Current Status of Neuromodulation-Induced Cortical Prehabilitation and Considerations for Treatment Pathways in Lower-Grade Glioma Surgery
Abstract
1. Introduction
2. Current Surgical Approaches and Outcomes Relating to Lower Grade Glioma Surgery
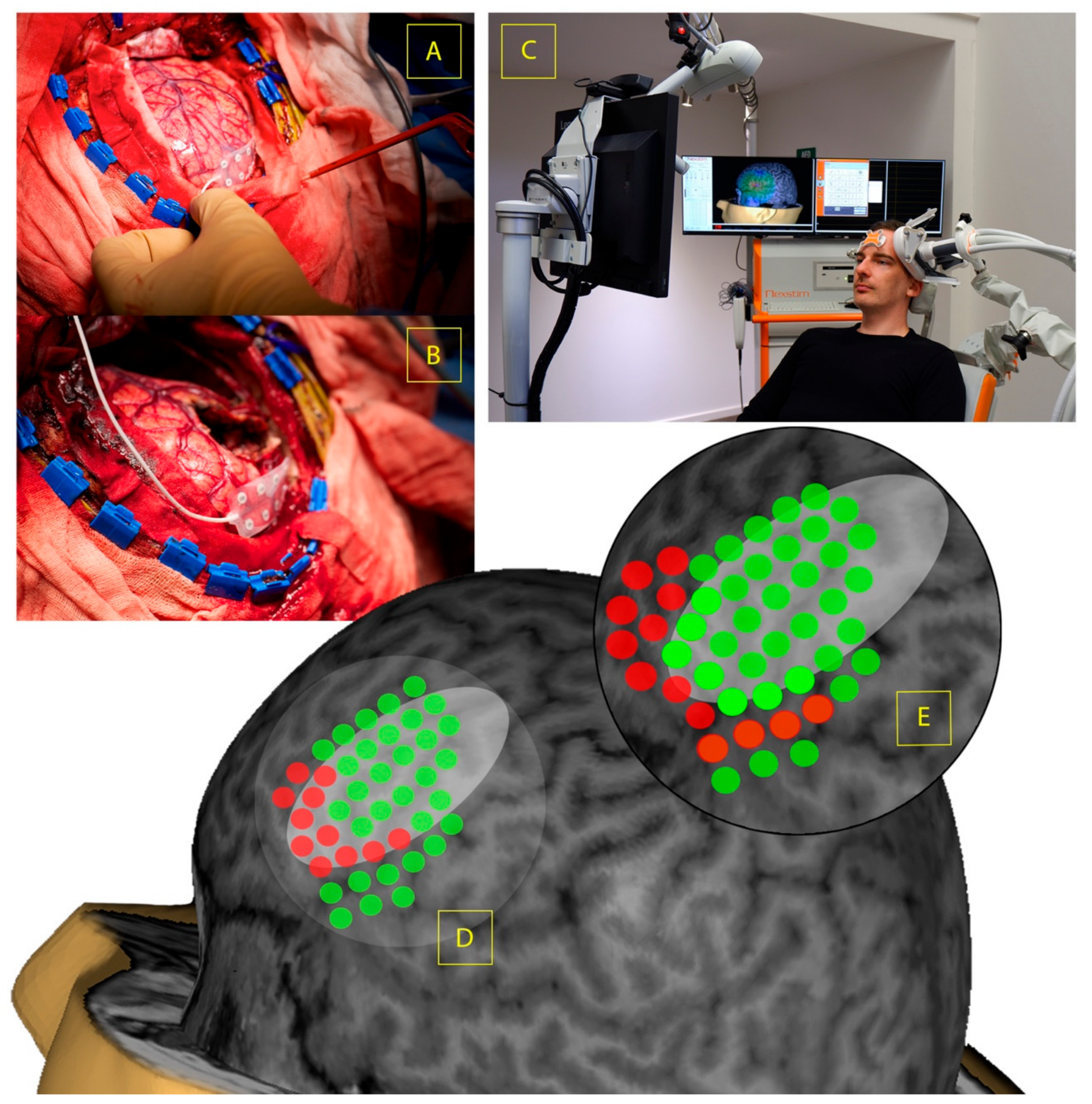
3. Electrophysiologic Mechanisms and Techniques Relating to Invasive and Non-Invasive Brain Mapping in Glioma Surgery
4. Current Applications of Non-Invasive Neuromodulation in Psychiatric and Rehabilitation Settings
5. Current Case Studies Attempting to Induce Cortical Plasticity in Brain Tumour Patients
6. Technical and Neuroethical Implications of Prehabilitation Relating to Clinical Decision Making in Lower Grade Glioma Surgery
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pascual-Leone, A.; Amedi, A.; Fregni, F.; Merabet, L.B. The plastic human brain cortex. Annu. Rev. Neurosci. 2005, 28, 377–401. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, S.; Caulo, M.; Pieri, V.; Falini, A.; Castellano, A. Role of Functional Imaging Techniques to Assess Motor and Language Cortical Plasticity in Glioma Patients: A Systematic Review. Neural Plast. 2019, 11, 4056436. [Google Scholar] [CrossRef]
- Carrera, E.; Tononi, G. Diaschisis: Past, present, future. Brain 2014, 137 Pt 9, 2408–2422. [Google Scholar] [CrossRef]
- Fornito, A.; Zalesky, A.; Breakspear, M. The connectomics of brain disorders. Nat. Rev. Neurosci. 2015, 16, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Herbet, G.; Duffau, H. Revisiting the Functional Anatomy of the Human Brain: Toward a Meta-Networking Theory of Cerebral Functions. Physiol. Rev. 2020, 100, 1181–1228. [Google Scholar] [CrossRef] [PubMed]
- Ojemann, G.; Ojemann, J.; Lettich, E.; Berger, M. Cortical language localization in left, dominant hemisphere. An electrical stimulation mapping investigation in 117 patients. J. Neurosurg. 1989, 71, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, P.; Dick, A.S. Broca and Wernicke are dead, or moving past the classic model of language neurobiology. Brain Lang. 2016, 162, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Duffau, H. The error of Broca: From the traditional localizationist concept to a connectomal anatomy of human brain. J. Chem. Neuroanat. 2018, 89, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Hickok, G.; Poeppel, D. Dorsal and ventral streams: A framework for understanding aspects of the functional anatomy of language. Cognition 2004, 92, 67–99. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.F.; Raygor, K.P.; Berger, M.S. Contemporary model of language organization: An overview for neurosurgeons. J. Neurosurg. 2015, 122, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Plaza, M.; Gatignol, P.; Leroy, M.; Duffau, H. Speaking without Broca’s area after tumor resection. Neurocase 2009, 15, 294–310. [Google Scholar] [CrossRef] [PubMed]
- Duffau, H.; Capelle, L.; Denvil, D.; Sichez, N.; Gatignol, P.; Lopes, M.; Mitchell, M.-C.; Sichez, J.-P.; Van Effenterre, R. Functional recovery after surgical resection of low grade gliomas in eloquent brain: Hypothesis of brain compensation. J. Neurol. Neurosurg. Psychiatry 2003, 74, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Duffau, H. Lessons from brain mapping in surgery for low-grade glioma: Insights into associations between tumour and brain plasticity. Lancet Neurol. 2005, 4, 476–486. [Google Scholar] [CrossRef]
- Duffau, H. New concepts in surgery of WHO grade II gliomas: Functional brain mapping, connectionism and plasticity—A review. J. Neurooncol. 2006, 79, 77–115. [Google Scholar] [CrossRef] [PubMed]
- Benzagmout, M.; Gatignol, P.; Duffau, H. Resection of World Health Organization Grade II gliomas involving Broca’s area: Methodological and functional considerations. Neurosurgery 2007, 61, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Duffau, H. What Direct Electrostimulation of the Brain Taught Us About the Human Connectome: A Three-Level Model of Neural Disruption. Front. Hum. Neurosci. 2020, 14, 315. [Google Scholar] [CrossRef] [PubMed]
- Giussani, C.; Roux, F.-E.; Ojemann, J.; Sganzerla, E.P.; Pirillo, D.; Papagno, C. Is preoperative functional magnetic resonance imaging reliable for language areas mapping in brain tumor surgery? Review of language functional magnetic resonance imaging and direct cortical stimulation correlation studies. Neurosurgery 2010, 66, 113–120. [Google Scholar] [CrossRef]
