How to Work with Electromyography Decomposition in Practical Classes of Exercise Physiology and Biomechanics
Abstract
:1. Introduction
2. Basis of Motor Unit Recruitment and Its Assessment
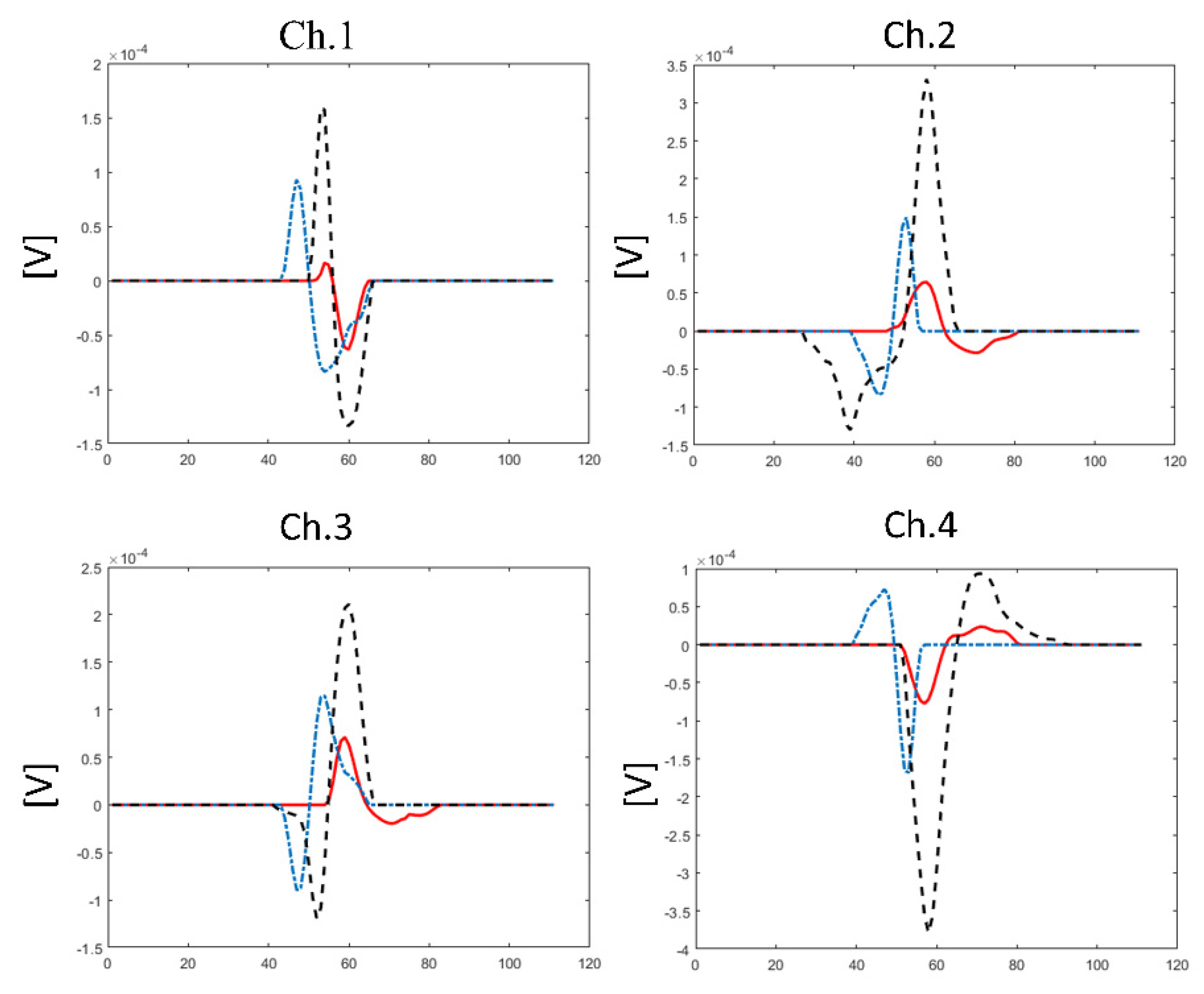
