Skull Base Approaches for Tuberculum Sellae Meningiomas: Institutional Experience in a Series of 34 Patients
Abstract
:1. Introduction
2. Materials and Methods
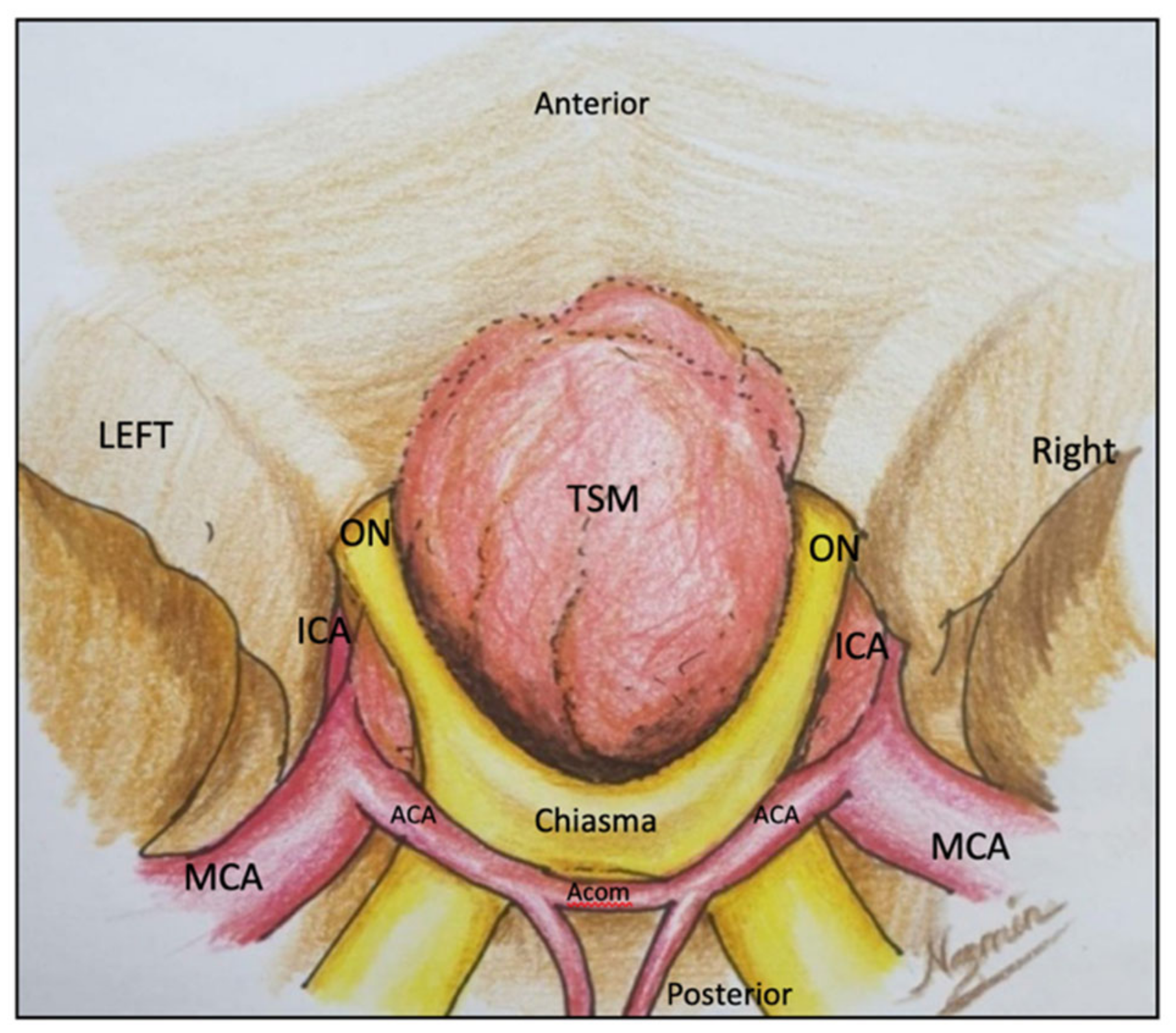
2.1. Minipterional Approach
2.1.1. Surgical Technique
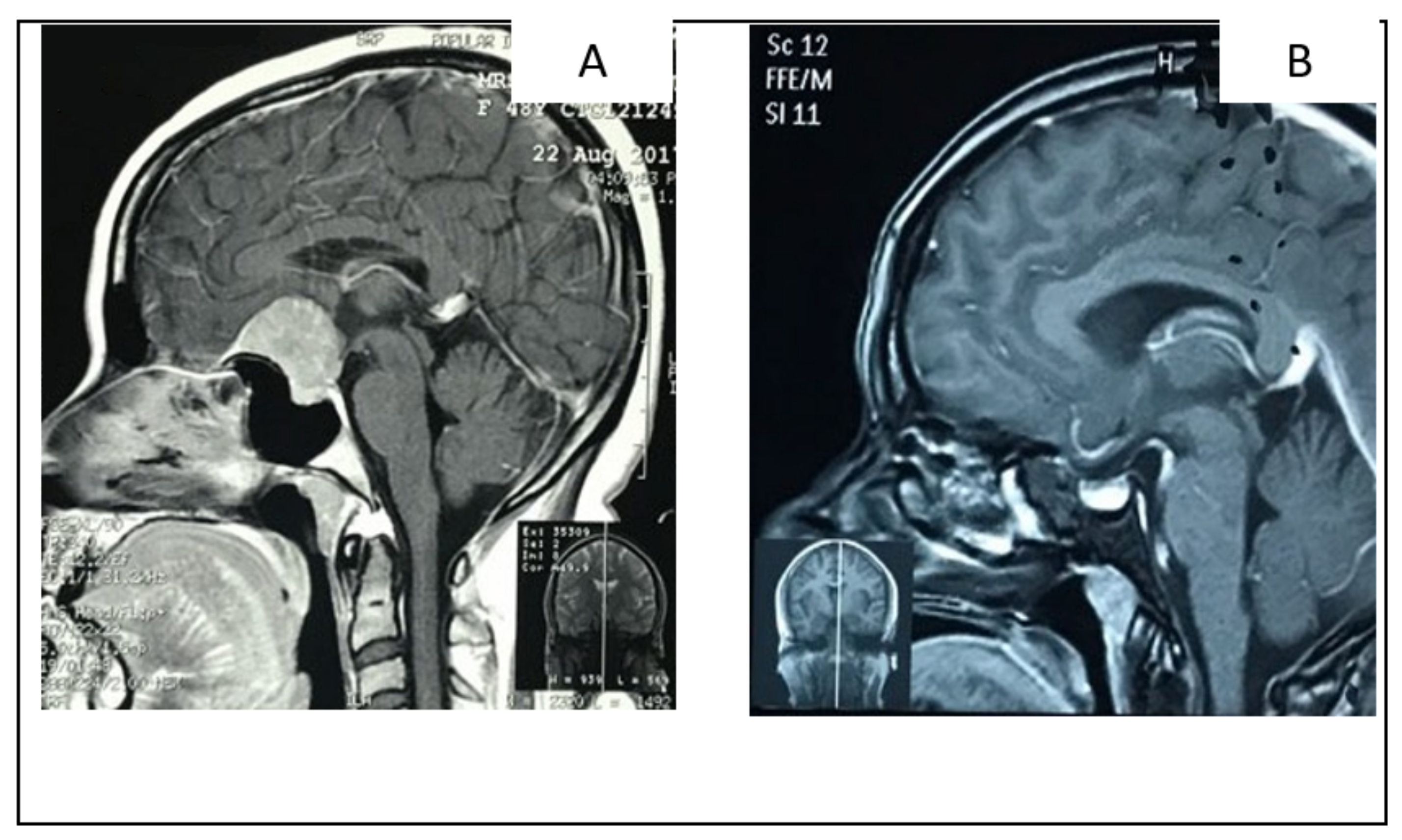
2.1.2. Illustrative Case: 1
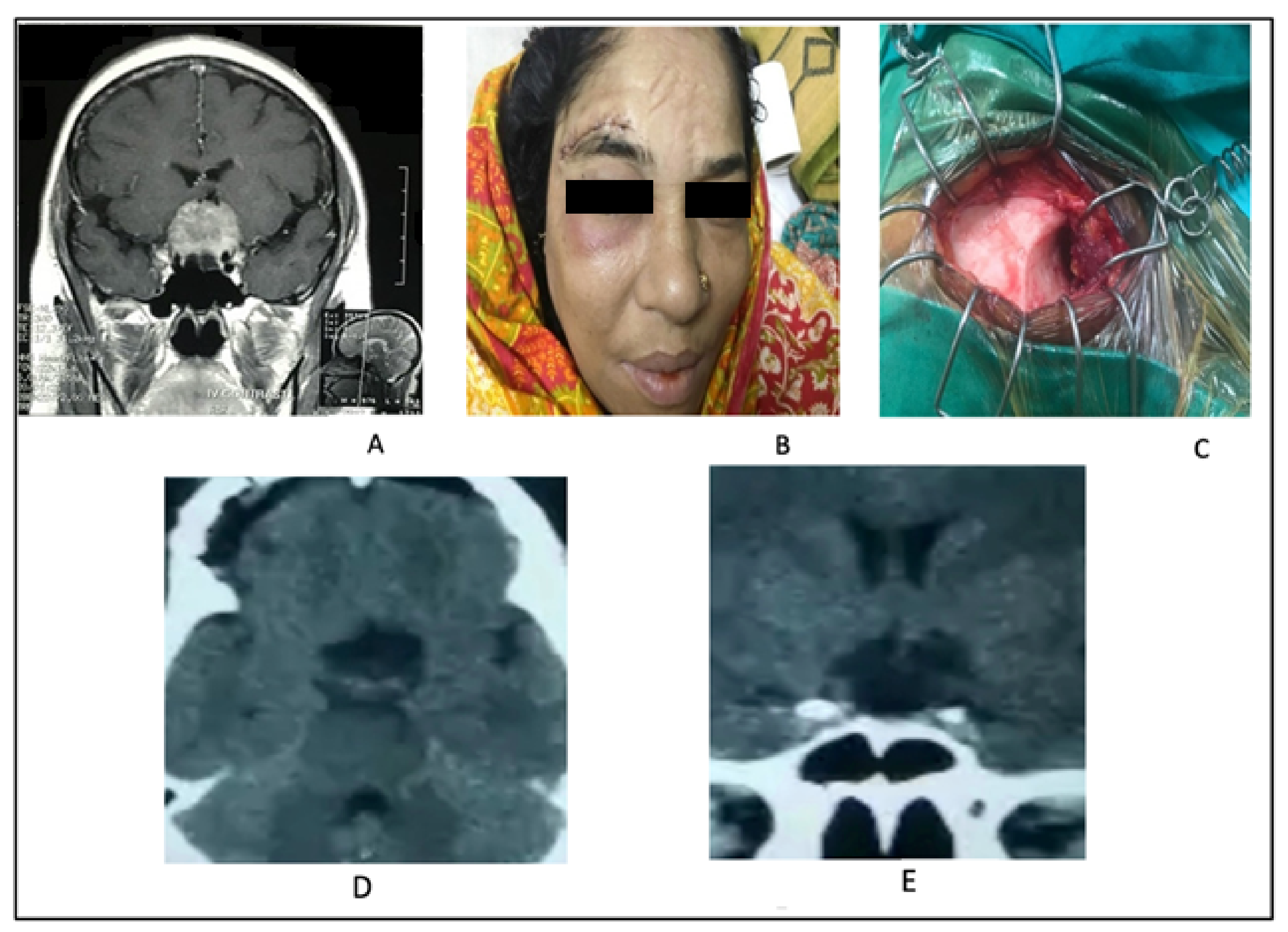
2.2. Supraciliary Keyhole Microsurgical Approach
2.2.1. Surgical Technique
2.2.2. Illustrative Case 2
2.3. Supraciliary Keyhole Endoscopic Approach
2.3.1. Surgical Technique
2.3.2. Illustrative Case 3
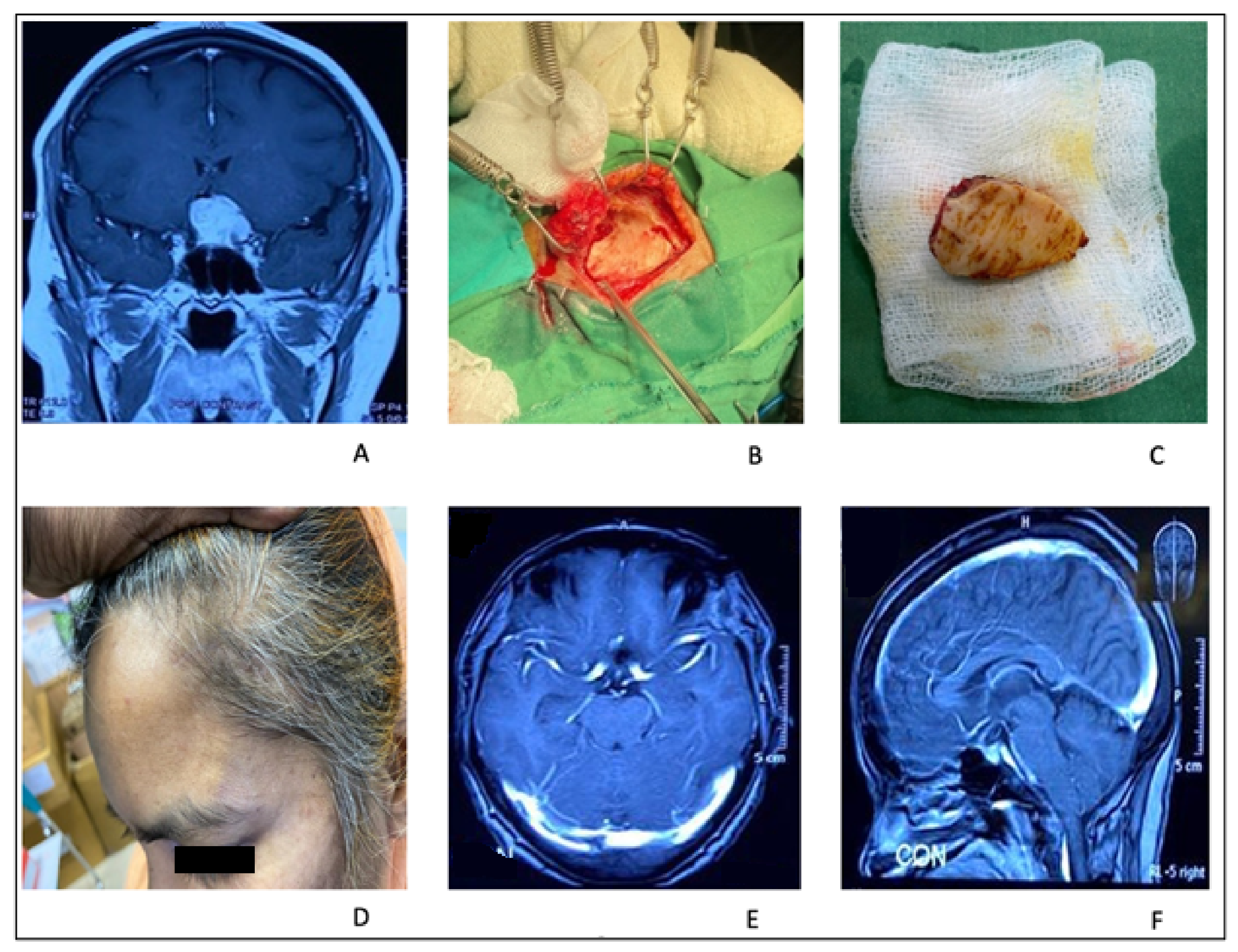
2.4. Minibifrontal Basal Approach
2.4.1. Surgical Technique
2.4.2. Illustrative Case 4
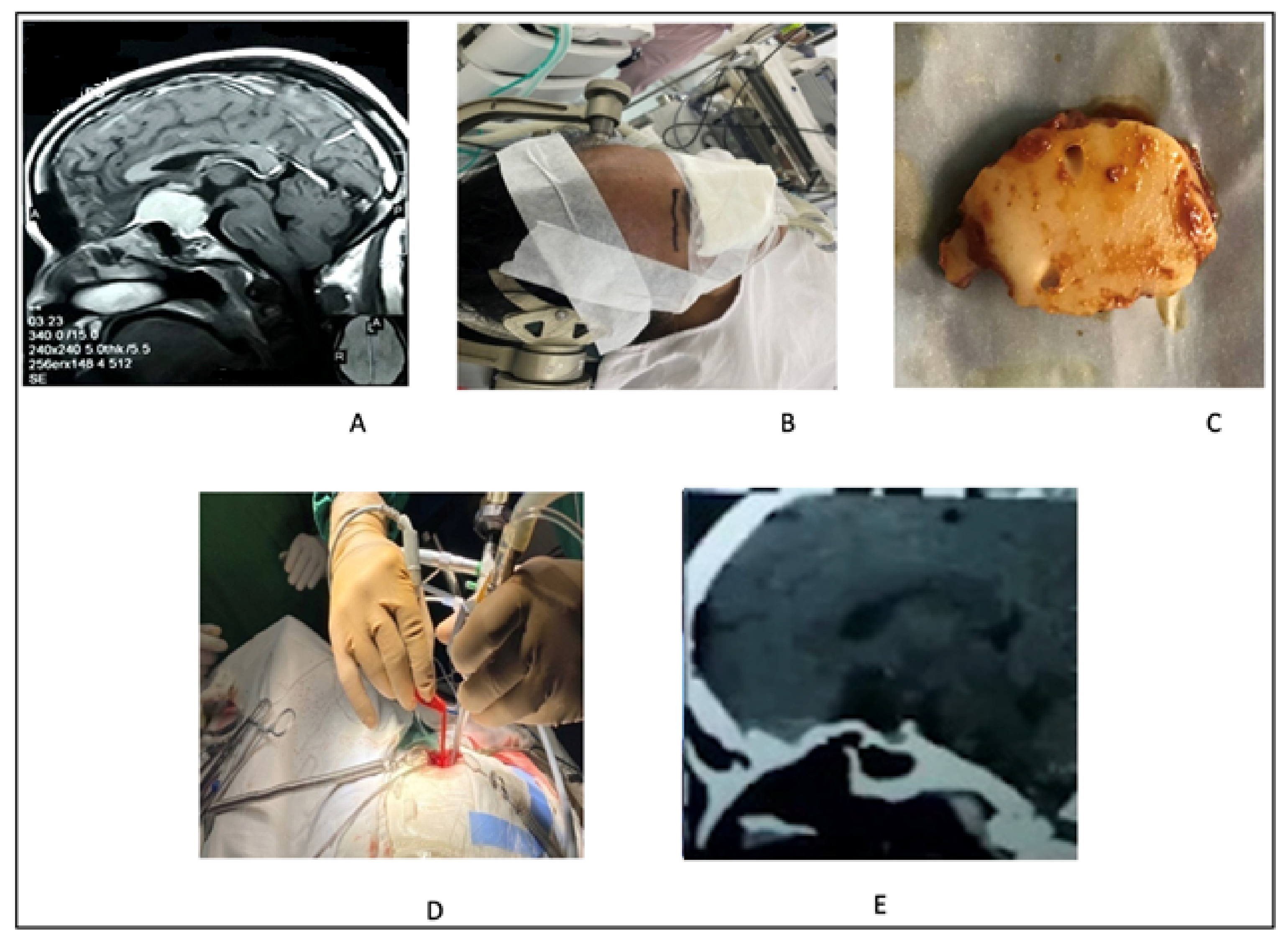
2.5. Extended Endoscopic Endonasal Approach
2.5.1. Surgical Technique
2.5.2. Illustrative Case 5
3. Results
4. Discussion
4.1. Minipterional Approach: Indications
4.2. Supraciliary Keyhole Microsurgical Approach: Indications
4.3. Supraciliary Keyhole Endoscopic Approach: Indications
4.4. Minibifrontal Basal Approach: Indications
4.5. Extended Endoscopic Endonasal Approach: Indications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mariniello, G.; de Divitiis, O.; Bonavolontà, G.; Maiuri, F. Surgical unroofing of the optic canal and visual outcome in basal meningiomas. Acta Neurochir. 2013, 155, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Turel, M.K.; Tsermoulas, G.; Reddy, D.; Andrade-Barazarte, H.; Zadeh, G.; Gentili, F. Endonasal endoscopic transsphenoidal excision of tuberculum sellae meningiomas: A systematic review. J. Neurosurg. Sci. 2016, 60, 463–475. [Google Scholar] [PubMed]
- Lee, S.; Hong, S.H.; Cho, Y.H.; Kim, J.H.; Kim, C.J. Anatomical origin of tuberculum sellae meningioma: Off-midline location and its clinical implications. World Neurosurg. 2016, 89, 552–561. [Google Scholar] [CrossRef] [PubMed]