- Roux, F.E.; Boulanouar, K.; Ranjeva, J.P.; Tremoulet, M.; Henry, P.; Manelfe, C.; Sabatier, J.; Berry, I. Usefulness of motor functional MRI correlated to cortical mapping in Rolandic low-grade astrocytomas. Acta Neurochir. 1999, 141, 71–79. [Google Scholar] [CrossRef]
- Lee, M.H.; Smyser, C.D.; Shimony, J.S. Resting-state fMRI: A review of methods and clinical applications. AJNR Am. J. Neuroradiol. 2013, 34, 1866–1872. [Google Scholar] [CrossRef] [PubMed]
- Korvenoja, A.; Kirveskari, E.; Aronen, H.J.; Avikainen, S.; Brander, A.; Huttunen, J.; Ilmoniemi, R.; Jääskeläinen, J.E.; Kovala, T.; Mäkelä, J.P.; et al. Sensorimotor cortex localization: Comparison of magnetoencephalography, functional MR imaging, and intraoperative cortical mapping. Radiology 2006, 241, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Schiffbauer, H.; Berger, M.S.; Ferrari, P.; Freudenstein, D.; Rowley, H.A.; Roberts, T.P.L. Preoperative magnetic source imaging for brain tumor surgery: A quantitative comparison with intraoperative sensory and motor mapping. J. Neurosurg. 2002, 97, 1333–1342. [Google Scholar] [PubMed]
- Tarapore, P.E.; Martino, J.; Guggisberg, A.G.; Owen, J.; Honma, S.M.; Findlay, A.; Berger, M.S.; Kirsch, H.E.; Nagarajan, S.S. Magnetoencephalographic imaging of resting-state functional connectivity predicts postsurgical neurological outcome in brain gliomas. Neurosurgery 2012, 71, 1012–1022. [Google Scholar] [PubMed]
- Wagner, T.; Valero-Cabre, A.; Pascual-Leone, A. Noninvasive human brain stimulation. Annu. Rev. Biomed. Eng. 2007, 9, 527–565. [Google Scholar] [CrossRef] [PubMed]
- Mills, K.R.; Murray, N.M.; Hess, C.W. Magnetic and electrical transcranial brain stimulation: Physiological mechanisms and clinical applications. Neurosurgery 1987, 20, 164–168. [Google Scholar]
- Haddad, A.F.; Young, J.S.; Berger, M.S.; Tarapore, P.E. Preoperative Applications of Navigated Transcranial Magnetic Stimulation. Front. Neurol. 2021, 11, 628903. [Google Scholar] [CrossRef] [PubMed]
- Sollmann, N.; Krieg, S.M.; Säisänen, L.; Julkunen, P. Mapping of Motor Function with Neuronavigated Transcranial Magnetic Stimulation: A Review on Clinical Application in Brain Tumors and Methods for Ensuring Feasible Accuracy. Brain Sci. 2021, 11, 897. [Google Scholar] [CrossRef] [PubMed]
- Jeltema, H.R.; Ohlerth, A.K.; de Wit, A.; Wagemakers, M.; Rofes, A.; Bastiaanse, R.; Drost, G. Comparing navigated transcranial magnetic stimulation mapping and “gold standard” direct cortical stimulation mapping in neurosurgery: A systematic review. Neurosurg. Rev. 2021, 44, 1903–1920. [Google Scholar] [CrossRef] [PubMed]
- Krieg, S.; Ringel, F.; Meyer, B. Functional guidance in intracranial tumor surgery. Perspect. Med. 2012, 1, 59–64. [Google Scholar] [CrossRef][Green Version]
- Henderson, F.; Abdullah, K.G.; Verma, R.; Brem, S. Tractography and the connectome in neurosurgical treatment of gliomas: The premise, the progress, and the potential. Neurosurg. Focus. 2020, 48, E6. [Google Scholar] [CrossRef] [PubMed]
- Krishna, V.; Sammartino, F.; Rezai, A. A Review of the Current Therapies, Challenges, and Future Directions of Transcranial Focused Ultrasound Technology: Advances in Diagnosis and Treatment. JAMA Neurol. 2018, 75, 246–254. [Google Scholar] [CrossRef]
- Smith, J.S.; Chang, E.F.; Lamborn, K.R.; Chang, S.M.; Prados, M.D.; Cha, S.; Tihan, T.; Vandenberg, S.; McDermott, M.W.; Berger, M.S. Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J. Clin. Oncol. 2008, 26, 1338–1345. [Google Scholar] [CrossRef]
- Keles, G.E.; Lundin, D.A.; Lamborn, K.R.; Chang, E.F.; Ojemann, G.; Berger, M.S. Intraoperative subcortical stimulation mapping for hemispherical perirolandic gliomas located within or adjacent to the descending motor pathways: Evaluation of morbidity and assessment of functional outcome in 294 patients. J. Neurosurg. 2004, 100, 369–375. [Google Scholar] [CrossRef]
- Chaichana, K.L.; McGirt, M.J.; Niranjan, A.; Olivi, A.; Burger, P.C.; Quinones-Hinojosa, A. Prognostic significance of contrast-enhancing low-grade gliomas in adults and a review of the literature. Neurol. Res. 2009, 31, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Fang, C.; Chen, G.; Sun, C. Relationship between the extent of resection and the survival of patients with low-grade gliomas: A systematic review and meta-analysis. BMC Cancer 2018, 18, 48. [Google Scholar] [CrossRef] [PubMed]