3. Decomposition of Surface Electromyography and Methodological Aspects for Practical Lessons
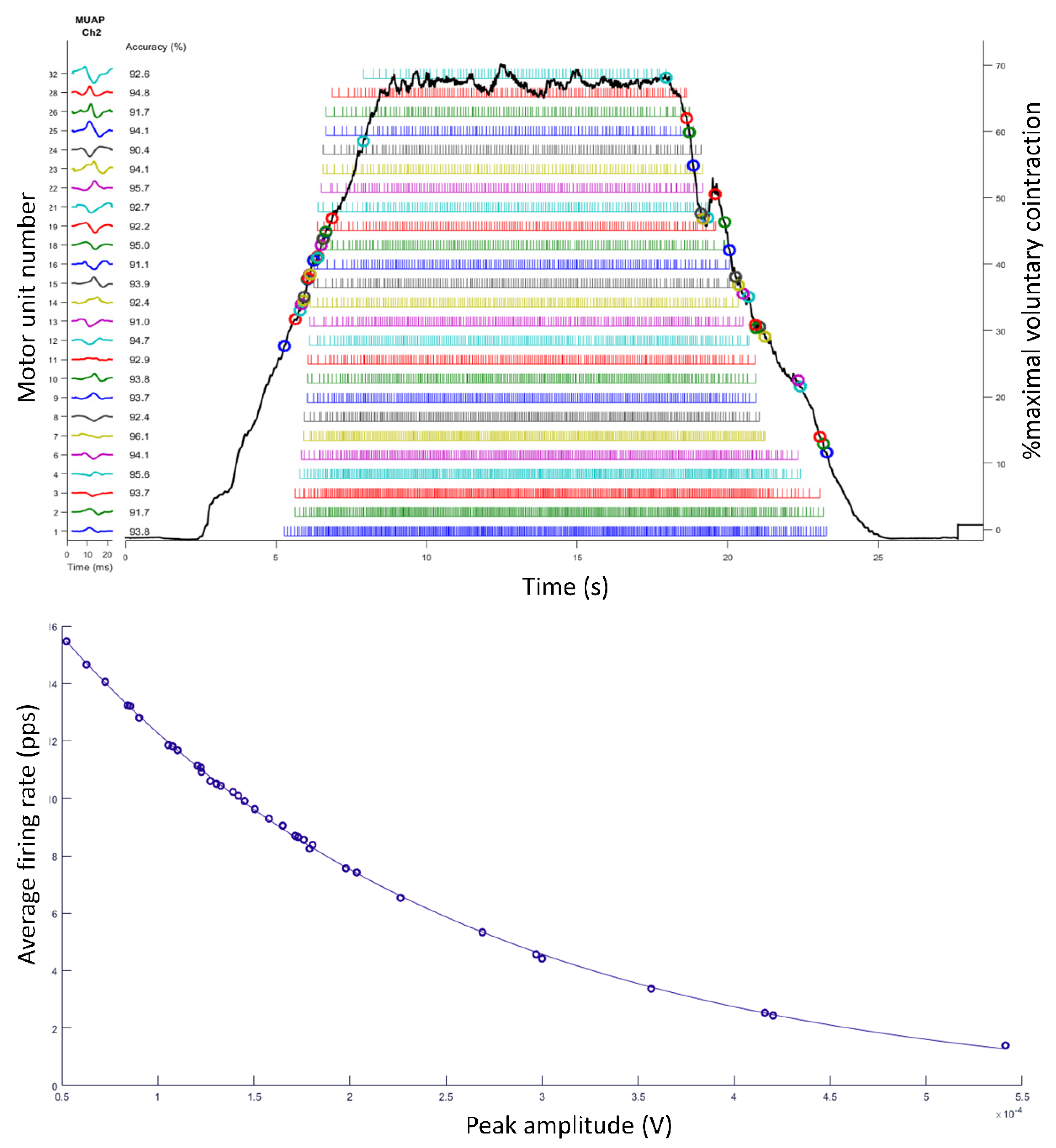
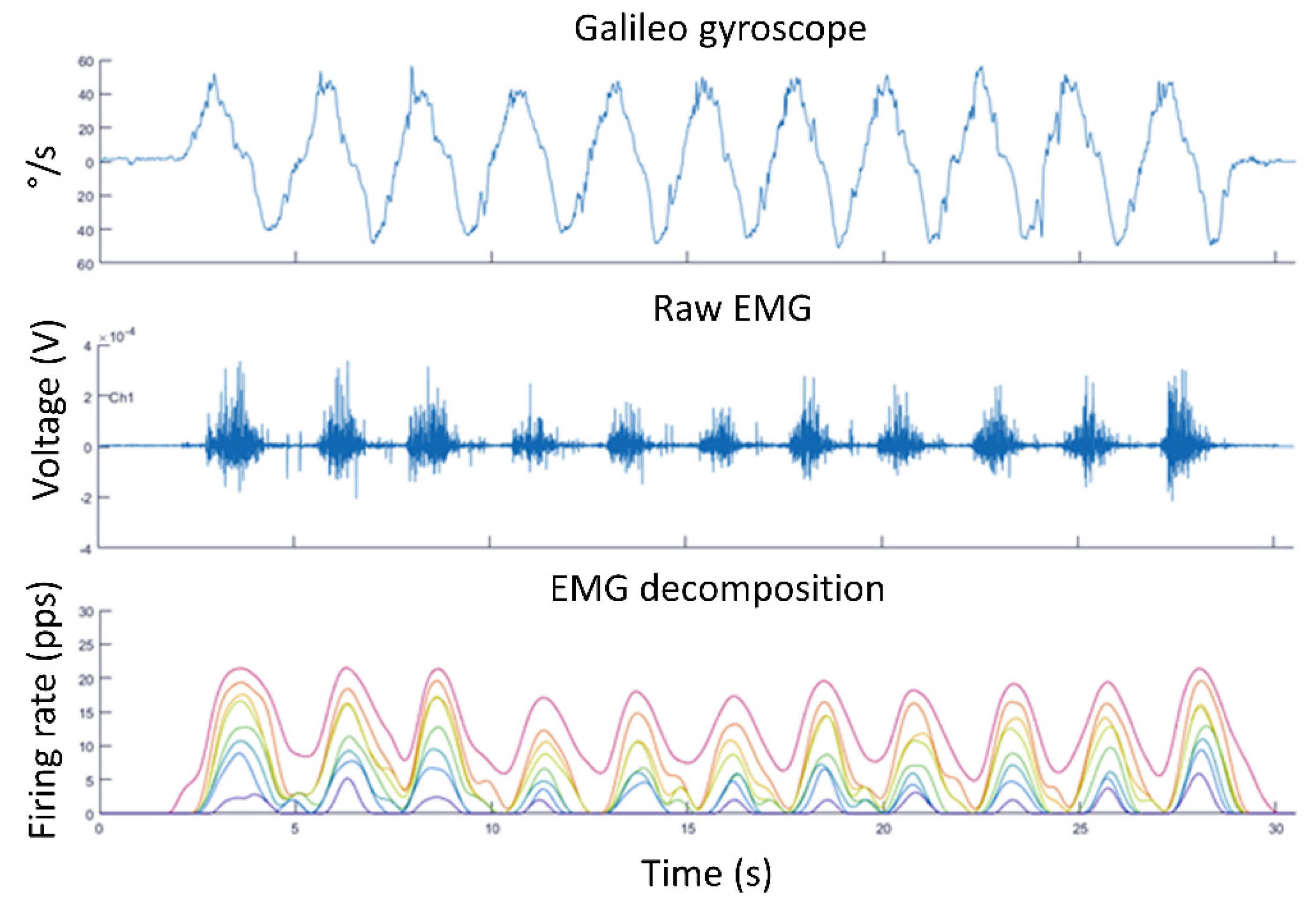
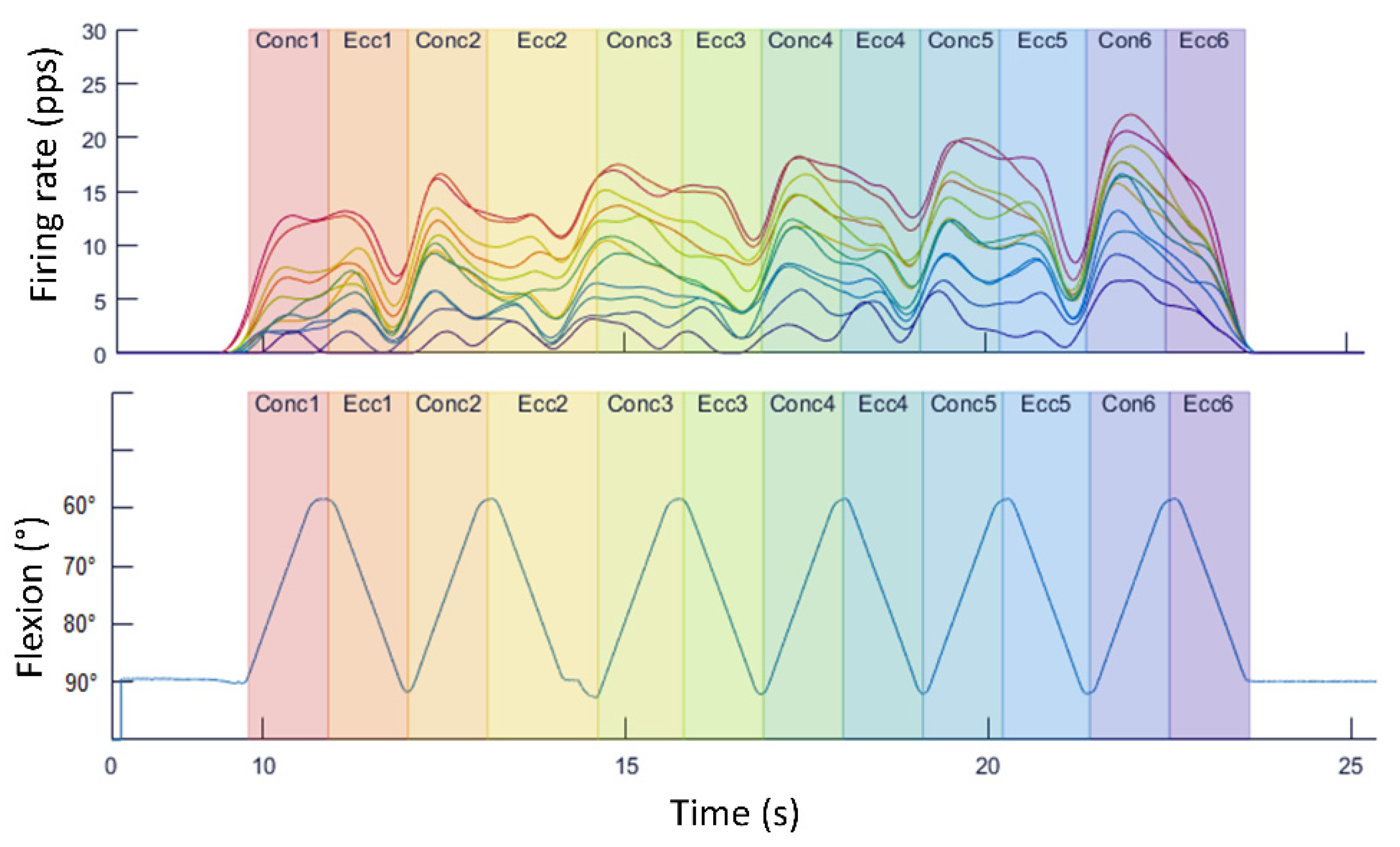
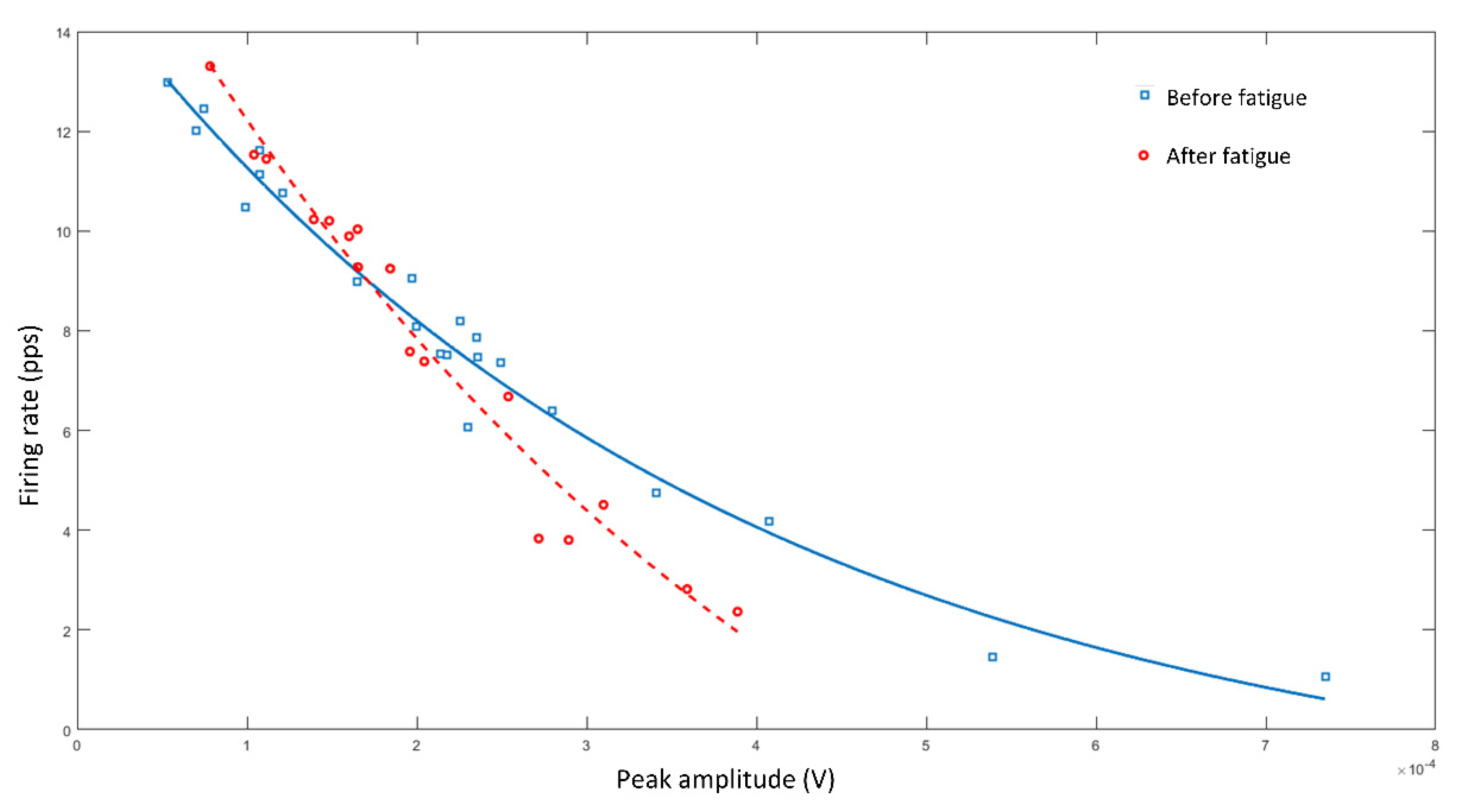
4. Examples of Practical Lessons Using Decomposition of Surface Electromyography for Teaching Motor Units’ Recruitment
- Data were collected using the Trigno Galileo Sensor (Delsys, Inc., Natick, MA, USA).
- Electrodes were placed according to the SENIAM guidelines [27,28]. Therefore, the electrodes were placed at 2/3 on the line from the anterior spina iliaca, superior to the lateral side of the patella, in the direction of the muscle fibers. For the determination of its location, the assessed person was sitting on a table with their knees in slight flexion and the upper body slightly bend backward.
- Data were recorded with a sampling rate of 2000 Hz.
- Data processing was performed by the software of Delsys that uses the Precision Decomposition III algorithm (version 1.1, Delsys, Inc., Natick, MA, USA).