- Refaat, M.I.; Eissa, E.M.; Ali, M.H. Surgical management of midline anterior skull base meningiomas: Experience of 30 cases. Turk. Neurosurg. 2015, 25, 432–437. [Google Scholar] [CrossRef]
- Al-Mefty, O.; Holoubi, A.; Rifai, A.; Fox, J.L. Microsurgical removal of suprasellar meningiomas. Neurosurgery 1985, 16, 364–372. [Google Scholar] [CrossRef]
- Schick, U.; Hassler, W. Surgical management of tuberculum sellae meningiomas: Involvement of the optic canal and visual outcome. J. Neurol. Neurosurg. Psychiatry 2005, 76, 977–983. [Google Scholar] [CrossRef] [Green Version]
- Mahmoud, M.; Nader, R.; Al-Mefty, O. Optic canal involvement in tuberculum sellae meningiomas: Influence on approach, recurrence, and visual recovery. Oper. Neurosurg. 2010, 67, ons108–ons119. [Google Scholar] [CrossRef]
- Turel, M.K.; Tsermoulas, G.; Yassin-Kassab, A.; Reddy, D.; Andrade-Barazarte, H.; Gonen, L.; Zadeh, G.; Gentili, F. Tuberculum sellae meningiomas: A systematic review of transcranial approaches in the endoscopic era. J. Neurosurg. Sci. 2016, 63, 200–215. [Google Scholar] [CrossRef] [Green Version]
- Umana, G.E.; Scalia, G.; Vats, A.; Pompili, G.; Barone, F.; Passanisi, M.; Graziano, F.; Maugeri, R.; Tranchina, M.G.; Cosentino, S.; et al. Primary Extracranial Meningiomas of the Head and Neck. Life 2021, 11, 942. [Google Scholar] [CrossRef]
- Song, S.W.; Kim, Y.H.; Kim, J.W.; Park, C.K.; Kim, J.E.; Kim, D.G.; Koh, Y.C.; Jung, H.W. Outcomes after transcranial and endoscopic endonasal approach for tuberculum meningiomas—A retrospective comparison. World Neurosurg. 2018, 109, e434–e445. [Google Scholar] [CrossRef]