- Chaichana, K.L.; Jusue-Torres, I.; Navarro-Ramirez, R.; Raza, S.M.; Pascual-Gallego, M.; Ibrahim, A.; Hernandez-Hermann, M.; Gomez, L.; Ye, X.; Weingart, J.D.; et al. Establishing percent resection and residual volume thresholds affecting survival and recurrence for patients with newly diagnosed intracranial glioblastoma. Neuro Oncol. 2014, 16, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Keles, G.E.; Chang, E.F.; Lamborn, K.R.; Tihan, T.; Chang, C.J.; Chang, S.M.; Berger, M.S. Volumetric extent of resection and residual contrast enhancement on initial surgery as predictors of outcome in adult patients with hemispheric anaplastic astrocytoma. J. Neurosurg. 2006, 105, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, M.; Abi-Said, D.; Fourney, D.R.; Gokaslan, Z.L.; Shi, W.; Demonte, F.; Lang, F.F.; McCutcheon, I.E.; Hassenbusch, S.J.; Holland, E.; et al. A multivariate analysis of 416 patients with glioblastoma multiforme: Prognosis, extent of resection, and survival. J. Neurosurg. 2001, 95, 190–198. [Google Scholar] [CrossRef]
- Lamborn, K.R.; Chang, S.M.; Prados, M.D. Prognostic factors for survival of patients with glioblastoma: Recursive partitioning analysis. Neuro Oncol. 2004, 6, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Jeremić, B.; Grulicić, D.; Samardzić, M.; Antunović, V.; Joksimović, M.; Djurić Lj Milicić, B.; Nikolić, N. Uticaj obima resekcije tumora na ishod kombinovanog lecenja bolesnika s multiformnim glioblastomom [The effect of extent of tumor resection on the outcome of combined therapy in patients with glioblastoma multiforme]. Srp. Arh. Celok. Lek. 1997, 125, 93–98. [Google Scholar] [PubMed]
- Stummer, W.; Reulen, H.J.; Meinel, T.; Pichlmeier, U.; Schumacher, W.; Tonn, J.C.; Rohde, V.; Oppel, F.; Turowski, B.; Woiciechowsky, C.; et al. Extent of resection and survival in glioblastoma multiforme: Identification of and adjustment for bias. Neurosurgery 2008, 62, 564–576. [Google Scholar] [CrossRef] [PubMed]
- Ushio, Y.; Kochi, M.; Hamada, J.; Kai, Y.; Nakamura, H. Effect of surgical removal on survival and quality of life in patients with supratentorial glioblastoma. Neurol. Med. Chir. 2005, 45, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Duffau, H. Can Non-invasive Brain Stimulation Be Considered to Facilitate Reoperation for Low-Grade Glioma Relapse by Eliciting Neuroplasticity? Front. Neurol. 2020, 11, 582489. [Google Scholar] [CrossRef] [PubMed]
- Obara, T.; Blonski, M.; Brzenczek, C.; Mézières, S.; Gaudeau, Y.; Pouget, C.; Gauchotte, G.; Verger, A.; Vogin, G.; Moureaux, J.M.; et al. Adult Diffuse Low-Grade Gliomas: 35-Year Experience at the Nancy France Neurooncology Unit. Front. Oncol. 2020, 10, 574–679. [Google Scholar] [CrossRef] [PubMed]
- Claus, E.B.; Walsh, K.M.; Wiencke, J.K.; Molinaro, A.M.; Wiemels, J.L.; Schildkraut, J.M.; Bondy, M.L.; Berger, M.; Jenkins, R.; Wrensch, M. Survival and low-grade glioma: The emergence of genetic information. Neurosurg. Focus. 2015, 38, E6. [Google Scholar] [CrossRef] [PubMed]
- Gogos, A.J.; Young, J.S.; Morshed, R.A.; Hervey-Jumper, S.L.; Berger, M.S. Awake glioma surgery: Technical evolution and nuances. J. Neurooncol. 2020, 147, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Sefcikova, V.; Sporrer, J.K.; Ekert, J.O.; Kirkman, M.A.; Samandouras, G. High Interrater Variability in Intraoperative Language Testing and Interpretation in Awake Brain Mapping Among Neurosurgeons or Neuropsychologists: An Emerging Need for Standardization. World Neurosurg. 2020, 141, e651–e660. [Google Scholar] [CrossRef] [PubMed]
- Magill, S.T.; Han, S.J.; Li, J.; Berger, M.S. Resection of primary motor cortex tumors: Feasibility and surgical outcomes. J. Neurosurg. 2018, 129, 961–972. [Google Scholar] [CrossRef]
- Sanai, N.; Mirzadeh, Z.; Berger, M.S. Functional outcome after language mapping for glioma resection. N. Engl. J. Med. 2008, 358, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Gerritsen, J.K.W.; Viëtor, C.L.; Rizopoulos, D.; Schouten, J.W.; Klimek, M.; Dirven, C.M.F.; Vincent, A.J.E. Awake craniotomy versus craniotomy under general anesthesia without surgery adjuncts for supratentorial glioblastoma in eloquent areas: A retrospective matched case-control study. Acta Neurochir. 2019, 161, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Bu, L.H.; Zhang, J.; Lu, J.F.; Wu, J.S. Glioma surgery with awake language mapping versus generalized anesthesia: A systematic review. Neurosurg. Rev. 2021, 44, 1997–2011. [Google Scholar] [CrossRef]
