 11, 11949. [Google Scholar] [CrossRef]
- Ceylan, Y.; Akpınar, G.; Doger, E.; Kasap, M.; Guzel, N.; Karaosmanoglu, K.; Kopuk, S.Y.; Yucesoy, I. Proteomic analysis in endometrial cancer and endometrial hyperplasia tissues by 2D-DIGE technique. J. Gynecol. Obstet. Hum. Reprod. 2020, 49, 101652. [Google Scholar] [CrossRef]
- Lv, M.; Luo, L.; Chen, X. The landscape of prognostic and immunological role of myosin light chain 9 (MYL9) in human tumors. Immun. Inflamm. Dis. 2021, 10, 241–254. [Google Scholar] [CrossRef]
- Mierke, C.T.; Kollmannsberger, P.; Zitterbart, D.P.; Diez, G.; Koch, T.M.; Marg, S.; Ziegler, W.H.; Goldmann, W.H.; Fabry, B. Vinculin facilitates cell invasion into three-dimensional collagen matrices. J. Biol. Chem. 2010, 285, 13121–13130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Z.; Zhuang, L.; Szatmary, P.; Wen, L.; Sun, H.; Lu, Y.; Xu, Q.; Chen, X. Upregulation of Heat Shock Proteins (HSPA12A, HSP90B1, HSPA4, HSPA5 and HSPA6) in Tumour Tissues Is Associated with Poor Outcomes from HBV-Related Early-Stage Hepatocellular Carcinoma. Int. J. Med. Sci. 2015, 12, 256–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Q.; Lin, X.; Zhang, X.; Jiang, G.; Zhang, Y.; Miao, Y.; Rong, X.; Zheng, X.; Han, Y.; Han, X.; et al. WWC3 regulates the Wnt and Hippo pathways via Dishevelled proteins and large tumour suppressor 1, to suppress lung cancer invasion and metastasis. J. Pathol. 2017, 242, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Kremerskothen, J.; Lin, X.; Zhang, X.; Rong, X.; Zhang, D.; Wang, E. WWC3 inhibits epithelial-mesenchymal transition of lung cancer by activating hippo-YAP signalling. Onco. Targets. Ther. 2018, 11, 2581–2591. [Google Scholar] [CrossRef] [Green Version]
- You, N.; Tan, Y.-X.; Zhou, L.; Huang, X.; Wang, W.; Wang, L.; Wu, K.; Mi, N.; Li, J.; Zheng, L. Tg737 acts as a key driver of invasion and migration in liver cancer stem cells and correlates with poor prognosis in patients with hepatocellular carcinoma. Exp. Cell Res. 2017, 358, 217–226. [Google Scholar] [CrossRef]
- Liu, L.; Sheng, J.-Q.; Wang, M.-R.; Gan, Y.; Wu, X.-L.; Liao, J.-Z.; Tian, D.-A.; He, X.-X.; Li, P.-Y. Primary Cilia Blockage Promotes the Malignant Behaviors of Hepatocellular Carcinoma via Induction of Autophagy. Biomed Res. Int. 2019, 2019, 5202750. [Google Scholar] [CrossRef] [Green Version]
- Xu, Q.; Chen, C.; Chen, G.; Chen, W.; Zhou, D.; Xie, Y. Significance of calreticulin as a prognostic factor in endometrial cancer. Oncol. Lett. 2018, 15, 8999–9008. [Google Scholar] [CrossRef] [Green Version]