- Haque, M.; Jahan, N.; Bari, S. Two cases of atypical foramen magnum meningioma presenting as rotatory paralysis. J. Neurol. Stroke 2019, 9, 180–182. [Google Scholar]
- Bassiouni, H.; Asgari, S.; Stolke, D. Tuberculum sellae meningiomas: Functional outcome in a consecutive series treated microsurgically. Surg. Neurol. 2006, 66, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.; Chaurasia, B.K.; Shalike, N.; Uddin, A.N.; Chowdhury, D.; Khan, A.H.; Ansari, A.; Barua, K.K.; Majumder, M.R. Surgical management of clinoidal meningiomas: 10 Cases analysis. Neuroimmunol. Nueroinflamm. 2018, 5, 21. [Google Scholar] [CrossRef] [Green Version]
- Goel, A.; Muzumdar, D.; Desai, K.I. Tuberculum sellae meningioma: A report on management on the basis of a surgical experience with 70 patients. Neurosurgery 2002, 51, 1358–1364. [Google Scholar] [CrossRef] [PubMed]
- Otani, N.; Muroi, C.; Yano, H.; Khan, N.; Pangalu, A.; Yonekawa, Y. Surgical manage-ment of tuberculum sellae meningiomas: Role of selective extradural anterior clinoidectomy. Br. J. Neurosurg. 2006, 20, 129–138. [Google Scholar] [CrossRef]
- Nakamura, M.; Roser, F.; Struck, M.; Vorkapic, P.; Samii, M. Tuberculum sellae meningiomas: Clinical outcome considering different surgical approaches. Neurosurgery 2006, 59, 1019–1029. [Google Scholar] [CrossRef]
- Olivecrona, H. The suprasellar meningiomas. In Handbuch der Neurochirurgie; Olivecrona, H., Tonnis, W., Eds.; Springer: Berlin, Germany, 1967; pp. 167–172. [Google Scholar]
- Solero, C.L.; Giombini, S.; Morello, G. Suprasellar and olfactory meningiomas. Report on a series of 153 personal cases. Acta Neurochir. 1983, 67, 181–194. [Google Scholar] [CrossRef]
- Kunicki, A.; Uhl, A. The clinical picture and results of surgical treatment of meningioma of the tuberculum sellae. Ceskoslovenská Neurol. 1968, 31, 80–92. [Google Scholar]