- Suarez-Meade, P.; Marenco-Hillembrand, L.; Prevatt, C.; Murguia-Fuentes, R.; Mohamed, A.; AlSaeed, T.; Lehrer, E.; Brigham, T.; Ruiz-Garcia, H.; Sabsevitz, D.; et al. Awake vs. asleep motor mapping for glioma resection: A systematic review and meta-analysis. Acta Neurochir. 2020, 162, 1709–1720. [Google Scholar] [CrossRef] [PubMed]
- Kong, N.W.; Gibb, W.R.; Tate, M.C. Neuroplasticity: Insights from Patients Harboring Gliomas. Neural Plast. 2016, 2016, 2365063. [Google Scholar] [CrossRef]
- Duffau, H.; Lopes, M.; Arthuis, F.; Bitar, A.; Sichez, J.P.; Van Effenterre, R.; Capelle, L. Contribution of intraoperative electrical stimulations in surgery of low grade gliomas: A comparative study between two series without (1985–96) and with (1996–2003) functional mapping in the same institution. J. Neurol. Neurosurg. Psychiatry 2005, 76, 845–851. [Google Scholar] [CrossRef] [PubMed]
- De Witt Hamer, P.C.; Robles, S.G.; Zwinderman, A.H.; Duffau, H.; Berger, M.S. Impact of intraoperative stimulation brain mapping on glioma surgery outcome: A meta-analysis. J. Clin. Oncol. 2012, 30, 2559–2565. [Google Scholar] [CrossRef]
- De Witt Hamer, P.C.; De Witt Hamer, P.C.; Klein, M.; Hervey-Jumper, S.L.; Wefel, J.S.; Berger, M.S. Functional Outcomes and Health-Related Quality of Life Following Glioma Surgery. Neurosurgery 2021, 88, 720–732. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.; Shah, A.H.; Bregy, A.; Shah, N.H.; Thambuswamy, M.; Barbarite, E.; Fuhrman, T.; Komotar, R.J. Awake craniotomy for brain tumor resection: The rule rather than the exception? J. Neurosurg. Anesthesiol. 2013, 25, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, R.; Kinoshita, M.; Yahata, T.; Nakada, M. Recovery time from supplementary motor area syndrome: Relationship to postoperative day 7 paralysis and damage of the cingulum. J. Neurosurg. 2019, 132, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Hervey-Jumper, S.L.; Li, J.; Lau, D.; Molinaro, A.M.; Perry, D.W.; Meng, L.; Berger, M.S. Awake craniotomy to maximize glioma resection: Methods and technical nuances over a 27-year period. J. Neurosurg. 2015, 123, 325–339. [Google Scholar] [CrossRef] [PubMed]
- Kombos, T.; Süss, O. Neurophysiological basis of direct cortical stimulation and applied neuroanatomy of the motor cortex: A review. Neurosurg. Focus 2009, 27, E3. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.V.; Sheth, S.A.; Eckhardt, C.A.; Kilbride, R.D.; Braver, D.; Williams, Z.; Curry, W.; Cahill, D.; Eskandar, E.N. Phase reversal technique decreases cortical stimulation time during motor mapping. J. Clin. Neurosci. 2014, 21, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
- Kombos, T.; Suess, O.; Kern, B.C.; Funk, T.; Hoell, T.; Kopetsch, O.; Brock, M. Comparison between monopolar and bipolar electrical stimulation of the motor cortex. Acta Neurochir. 1999, 141, 1295–1301. [Google Scholar] [CrossRef]
- Kombos, T.; Suess, O.; Funk, T.; Kern, B.C.; Brock, M. Intra-operative mapping of the motor cortex during surgery in and around the motor cortex. Acta Neurochir. 2000, 142, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Szelényi, A.; Bello, L.; Duffau, H.; Fava, E.; Feigl, G.C.; Galanda, M.; Neuloh, G.; Signorelli, F.; Sala, F.; Workgroup for Intraoperative Management in Low-Grade Glioma Surgery within the European Low-Grade Glioma Network. Intraoperative electrical stimulation in awake craniotomy: Methodological aspects of current practice. Neurosurg. Focus 2010, 28, E7. [Google Scholar] [CrossRef]
- Bello, L.; Riva, M.; Fava, E.; Ferpozzi, V.; Castellano, A.; Raneri, F.; Pessina, F.; Bizzi, A.; Falini, A.; Cerri, G. Tailoring neurophysiological strategies with clinical context enhances resection and safety and expands indications in gliomas involving motor pathways. Neuro Oncol. 2014, 16, 1110–1128. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Sciortino, T.; Nibali, M.C.; Gay, L.; Viganò, L.; Puglisi, G.; Leonetti, A.; Howells, H.; Fornia, L.; Cerri, G.; et al. Clinical Pearls and Methods for Intraoperative Motor Mapping. Neurosurgery 2021, 88, 457–467. [Google Scholar] [CrossRef]