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Holmes, J.W. Teaching from Classic Papers: Hill’s Model of Muscle Contraction. Adv. Physiol. Educ. 2006, 30, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Dobson, J.L. Learning Style Preferences and Course Performance in an Undergraduate Physiology Class. Adv. Physiol. Educ. 2009, 33, 308–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahaffey, A.L. Interfacing Virtual and Face-to-Face Teaching Methods in an Undergraduate Human Physiology Course for Health Professions Students. Adv. Physiol. Educ. 2018, 42, 477–481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samsel, R.W.; Schmidt, G.A.; Hall, J.B.; Wood, L.D.; Shroff, S.G.; Schumacker, P.T. Cardiovascular Physiology Teaching: Computer Simulations vs. Animal Demonstrations. Adv. Physiol. Educ. 1994, 266, S36. [Google Scholar] [CrossRef] [PubMed]
- Kojic, Z.Z.; Dewhurst, D.G. The Impact of Introducing Computer-Based Alternatives to the Use of Animals in the Teaching of Physiology and Pharmacology at Balkan Universities—A Pilot Study. Altern. Lab. Anim. 2009, 37, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Felici, F.; Del Vecchio, A. Surface Electromyography: What Limits Its Use in Exercise and Sport Physiology? Front. Neurol. 2020, 11, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Del Vecchio, A.; Holobar, A.; Falla, D.; Felici, F.; Enoka, R.M.; Farina, D. Tutorial: Analysis of Motor Unit Discharge Characteristics from High-Density Surface EMG Signals. J. Electromyogr. Kinesiol. 2020, 53, 102426. [Google Scholar] [CrossRef]
- Kapelner, T.; Jiang, N.; Holobar, A.; Vujaklija, I.; Roche, A.D.; Farina, D.; Aszmann, O.C. Motor Unit Characteristics after Targeted Muscle Reinnervation. PLoS ONE 2016, 11, e0149772. [Google Scholar] [CrossRef]
- De Luca, C.J.; Contessa, P. Biomechanical Benefits of the Onion-Skin Motor Unit Control Scheme. J. Biomech. 2015, 48, 195–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Girts, R.M.; Mota, J.A.; Harmon, K.K.; MacLennan, R.J.; Stock, M.S. Vastus Lateralis Motor Unit Recruitment Thresholds Are Compressed Towards Lower Forces in Older Men. J. Frailty Aging 2020, 9, 191–196. [Google Scholar] [CrossRef]
- Henneman, E. Relation between Size of Neurons and Their Susceptibility to Discharge. Science 1957, 126, 1345–1347. [Google Scholar] [CrossRef] [PubMed]
- De Luca, C.J.; Erim, Z. Common Drive of Motor Units in Regulation of Muscle Force. Trends Neurosci. 1994, 17, 299–305. [Google Scholar] [CrossRef]
- Priego Quesada, J.I.; Carpes, F.P.; Bini, R.R.; Salvador Palmer, R.; Pérez-Soriano, P.; Cibrián Ortiz de Anda, R.M. Relationship between Skin Temperature and Muscle Activation during Incremental Cycle Exercise. J. Therm. Biol. 2015, 48, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Diefenthaeler, F.; Bini, R.R.; Vaz, M.A. Frequency Band Analysis of Muscle Activation during Cycling to Exhaustion. Rev. Bras. Cineantropometria Desempenho Hum. 2012, 14, 243–253. [Google Scholar] [CrossRef]
- Priego Quesada, J.; Quesada, J.I.P.; Bini, R.R.; Diefenthaeler, F.; Carpes, F.P. Spectral Properties of Muscle Activation during Incremental Cycling Test. J. Sci. Cycl. 2015, 4, 7–13. [Google Scholar]