- Kadis, G.N.; Mount, L.A.; Ganti, S.R. The importance of early diagnosis and treatment of the meningiomas of the planum sphenoidale and tuberculum sellae: A retrospective study of 105 cases. Surg. Neurol. 1979, 12, 367–371. [Google Scholar]
- Guiot, G.; Montrieul, B.; Goutelle, A.; Comoy, J.; Langie, S. Retro-chiasmatic suprasellar meningiomas. Neuro-Chir. 1970, 16, 273–285. [Google Scholar]
- Cushing, H.; Eisenhardt, L. Suprasellar meningiomas. In Meningiomas: Their Classification, Regional Behaviour, Life History, and Surgical End Results; British Journal of Surgery: Oxford, UK, 1938; Volume 26, pp. 224–249. [Google Scholar]
- Ferini, G.; Umana, G.E. Radiotherapy in Current Neuro-Oncology: There Is Still Much to Reveal. Life 2021, 11, 1412. [Google Scholar] [CrossRef] [PubMed]
- Barone, F.; Inserra, F.; Scalia, G.; Ippolito, M.; Cosentino, S.; Crea, A.; Sabini, M.G.; Valastro, L.; Patti, I.V.; Mele, S.; et al. 68Ga-DOTATOC PET/CT Follow Up after Single or Hypofractionated Gamma Knife ICON Radiosurgery for Meningioma Patients. Brain Sci. 2021, 11, 375. [Google Scholar] [CrossRef] [PubMed]
- Symon, L.; Jakubowski, J. Clinical features, technical problems, and results of treatment of anterior parasellar meningiomas. Acta Neurochir. Suppl. 1979, 28, 367–370. [Google Scholar] [PubMed]
- Finn, J.E.; Mount, L.A. Meningiomas of the tuberculum sellae and planum sphenoidale: A review of 83 cases. Arch. Ophthalmol. 1974, 92, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, N.; Malmros, R. The suprasellar meningioma. A review of the literature and presentation of a series of 31 cases. Acta Ophthalmol. Suppl. 1973, 1–74. [Google Scholar]
- Krenkel, W.; Frowein, R.A. Proceedings: Suprasellar meningiomas. Acta Neurochir. 1975, 31, 280. [Google Scholar]