- Boden, G.; Duan, X.; Homko, C.; Molina, E.J.; Song, W.; Perez, O.; Cheung, P.; Merali, S. Increase in endoplasmic reticulum stress-related proteins and genes in adipose tissue of obese, insulin-resistant individuals. Diabetes 2008, 57, 2438–2444. [Google Scholar] [CrossRef] [Green Version]
- Zhao, M.; Fang, W.; Wang, Y.; Guo, S.; Shu, L.; Wang, L.; Chen, Y.; Fu, Q.; Liu, Y.; Hua, S.; et al. Enolase-1 is a therapeutic target in endometrial carcinoma. Oncotarget 2015, 6, 15610–15627. [Google Scholar] [CrossRef] [Green Version]
- Cho, H.; Um, J.; Lee, J.-H.; Kim, W.-H.; Kang, W.S.; Kim, S.H.; Ha, H.-H.; Kim, Y.-C.; Ahn, Y.-K.; Jung, D.-W.; et al. ENOblock, a unique small molecule inhibitor of the non-glycolytic functions of enolase, alleviates the symptoms of type 2 diabetes. Sci. Rep. 2017, 7, 44186. [Google Scholar] [CrossRef]


| Parameters | EC Diabetic (n = 7) Mean ± SD | EC Non-Diabetic (n = 7) Mean ± SD | p-Value |
|---|---|---|---|
| Age (years) | 63.7 ± 10.1 | 58.4 ± 11.4 | 0.375 |
| Height (cm) | 151.8 ± 1.3 | 151.6 ± 1.5 | 1.0000 |
| Weight (kg) | 96.8 ± 10.3 | 67.1 ±12.9 | 0.0005 * |
| BMI (kg/m2) | 42.0 ± 4.9 | 29.1 ± 5.3 | 0.0005 * |
| HbA1C (%) | 7.0 ± 0.2 | 6.0 ± 0.3 | <0.0001 * |
| Total cholesterol (mmol/L) | 4.2 ± 1.0 | 3.7 ± 1.6 | 0.496 |
| LDL (mmol/L) | 2.5 ± 1.0 | 2.1 ± 1.5 | 0.496 |
| HDL (mmol/L) | 1.1± 0.5 | 1.2 ± 0.2 | 0.632 |
| TG (mmol/L) | 1.4 ± 0.7 | 0.7 ± 0.6 | 0.110 |
| Urea (mmol/L) | 4.8 ± 2.0 | 4.3 ± 1.5 | 0.606 |
| Creatinine (µmol/L) | 56.0 ± 9.3 | 52.6 ± 9.5 | 0.511 |
| Glucose (mmol/l) | 10.5 ± 5.5 | 5.4 ± 0.5 | 0.031 |
| Sl No. | Spot No. a | Accession No. | Protein Name | MASCOT ID | p-Value b (ANOVA) | Ratio ECD/ECND c | Exp d |
|---|---|---|---|---|---|---|---|
| 1 | 633 | Q96LW4 | DNA-directed primase/polymerase protein | PRIPO_HUMAN | 8.29 × 10−4 | −1.5 | DOWN |
| 2 | 274 | P14625 | Endoplasmin | ENPL_HUMAN | 0.002 | 2.44 | UP |
| 3 | 2462 | P24844 | Myosin regulatory light polypeptide 9 | MYL9_HUMAN | 0.01 | −2.47 | DOWN |
| 4 | 1627 | P04406 | Glyceraldehyde-3-phosphate dehydrogenase | G3P_HUMAN | 0.01 | 1.78 | UP |
| 5 | 1691 | P08758 | Annexin A5 | ANXA5_HUMAN | 0.01 | 2.81 | UP |
| 6 | 3130 | Q13099 | Intraflagellar transport protein 88 homolog | IFT88_HUMAN | 0.01 | −2.05 | DOWN |
| 7 | 109 | P14625 | Endoplasmin | ENPL_HUMAN | 0.01 | 2.86 | UP |
| 8 | 2608 | P60174 | Triosephosphate isomerase | TPIS_HUMAN | 0.01 | −1.77 | DOWN |