- Cogliati, M.; Cudicio, A.; Martinez-Valdes, E.; Tarperi, C.; Schena, F.; Orizio, C.; Negro, F. Half Marathon Induces Changes in Central Control and Peripheral Properties of Individual Motor Units in Master Athletes. J. Electromyogr. Kinesiol. 2020, 55, 102472. [Google Scholar] [CrossRef]
- Negro, F.; Bathon, K.E.; Nguyen, J.N.; Bannon, C.G.; Orizio, C.; Hunter, S.K.; Hyngstrom, A.S. Impaired Firing Behavior of Individually Tracked Paretic Motor Units During Fatiguing Contractions of the Dorsiflexors and Functional Implications Post Stroke. Front. Neurol. 2020, 11, 1310. [Google Scholar] [CrossRef]
- Cohen, J.W.; Vieira, T.; Ivanova, T.D.; Cerone, G.L.; Garland, S.J. Maintenance of Standing Posture during Multi-Directional Leaning Demands the Recruitment of Task-Specific Motor Units in the Ankle Plantarflexors. Exp. Brain Res. 2021, 239, 2569–2581. [Google Scholar] [CrossRef]
- Héroux, M.E.; Dakin, C.J.; Luu, B.L.; Inglis, J.T.; Blouin, J.-S. Absence of Lateral Gastrocnemius Activity and Differential Motor Unit Behavior in Soleus and Medial Gastrocnemius during Standing Balance. J. Appl. Physiol. 2014, 116, 140–148. [Google Scholar] [CrossRef] [Green Version]
- Hodges, P.W.; Ervilha, U.F.; Graven-Nielsen, T. Changes in Motor Unit Firing Rate in Synergist Muscles Cannot Explain the Maintenance of Force during Constant Force Painful Contractions. J. Pain 2008, 9, 1169–1174. [Google Scholar] [CrossRef]
- Mani, D.; Almuklass, A.M.; Hamilton, L.D.; Vieira, T.M.; Botter, A.; Enoka, R.M. Motor Unit Activity, Force Steadiness, and Perceived Fatigability Are Correlated with Mobility in Older Adults. J. Neurophysiol. 2018, 120, 1988–1997. [Google Scholar] [CrossRef] [PubMed]
- Hoshizaki, T.; Clancy, E.A.; Gabriel, D.A.; Green, L.A. The Reliability of Surface EMG Derived Motor Unit Variables. J. Electromyogr. Kinesiol. 2020, 52, 102419. [Google Scholar] [CrossRef] [PubMed]
- Farina, D. Counterpoint: Spectral Properties of the Surface EMG Do Not Provide Information about Motor Unit Recruitment and Muscle Fiber Type. J. Appl. Physiol. 2008, 105, 1673–1674. [Google Scholar] [CrossRef] [PubMed]
- De Luca, C.J.; Adam, A.; Wotiz, R.; Gilmore, L.D.; Nawab, S.H. Decomposition of Surface EMG Signals. J. Neurophysiol. 2006, 96, 1646–1657. [Google Scholar] [CrossRef]
- Contessa, P.; de Luca, C.J. Neural Control of Muscle Force: Indications from a Simulation Model. J. Neurophysiol. 2013, 109, 1548–1570. [Google Scholar] [CrossRef] [Green Version]
- Kapelner, T.; Vujaklija, I.; Jiang, N.; Negro, F.; Aszmann, O.C.; Principe, J.; Farina, D. Predicting Wrist Kinematics from Motor Unit Discharge Timings for the Control of Active Prostheses. J. Neuroeng. Rehabil. 2019, 16, 47. [Google Scholar] [CrossRef]
- Hermens, H.J.; Freriks, B.; Merletti, R.; Stegeman, D.; Blok, J.; Rau, G.; Disselhorst-Klug, C.; Hägg, G. European Recommendations for Surface Electromyography. Roessingh Res. Dev. 1999, 8, 13–54. [Google Scholar]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of Recommendations for SEMG Sensors and Sensor Placement Procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef]
- Sensor Locations. Available online: http://seniam.org/quadricepsfemorisvastuslateralis.html (accessed on 13 September 2021).