- Kedar, S. Optic Chiasm and Tract. In Encyclopedia of the Neurological Sciences; Elsevier Inc.: Amsterdam, The Netherlands, 2014; pp. 669–671. [Google Scholar] [CrossRef]
- Jane, J.A.; McKissock, W. Importance of failing vision in early diagnosis of suprasellar meningiomas. Br. Med. J. 1962, 2, 5. [Google Scholar] [CrossRef] [Green Version]
- Holmes, G.; Sargent, P. Suprasellar endotheliomata. Brain 1927, 50, 518–523. [Google Scholar] [CrossRef]
- Chambers, B.M. Meningioma of the tuberculum sellae. Am. J. Ophthalmol. 1950, 33, 1447–1449. [Google Scholar]
- Gregorius, F.K.; Hepler, R.S.; Stern, W.E. Loss and recovery of vision with suprasellar meningiomas. J. Neurosurg. 1975, 42, 69–75. [Google Scholar] [CrossRef]
- Brihaye, J.; Brihaye-van Geertruyden, M. Management and surgical outcome of suprasellar meningiomas. In Proceedings of the 8th European Congress of Neurosurgery, Barcelona, Spain, 6–11 September 1987; pp. 124–129. [Google Scholar]
- Bristot, A.R.; Domenicucci, M.; Cantore, G. Meningiomas of the tuberculum sellae: Our experience in 69 cases surgically treated between 1973 and 1993. J. Neurosurg. Sci. 1999, 43, 253. [Google Scholar]
- Koos, W.T.H.; Kletter, G.; Schuster, H.; Perneczky, A. Microsurgery of suprasellar meningiomas. Adv. Neurosurg. 1975, 2, 62–67. [Google Scholar]
- Grisoli, F.; Diaz-Vasquez, P.; Riss, M.; Vincentelli, F.; Leclercq, T.A.; Hassoun, J.; Salamon, G. Microsurgical management of tuberculum sellae meningiomas. Results in 28 consecutive cases. Surg. Neurol. 1986, 26, 37–44. [Google Scholar] [CrossRef]