| 9 | 282 | P02787 | Serotransferrin | TRFE_HUMAN | 0.01 | 2.14 | UP |
| 10 | 986 | P27797 | Calreticulin | CALR_HUMAN | 0.02 | 2.24 | UP |
| 11 | 131 | P13639 | Elongation factor 2 | EF2_HUMAN | 0.02 | 1.78 | UP |
| 12 | 2167 | Q06830 | Peroxiredoxin-1 | PRDX1_HUMAN | 0.02 | 2.87 | UP |
| 13 | 46 | O15020 | Spectrin beta chain, non-erythrocytic 2 | SPTN2_HUMAN | 0.02 | −1.6 | DOWN |
| 14 | 2537 | P02766 | Transthyretin | TTHY_HUMAN | 0.02 | −1.5 | DOWN |
| 15 | 1322 | P06733 | Alpha-enolase | ENOA_HUMAN | 0.02 | −1.9 | DOWN |
| 16 | 2694 | P00441 | Superoxide dismutase [Cu-Zn] | SODC_HUMAN | 0.02 | −1.92 | DOWN |
| 17 | 518 | P18206 | Vinculin | VINC_HUMAN | 0.02 | 1.79 | UP |
| 18 | 172 | P21333 | Filamin-A | FLNA_HUMAN | 0.02 | 1.67 | UP |
| 19 | 548 | P12814 | Alpha-actinin-1 | ACTN1_HUMAN | 0.02 | −1.5 | DOWN |
| 20 | 2485 | P23528 | Cofilin-1 | COF1_HUMAN | 0.03 | −1.6 | DOWN |
| 21 | 2823 | P05413 | Fatty acid-binding protein, heart | FABPH_HUMAN | 0.03 | 1.77 | UP |
| 22 | 110 | P21333 | Filamin-A | FLNA_HUMAN | 0.03 | 1.79 | UP |
| 23 | 234 | P12110 | Collagen alpha-2(VI) chain | CO6A2_HUMAN | 0.03 | 1.79 | UP |
| 24 | 224 | P18206 | Vinculin | VINC_HUMAN | 0.03 | 2.86 | UP |
| 25 | 2687 | Q96NR8 | Retinol dehydrogenase 12 | RDH12_HUMAN | 0.02 | −2.1 | DOWN |
| 26 | 346 | Q9HAE3 | EF-hand calcium-binding domain-containing protein 1 | EFCB1_HUMAN | 0.03 | −1.8 | DOWN |
| 27 | 2319 | Q9ULE0 | Protein WWC3 | WWC3_HUMAN | 0.04 | −2.1 | DOWN |
| 28 | 341 | P11142 | Heat shock cognate 71 kDa protein | HSP7C_HUMAN | 0.04 | 1.9 | UP |
| 29 | 307 | P12814 | Alpha-actinin-1 | ACTN1_HUMAN | 0.04 | 1.7 | UP |
| 30 | 1860 | P62736 | Actin, aortic smooth muscle | ACTA_HUMAN | 0.09 | 1.5 | UP |
| 31 | 603 | Q92737 | Ras-like protein family member 10A | RSLAA_HUMAN | 0.04 | 2.1 | UP |
| 32 | 1569 | Q96S15 | GATOR complex protein WDR24 | WDR24_HUMAN | 0.04 | 1.7 | UP |
| 33 | 2827 | Q9NQ76 | Matrix extracellular phosphoglycoprotein | MEPE_HUMAN | 0.05 | −1.6 | DOWN |
| 34 | 2846 | Q5VWT5 | FYN-binding protein 2 | FYB2_HUMAN | 0.05 | −1.56 | DOWN |
| 35 | 1114 | P00352 | Retinal dehydrogenase 1 | AL1A1_HUMAN | 0.05 | −1.91 | DOWN |
| 36 | 2559 | Q01995 | Transgelin | TAGL_HUMAN | 0.05 | −1.5 | DOWN |
| 37 | 2928 | P06702 | Protein S100-A9 | S10A9_HUMAN | 0.05 | −1.5 | DOWN |
| 38 | 1842 | P35232 | Prohibitin | PHB_HUMAN | 0.05 | −1.91 | DOWN |
| 39 | 1050 | P08670 | Vimentin | VIME_HUMAN | 0.05 | 1.5 | UP |