- Bello, L.; Acerbi, F.; Giussani, C.; Baratta, P.; Taccone, P.; Songa, V.; Fava, M.; Stocchetti, N.; Papagno, C.; Gaini, S.M. Intraoperative language localization in multilingual patients with gliomas. Neurosurgery 2006, 59, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Bello, L.; Gallucci, M.; Fava, M.; Carrabba, G.; Giussani, C.; Acerbi, F.; Baratta, P.; Songa, V.; Conte, V.; Branca, V.; et al. Intraoperative subcortical language tract mapping guides surgical removal of gliomas involving speech areas. Neurosurgery 2007, 60, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Riva, M.; Fava, E.; Gallucci, M.; Comi, A.; Casarotti, A.; Alfiero, T.; Raneri, F.A.; Pessina, F.; Bello, L. Monopolar high-frequency language mapping: Can it help in the surgical management of gliomas? A comparative clinical study. J. Neurosurg. 2016, 124, 1479–1489. [Google Scholar] [CrossRef] [PubMed]
- Verst, S.M.; de Aguiar, P.; Joaquim, M.; Vieira, V.G.; Sucena, A.; Maldaun, M. Monopolar 250–500 Hz language mapping: Results of 41 patients. Clin. Neurophysiol. Pract. 2018, 4, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pouratian, N.; Bookheimer, S.Y. The reliability of neuroanatomy as a predictor of eloquence: A review. Neurosurg. Focus 2010, 28, E3. [Google Scholar] [CrossRef]
- Penfield, W.; Boldrey, E. Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain 1937, 60, 389–443. [Google Scholar] [CrossRef]
- Ojemann, G.A.; Whitaker, H.A. The bilingual brain. Arch. Neurol. 1978, 35, 409–412. [Google Scholar] [CrossRef]
- Ojemann, G.A. Functional mapping of cortical language areas in adults. Intraoperative approaches. Adv. Neurol. 1993, 63, 155–163. [Google Scholar] [PubMed]
- Berger, M.S.; Ojemann, G.A. Intraoperative brain mapping techniques in neuro-oncology. Stereotact. Funct. Neurosurg. 1992, 58, 153–161. [Google Scholar] [CrossRef]
- Seidel, K.; Beck, J.; Stieglitz, L.; Schucht, P.; Raabe, A. The warning-sign hierarchy between quantitative subcortical motor mapping and continuous motor evoked potential monitoring during resection of supratentorial brain tumors. J. Neurosurg. 2013, 118, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Hamer, R.P.; Jain, S.; Teo, C.; Loh, W.N.; Chan, H.M.; Yeo, T.T.; Teo, K. Optimizing the onco-functional balance in supratentorial brain tumour surgery: A single institution’s initial experience with intraoperative cortico-subcortical mapping and monitoring in Singapore. J. Clin. Neurosci. 2020, 79, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Szelényi, A.; Joksimovic, B.; Seifert, V. Intraoperative risk of seizures associated with transient direct cortical stimulation in patients with symptomatic epilepsy. J. Clin. Neurophysiol. 2007, 24, 39–43. [Google Scholar] [CrossRef]
- Asimakidou, E.; Abut, P.A.; Raabe, A.; Seidel, K. Motor Evoked Potential Warning Criteria in Supratentorial Surgery: A Scoping Review. Cancers 2021, 13, 2803. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Nibali, M.C.; Viganò, L.; Puglisi, G.; Howells, H.; Gay, L.; Sciortino, T.; Leonetti, A.; Riva, M.; Fornia, L.; et al. Resection of tumors within the primary motor cortex using high-frequency stimulation: Oncological and functional efficiency of this versatile approach based on clinical conditions. J. Neurosurg. 2019, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Gogos, A.J.; Young, J.S.; Morshed, R.A.; Avalos, L.N.; Noss, R.S.; Villanueva-Meyer, J.E.; Hervey-Jumper, S.L.; Berger, M.S. Triple motor mapping: Transcranial, bipolar, and monopolar mapping for supratentorial glioma resection adjacent to motor pathways. J. Neurosurg. 2020, 134, 1728–1737. [Google Scholar] [CrossRef]
- Mandonnet, E.; Duffau, H. An attempt to conceptualize the individual onco-functional balance: Why a standardized treatment is an illusion for diffuse low-grade glioma patients. Crit. Rev. Oncol./Hematol. 2018, 122, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Połczyńska, M.M.; Bookheimer, S.Y. Factors Modifying the Amount of Neuroanatomical Overlap between Languages in Bilinguals-A Systematic Review of Neurosurgical Language Mapping Studies. Brain Sci. 2020, 10, 983. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Chan, H.M.; Yeo, T.T.; Teo, K. Language Mapping of Hindi and English in a Bilingual Patient During Resection of a Right Frontal Glioma. World Neurosurg. 2019, 125, 106–110. [Google Scholar] [CrossRef]
- De Martino, M.; Talacchi, A.; Capasso, R.; Mazzotta, A.; Miceli, G. Language Assessment in Multilingualism and Awake Neurosurgery. Front. Hum. Neurosci. 2021, 15, 750013. [Google Scholar] [CrossRef] [PubMed]