- De Divitiis, E.; Esposito, F.; Cappabianca, P.; Cavallo, L.M.; de Divitiis, O. Tuberculum sellae meningiomas: High route or low route? A series of 51 consecutive cases. Neurosurgery 2008, 62, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Al-Mefty, O.; Smith, R.R. Tuberculum sellae meningiomas. In Meningiomas; Al-Mefty, O., Ed.; Raven Press: New York, NY, USA, 1991; pp. 311–395. [Google Scholar]
- Mathiesen, T.; Kihlström, L. Visual outcome of tuberculum sellae meningiomas after extradural optic nerve decompression. Neurosurgery 2006, 59, 570–576. [Google Scholar] [CrossRef]
- Jho, H.D. Endoscopic transsphenoidal surgery. J. Neuro-Oncol. 2001, 54, 187–195. [Google Scholar] [CrossRef]
- Dusick, J.R.; Esposito, F.; Kelly, D.F. Extended transsphenoidal approach. J. Neurosurg. 2005, 102, 825–828. [Google Scholar] [CrossRef] [Green Version]
- Cafiero, T.; Cavallo, L.M.; Frangiosa, A.; Burrelli, R.; Gargiulo, G.; Cappabianca, P.; de Divitiis, E. Clinical comparison of remifentanil-sevoflurane vs. remifentanil-propofol for endoscopic endonasal transphenoidal surgery. Eur. J. Anaesthesiol. 2007, 24, 441–446. [Google Scholar] [CrossRef]
- Palani, A.; Panigrahi, M.K.; Purohit, A.K. Tuberculum sellae meningiomas: A series of 41 cases; surgical and ophthalmological outcomes with proposal of a new prognostic scoring system. J. Neurosci. Rural. Pract. 2012, 3, 286–293. [Google Scholar] [CrossRef]

| No of Patients | |
|---|---|
| Age Group(years) | Frequency |
| 11–20 | 1 |
| 21–30 | 6 |
| 31–40 | 11 |
| 41–50 | 10 |
| 51–60 | 5 |
| 61–70 | 1 |
| Total | 34 |
| No of Patients | ||
|---|---|---|
| Sex | Frequency | Percentage (%) |
| Male | 6 | 17.64 |
| Female | 28 | 82.35 |
| Total | 34 | 100 |
| Male:Female | 1:4.66 | |
| Visual Presentation | No of Patients |
|---|---|
| Frequency (Percentage %) | |
| No visual field defect | 4 (11.76%) |
| Bilateral blind | 6 (17.64%) |
| Bitemporal field defect | 16 (47.05%) |