| 40 | 2399 | P35228 | Nitric oxide synthase, inducible | NOS2_HUMAN | 0.01 | 2.02 | UP |
| 41 | 865 | P02545 | Prelamin-A/C | LMNA_HUMAN | 0.01 | −1.82 | DOWN |
| 42 | 702 | P02787 | Serotransferrin | TRFE_HUMAN | 0.01 | 1.94 | UP |
| 43 | 2847 | Q9BWT1 | Cell division cycle-associated protein 7 | CDCA7_HUMAN | 0.01 | −1.5 | DOWN |
| 44 | 1344 | P05787 | Keratin, type II cytoskeletal 8 | K2C8_HUMAN | 0.01 | −1.5 | DOWN |
| 45 | 1341 | P68032 | Actin, alpha cardiac muscle 1 | ACTC_HUMAN | 0.01 | 1.96 | UP |
| 46 | 571 | Q9C0H9 | SRC kinase signaling inhibitor 1 | SRCN1_HUMAN | 0.01 | −1.5 | DOWN |
| 47 | 1696 | P68032 | Actin, alpha cardiac muscle 1 | ACTC_HUMAN | 0.01 | 1.5 | UP |
| 48 | 2478 | P63267 | Actin, gamma-enteric smooth muscle | ACTH_HUMAN | 0.01 | 1.56 | UP |
| 49 | 1633 | P62937 | Peptidyl-prolyl cis-trans isomerase A | PPIA_HUMAN | 0.01 | −1.61 | DOWN |
| 50 | 2442 | O95789 | Zinc finger MYM-type protein 6 | ZMYM6_HUMAN | 0.01 | 1.55 | UP |
| 51 | 239 | Q8IYX0 | Zinc finger protein 679 | ZN679_HUMAN | 0.01 | 1.59 | UP |
| 52 | 1588 | P17661 | Desmin | DESM_HUMAN | 0.01 | 2.17 | UP |
| 53 | 649 | P11142 | Heat shock cognate 71 kDa protein | HSP7C_HUMAN | 0.01 | 1.55 | UP |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mujammami, M.; Rafiullah, M.; Alfadda, A.A.; Akkour, K.; Alanazi, I.O.; Masood, A.; Musambil, M.; Alhalal, H.; Arafah, M.; Rahman, A.M.A.; et al. Proteomic Analysis of Endometrial Cancer Tissues from Patients with Type 2 Diabetes Mellitus. Life 2022, 12, 491. https://doi.org/10.3390/life12040491
Mujammami M, Rafiullah M, Alfadda AA, Akkour K, Alanazi IO, Masood A, Musambil M, Alhalal H, Arafah M, Rahman AMA, et al. Proteomic Analysis of Endometrial Cancer Tissues from Patients with Type 2 Diabetes Mellitus. Life. 2022; 12(4):491. https://doi.org/10.3390/life12040491
Chicago/Turabian StyleMujammami, Muhammad, Mohamed Rafiullah, Assim A. Alfadda, Khalid Akkour, Ibrahim O. Alanazi, Afshan Masood, Mohthash Musambil, Hani Alhalal, Maria Arafah, Anas M. Abdel Rahman, and et al. 2022. "Proteomic Analysis of Endometrial Cancer Tissues from Patients with Type 2 Diabetes Mellitus" Life 12, no. 4: 491. https://doi.org/10.3390/life12040491
APA StyleMujammami, M., Rafiullah, M., Alfadda, A. A., Akkour, K., Alanazi, I. O., Masood, A., Musambil, M., Alhalal, H., Arafah, M., Rahman, A. M. A., & Benabdelkamel, H. (2022). Proteomic Analysis of Endometrial Cancer Tissues from Patients with Type 2 Diabetes Mellitus. Life, 12(4), 491. https://doi.org/10.3390/life12040491