- De Luca, C.J.; Hostage, E.C. Relationship Between Firing Rate and Recruitment Threshold of Motoneurons in Voluntary Isometric Contractions. J. Neurophysiol. 2010, 104, 1034–1046. [Google Scholar] [CrossRef]
- Staron, R.S.; Hagerman, F.C.; Hikida, R.S.; Murray, T.F.; Hostler, D.P.; Crill, M.T.; Ragg, K.E.; Toma, K. Fiber Type Composition of the Vastus Lateralis Muscle of Young Men and Women. J. Histochem. Cytochem. 2000, 48, 623–629. [Google Scholar] [CrossRef] [Green Version]
- Van Tiggelen, D.; Cowan, S.; Coorevits, P.; Duvigneaud, N.; Witvrouw, E. Delayed Vastus Medialis Obliquus to Vastus Lateralis Onset Timing Contributes to the Development of Patellofemoral Pain in Previously Healthy Men: A Prospective Study. Am. J. Sports Med. 2009, 37, 1099–1105. [Google Scholar] [CrossRef]
- Hinman, R.S.; Bennell, K.L.; Metcalf, B.R.; Crossley, K.M. Delayed Onset of Quadriceps Activity and Altered Knee Joint Kinematics during Stair Stepping in Individuals with Knee Osteoarthritis. Arch. Phys. Med. Rehabil. 2002, 83, 1080–1086. [Google Scholar] [CrossRef] [PubMed]
- Stock, M.S.; Beck, T.W.; Defreitas, J.M. Effects of Fatigue on Motor Unit Firing Rate versus Recruitment Threshold Relationships. Muscle Nerve 2012, 45, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Contessa, P.; de Luca, C.J.; Kline, J.C. The Compensatory Interaction between Motor Unit Firing Behavior and Muscle Force during Fatigue. J. Neurophysiol. 2016, 116, 1579–1585. [Google Scholar] [CrossRef] [Green Version]
- Conwit, R.A.; Stashuk, D.; Tracy, B.; McHugh, M.; Brown, W.F.; Metter, E.J. The Relationship of Motor Unit Size, Firing Rate and Force. Clin. Neurophysiol. 1999, 110, 1270–1275. [Google Scholar] [CrossRef]
- Adam, A.; de Luca, C.J. Firing Rates of Motor Units in Human Vastus Lateralis Muscle during Fatiguing Isometric Contractions. J. Appl. Physiol. 2005, 99, 268–280. [Google Scholar] [CrossRef]
- Neyroud, D.; Samararatne, J.; Kayser, B.; Place, N. Neuromuscular Fatigue After Repeated Jumping With Concomitant Electrical Stimulation. Int. J. Sports Physiol. Perform. 2017, 12, 1335–1340. [Google Scholar] [CrossRef]
- Lessi, G.C.; Silva, R.S.; Serrão, F.V. Comparison of the Effects of Fatigue on Kinematics and Muscle Activation between Men and Women after Anterior Cruciate Ligament Reconstruction. Phys. Ther. Sport 2018, 31, 29–34. [Google Scholar] [CrossRef]
- Mettler, J.A.; Griffin, L. Muscular Endurance Training and Motor Unit Firing Patterns during Fatigue. Exp. Brain Res. 2016, 234, 267–276. [Google Scholar] [CrossRef]
- Potvin, J.R.; Fuglevand, A.J. A Motor Unit-Based Model of Muscle Fatigue. PLOS Comput. Biol. 2017, 13, e1005581. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Priego-Quesada, J.I.; Goethel, M.F.; Becker, K.M.; Fernandes, R.J.; Vilas-Boas, J.P. How to Work with Electromyography Decomposition in Practical Classes of Exercise Physiology and Biomechanics. Life 2022, 12, 483. https://doi.org/10.3390/life12040483
Priego-Quesada JI, Goethel MF, Becker KM, Fernandes RJ, Vilas-Boas JP. How to Work with Electromyography Decomposition in Practical Classes of Exercise Physiology and Biomechanics. Life. 2022; 12(4):483. https://doi.org/10.3390/life12040483
Chicago/Turabian StylePriego-Quesada, Jose I., Márcio F. Goethel, Klaus Magno Becker, Ricardo J. Fernandes, and João Paulo Vilas-Boas. 2022. "How to Work with Electromyography Decomposition in Practical Classes of Exercise Physiology and Biomechanics" Life 12, no. 4: 483. https://doi.org/10.3390/life12040483
APA StylePriego-Quesada, J. I., Goethel, M. F., Becker, K. M., Fernandes, R. J., & Vilas-Boas, J. P. (2022). How to Work with Electromyography Decomposition in Practical Classes of Exercise Physiology and Biomechanics. Life, 12(4), 483. https://doi.org/10.3390/life12040483