| Tunnel vision | 8 (23.52%) |
| Total: | 34 (100%) |
| Transcranial Approach (%) | Transsphenoidal Approach. (%) |
|---|---|
| Microscopic | (70.58%) |
| Endoscopic-assisted | (14.70%) |
| transcranial/supraciliary keyhole endoscopic-assisted | (14.70%) |
| Total: | (85.29%) |
| No of Patients | ||
|---|---|---|
| Size | Frequency | Percentage (%) |
| <3 cm | 15 | (44.11%) |
| 3–6 cm | 17 | (50%) |
| >6 cm | 2 | (5.88%) |
| Total: | 34 | 100% |
| No of Patients (%) | ||
|---|---|---|
| Yes | No | |
| Vascular encasement | 11 (32.35%) | 23 (67.64%) |
| No of Patients (%) | ||
|---|---|---|
| Transcranial | Transsphenoidal | |
| Improved Vision | 25 (86.20%) | 4 (80%) |
| Static vision | 3 (10.34%) | 1 (20%) |
| Deteriorated vision | 1 (03.45%) | 0 (%) |
| No of Patients (%) | |||
|---|---|---|---|
| Transcranial Microscopic | Transcranial/Superciliary Keyhole Endoscopic-Assisted | Transsphenoidal | |
| Gross total | 20 (58.82%) | 4 (80%) | 3 (60%) |
| Near total | 3 (10.34%) | 1 (20%) | 1 (20%) |
| Partial | 1 (03.45%) | 0 | 1 (20%) |
| No of Patients (%) | ||
|---|---|---|
| Transcranial | Transsphenoidal | |
| CSF leak | 2 (6.89%) | 1 (20%) |
| Meningitis | 3 (10.34%) | 1 (20%) |
| Vascular injury | Nil | Nil |
| Would infection | 2 (6.89%) | Nil |
| Series (Ref. No) | No of Cases | Approach | Gross Total Removal | Visual Outcome Improved | Complication Mortality |
|---|---|---|---|---|---|
| Al-Mefty and Smith, 1991 [39] | 35 | Transcranial | 91% | 25% | 8.6% |
| Mathiesen and Kihlstrom, 2006 [40] | 29 | Transcranial | 90% | 75.9% | 0% |
| Jho, 2001 [41] | 1 | Endoscopic endonasal transphenoidal | 100% | 100% | 0% |
| Dusick et al., 2005 [42] | 7 | Microsurgical endoscopic-assisted | 57.14% | Not recorded | 0% |