- Landers, M.J.F.; Sitskoorn, M.M.; Rutten, G.M.; Mandonnet, E.; De Baene, W. A systematic review of the use of subcortical intraoperative electrical stimulation mapping for monitoring of executive deficits and neglect: What is the evidence so far? Acta Neurochir. 2022, 164, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Samandouras, G. Extended testing for cognition: Has awake brain mapping moved to the next level? Acta Neurochir. 2022, 164, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Erez, Y.; Assem, M.; Coelho, P.; Romero-Garcia, R.; Owen, M.; McDonald, A.; Woodberry, E.; Morris, R.C.; Price, S.J.; Suckling, J.; et al. Intraoperative mapping of executive function using electrocorticography for patients with low-grade gliomas. Acta Neurochir. 2021, 163, 1299–1309. [Google Scholar] [CrossRef] [PubMed]
- Puglisi, G.; Sciortino, T.; Rossi, M.; Leonetti, A.; Fornia, L.; Conti Nibali, M.; Casarotti, A.; Pessina, F.; Riva, M.; Cerri, G.; et al. Preserving executive functions in nondominant frontal lobe glioma surgery: An intraoperative tool. J. Neurosurg. 2018, 131, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Wager, M.; Du Boisgueheneuc, F.; Pluchon, C.; Bouyer, C.; Stal, V.; Bataille, B.; Guillevin, C.M.; Gil, R. Intraoperative monitoring of an aspect of executive functions: Administration of the Stroop test in 9 adult patients during awake surgery for resection of frontal glioma. Neurosurgery 2013, 72, ons169–ons180; discussion ons180–ons181. [Google Scholar] [CrossRef] [PubMed]
- Vallar, G.; Bello, L.; Bricolo, E.; Castellano, A.; Casarotti, A.; Falini, A.; Riva, M.; Fava, E.; Papagno, C. Cerebral correlates of visuospatial neglect: A direct cerebral stimulation study. Hum. Brain Mapp. 2014, 35, 1334–1350. [Google Scholar] [CrossRef] [PubMed]
- Nakada, M.; Nakajima, R.; Okita, H.; Nakade, Y.; Yuno, T.; Tanaka, S.; Kinoshita, M. Awake surgery for right frontal lobe glioma can preserve visuospatial cognition and spatial working memory. J. Neurooncol. 2021, 151, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Herbet, G.; Duffau, H. Contribution of the medial eye field network to the voluntary deployment of visuospatial attention. Nat. Commun. 2022, 13, 328. [Google Scholar] [CrossRef] [PubMed]
- Motomura, K.; Chalise, L.; Ohka, F.; Aoki, K.; Tanahashi, K.; Hirano, M.; Nishikawa, T.; Wakabayashi, T.; Natsume, A. Supratotal Resection of Diffuse Frontal Lower Grade Gliomas with Awake Brain Mapping, Preserving Motor, Language, and Neurocognitive Functions. World Neurosurg. 2018, 119, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Riva, M.; Casarotti, A.; Comi, A.; Pessina, F.; Bello, L. Brain and Music: An Intraoperative Stimulation Mapping Study of a Professional Opera Singer. World Neurosurg. 2016, 93, 486.e13–486.e18. [Google Scholar] [CrossRef] [PubMed]
- Dziedzic, T.A.; Bala, A.; Podgórska, A.; Piwowarska, J.; Marchel, A. Awake intraoperative mapping to identify cortical regions related to music performance: Technical note. J. Clin. Neurosci. 2021, 83, 64–67. [Google Scholar] [CrossRef] [PubMed]
- Delion, M.; Klinger, E.; Bernard, F.; Aubin, G.; Minassian, A.T.; Menei, P. Immersing Patients in a Virtual Reality Environment for Brain Mapping During Awake Surgery: Safety Study. World Neurosurg. 2020, 134, e937–e943. [Google Scholar] [CrossRef] [PubMed]
- Sala, F.; Giampiccolo, D.; Cattaneo, L. Novel Asleep Techniques for Intraoperative Assessment of Brain Connectivity. Front. Neurol. 2021, 12, 687030. [Google Scholar] [CrossRef] [PubMed]
- Giampiccolo, D.; Parmigiani, S.; Basaldella, F.; Russo, S.; Pigorini, A.; Rosanova, M.; Cattaneo, L.; Sala, F. Recording cortico-cortical evoked potentials of the human arcuate fasciculus under general anaesthesia. Clin. Neurophysiol. 2021, 132, 1966–1973. [Google Scholar] [CrossRef]
- Lefaucheur, J.-P.; André-Obadia, N.; Antal, A.; Ayache, S.S.; Baeken, C.; Benninger, D.; Cantello, R.M.; Cincotta, M.; De Carvalho, M.; De Ridder, D.; et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clin. Neurophysiol. 2014, 125, 2150–2206. [Google Scholar] [CrossRef]
- Oliviero, A.; Carrasco-López, M.C.; Campolo, M.; Perez-Borrego, Y.A.; Soto-León, V.; Gonzalez-Rosa, J.J.; Higuero, A.M.; Strange, B.A.; Abad-Rodriguez, J.; Foffani, G. Safety Study of Transcranial Static Magnetic Field Stimulation (tSMS) of the Human Cortex. Brain Stimul. 2015, 8, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Tarkiainen, A.; Liljeström, M.; Seppä, M.; Salmelin, R. The 3D topography of MEG source localization accuracy: Effects of conductor model and noise. Clin. Neurophysiol. 2003, 114, 1977–1992. [Google Scholar] [CrossRef]