| de Divitiis et al., 2007 [43] | 44 | Transcranial | 86.4% | 61.4% | 0% |
| 11 | Endoscopic endonasal | 83% | 71.4% | 0% | |
| Palani et al., 2012 [44] | 41 | Transcranial | 73% | 27% | 4.9% |
| Our Series 2021 | 24 | Transcranial microscopic | 58.82% | 86.20% | 0% |
| 5 | Transcranial endoscopic | 80% | 86.20% | 0% | |
| 5 | Extended endonasal transsphenoidal approach | 60% | 80% | 0% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alam, S.; Ferini, G.; Muhammad, N.; Ahmed, N.; Wakil, A.N.M.; Islam, K.M.A.; Arifin, M.S.; Al Mahbub, A.; Habib, R.; Mojumder, M.R.; et al. Skull Base Approaches for Tuberculum Sellae Meningiomas: Institutional Experience in a Series of 34 Patients. Life 2022, 12, 492. https://doi.org/10.3390/life12040492
Alam S, Ferini G, Muhammad N, Ahmed N, Wakil ANM, Islam KMA, Arifin MS, Al Mahbub A, Habib R, Mojumder MR, et al. Skull Base Approaches for Tuberculum Sellae Meningiomas: Institutional Experience in a Series of 34 Patients. Life. 2022; 12(4):492. https://doi.org/10.3390/life12040492
Chicago/Turabian StyleAlam, Shamsul, Gianluca Ferini, Nur Muhammad, Nazmin Ahmed, Abu Naim Mohammad Wakil, Kazi Mohammad Atiqul Islam, Mohammad Samsul Arifin, Abdullah Al Mahbub, Riad Habib, Mosiur Rahman Mojumder, and et al. 2022. "Skull Base Approaches for Tuberculum Sellae Meningiomas: Institutional Experience in a Series of 34 Patients" Life 12, no. 4: 492. https://doi.org/10.3390/life12040492
APA StyleAlam, S., Ferini, G., Muhammad, N., Ahmed, N., Wakil, A. N. M., Islam, K. M. A., Arifin, M. S., Al Mahbub, A., Habib, R., Mojumder, M. R., Vats, A., & Chaurasia, B. (2022). Skull Base Approaches for Tuberculum Sellae Meningiomas: Institutional Experience in a Series of 34 Patients. Life, 12(4), 492. https://doi.org/10.3390/life12040492