- He, W.; Fong, P.Y.; Leung, T.W.H.; Huang, Y.Z. Protocols of non-invasive brain stimulation for neuroplasticity induction. Neurosci. Lett. 2020, 719, 133437. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Blumberger, D.M.; Downar, J.; Daskalakis, Z.J. Systematic review of biological markers of therapeutic repetitive transcranial magnetic stimulation in neurological and psychiatric disorders. Clin. Neurophysiol. 2021, 132, 429–448. [Google Scholar] [CrossRef] [PubMed]
- Picht, T.; Schulz, J.; Hanna, M.; Schmidt, S.; Suess, O.; Vajkoczy, P. Assessment of the influence of navigated transcranial magnetic stimulation on surgical planning for tumors in or near the motor cortex. Neurosurgery 2012, 70, 1248–1256. [Google Scholar] [CrossRef]
- Frey, D.; Schilt, S.; Strack, V.; Zdunczyk, A.; Rösler, J.; Niraula, B.; Vajkoczy, P.; Picht, T. Navigated transcranial magnetic stimulation improves the treatment outcome in patients with brain tumors in motor eloquent locations. Neuro Oncol. 2014, 16, 1365–1372. [Google Scholar] [CrossRef] [PubMed]
- Sollmann, N.; Ille, S.; Hauck, T.; Maurer, S.; Negwer, C.; Zimmer, C.; Ringel, F.; Meyer, B.; Krieg, S.M. The impact of preoperative language mapping by repetitive navigated transcranial magnetic stimulation on the clinical course of brain tumor patients. BMC Cancer 2015, 15, 261. [Google Scholar] [CrossRef] [PubMed]
- Raffa, G.; Conti, A.; Scibilia, A.; Cardali, S.M.; Esposito, F.; Angileri, F.F.; La Torre, D.; Sindorio, C.; Abbritti, R.V.; Germanò, A.; et al. The Impact of Diffusion Tensor Imaging Fiber Tracking of the Corticospinal Tract Based on Navigated Transcranial Magnetic Stimulation on Surgery of Motor-Eloquent Brain Lesions. Neurosurgery 2018, 83, 768–782. [Google Scholar] [CrossRef] [PubMed]
- Raffa, G.; Picht, T.; Angileri, F.F.; Youssef, M.; Conti, A.; Esposito, F.; Cardali, S.M.; Vajkoczy, P.; Germanò, A. Surgery of malignant motor-eloquent gliomas guided by sodium-fluorescein and navigated transcranial magnetic stimulation: A novel technique to increase the maximal safe resection. J. Neurosurg. Sci. 2019, 63, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Krieg, S.M.; Sollmann, N.; Obermueller, T.; Sabih, J.; Bulubas, L.; Negwer, C.; Moser, T.; Droese, D.; Boeckh-Behrens, T.; Ringel, F.; et al. Changing the clinical course of glioma patients by preoperative motor mapping with navigated transcranial magnetic brain stimulation. BMC Cancer 2015, 15, 231. [Google Scholar] [CrossRef] [PubMed]
- Krieg, S.M.; Sabih, J.; Bulubasova, L.; Obermueller, T.; Negwer, C.; Janssen, I.; Shiban, E.; Meyer, B.; Ringel, F. Preoperative motor mapping by navigated transcranial magnetic brain stimulation improves outcome for motor eloquent lesions. Neuro Oncol. 2014, 16, 1274–1282. [Google Scholar] [CrossRef]
- Tarapore, P.E.; Tate, M.C.; Findlay, A.M.; Honma, S.M.; Mizuiri, D.; Berger, M.S.; Nagarajan, S.S. Preoperative multimodal motor mapping: A comparison of magnetoencephalography imaging, navigated transcranial magnetic stimulation, and direct cortical stimulation. J. Neurosurg. 2012, 117, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Sollmann, N.; Negwer, C.; Tussis, L.; Hauck, T.; Ille, S.; Maurer, S.; Giglhuber, K.; Bauer, J.S.; Ringel, F.; Meyer, B.; et al. Interhemispheric connectivity revealed by diffusion tensor imaging fiber tracking derived from navigated transcranial magnetic stimulation maps as a sign of language function at risk in patients with brain tumors. J. Neurosurg. 2017, 126, 222–233. [Google Scholar] [CrossRef] [PubMed]
- Sollmann, N.; Zhang, H.; Fratini, A.; Wildschuetz, N.; Ille, S.; Schröder, A.; Zimmer, C.; Meyer, B.; Krieg, S.M. Risk Assessment by Presurgical Tractography Using Navigated TMS Maps in Patients with Highly Motor- or Language-Eloquent Brain Tumors. Cancers 2020, 12, 1264. [Google Scholar] [CrossRef] [PubMed]
- Raffa, G.; Scibilia, A.; Conti, A.; Ricciardo, G.; Rizzo, V.; Morelli, A.; Angileri, F.F.; Cardali, S.M.; Germanò, A. The role of navigated transcranial magnetic stimulation for surgery of motor-eloquent brain tumors: A systematic review and meta-analysis. Clin. Neurol. Neurosurg. 2019, 180, 7–17. [Google Scholar] [CrossRef]
- Seidel, K.; Häni, L.; Lutz, K.; Zbinden, C.; Redmann, A.; Consuegra, A.; Raabe, A.; Schucht, P. Postoperative navigated transcranial magnetic stimulation to predict motor recovery after surgery of tumors in motor eloquent areas. Clin. Neurophysiol. 2019, 130, 952–959. [Google Scholar] [CrossRef]
- Rosenstock, T.; Picht, T.; Schneider, H.; Vajkoczy, P.; Thomale, U.W. Pediatric navigated transcranial magnetic stimulation motor and language mapping combined with diffusion tensor imaging tractography: Clinical experience. J. Neurosurg. Pediatr. 2020, 24, 583–593. [Google Scholar] [CrossRef]
- Rosenstock, T.; Tuncer, M.S.; Münch, M.R.; Vajkoczy, P.; Picht, T.; Faust, K. Preoperative nTMS and Intraoperative Neurophysiology—A Comparative Analysis in Patients with Motor-Eloquent Glioma. Front. Oncol. 2021, 11, 676626. [Google Scholar] [CrossRef] [PubMed]
- Lavrador, J.P.; Gioti, I.; Hoppe, S.; Jung, J.; Patel, S.; Gullan, R.; Ashkan, K.; Bhangoo, R.; Vergani, F. Altered Motor Excitability in Patients with Diffuse Gliomas Involving Motor Eloquent Areas: The Impact of Tumor Grading. Neurosurgery 2020, 88, 183–192. [Google Scholar] [CrossRef]
- Ille, S.; Sollmann, N.; Butenschoen, V.M.; Meyer, B.; Ringel, F.; Krieg, S.M. Resection of highly language-eloquent brain lesions based purely on rTMS language mapping without awake surgery. Acta Neurochir. 2016, 158, 2265–2275. [Google Scholar] [CrossRef]
- Maurer, S.; Giglhuber, K.; Sollmann, N.; Kelm, A.; Ille, S.; Hauck, T.; Tanigawa, N.; Ringel, F.; Boeckh-Behrens, T.; Meyer, B.; et al. Non-invasive Mapping of Face Processing by Navigated Transcranial Magnetic Stimulation. Front. Hum. Neurosci. 2017, 11, 4. [Google Scholar] [CrossRef]
- Raffa, G.; Quattropani, M.C.; Marzano, G.; Curcio, A.; Rizzo, V.; Sebestyén, G.; Tamás, V.; Büki, A.; Germanò, A. Mapping and Preserving the Visuospatial Network by repetitive nTMS and DTI Tractography in Patients with Right Parietal Lobe Tumors. Front. Oncol. 2021, 11, 677172. [Google Scholar] [CrossRef]
- Giglhuber, K.; Maurer, S.; Zimmer, C.; Meyer, B.; Krieg, S.M. Mapping visuospatial attention: The greyscales task in combination with repetitive navigated transcranial magnetic stimulation. BMC Neurosci. 2018, 19, 40. [Google Scholar] [CrossRef] [PubMed]
- Salatino, A.; Chillemi, G.; Gontero, F.; Poncini, M.; Pyasik, M.; Berti, A.; Ricci, R. Transcranial Magnetic Stimulation of Posterior Parietal Cortex Modulates Line-Length Estimation but Not Illusory Depth Perception. Front. Psychol. 2019, 10, 1169. [Google Scholar] [CrossRef] [PubMed]
- Bodart, O.; Amico, E.; Gómez, F.; Casali, A.G.; Wannez, S.; Heine, L.; Thibaut, A.; Annen, J.; Boly, M.; Casarotto, S.; et al. Global structural integrity and effective connectivity in patients with disorders of consciousness. Brain Stimul. 2018, 11, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Baro, V.; Caliri, S.; Sartori, L.; Facchini, S.; Guarrera, B.; Zangrossi, P.; Anglani, M.; Denaro, L.; D’Avella, D.; Ferreri, F.; et al. Preoperative Repetitive Navigated TMS and Functional White Matter Tractography in a Bilingual Patient with a Brain Tumor in Wernike Area. Brain Sci. 2021, 11, 557. [Google Scholar] [CrossRef] [PubMed]
- Krieg, S.M.; Sollmann, N.; Hauck, T.; Ille, S.; Foerschler, A.; Meyer, B.; Ringel, F. Functional language shift to the right hemisphere in patients with language-eloquent brain tumors. PLoS ONE 2013, 8, e75403. [Google Scholar] [CrossRef] [PubMed]
- Cargnelutti, E.; Ius, T.; Skrap, M.; Tomasino, B. What do we know about pre- and postoperative plasticity in patients with glioma? A review of neuroimaging and intraoperative mapping studies. Neuroimage Clin. 2020, 28, 102435. [Google Scholar] [CrossRef] [PubMed]
- Lang, S.; Gan, L.S.; McLennan, C.; Kirton, A.; Monchi, O.; Kelly, J.J.P. Preoperative Transcranial Direct Current Stimulation in Glioma Patients: A Proof of Concept Pilot Study. Front. Neurol. 2020, 11, 593950. [Google Scholar] [CrossRef]
- Hoogendam, J.M.; Ramakers, G.M.; Di Lazzaro, V. Physiology of repetitive transcranial magnetic stimulation of the human brain. Brain Stimul. 2010, 3, 95–118. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, P.B.; George, M.S.; Pridmore, S. The evidence is in: Repetitive transcranial magnetic stimulation is an effective, safe and well-tolerated treatment for patients with major depressive disorder. Aust. N. Z. J. Psychiatry 2021, 48674211043047. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.D.; Hieronymus, F.; Lorentzen, R.; McGirr, A.; Østergaard, S.D. The efficacy of repetitive transcranial magnetic stimulation (rTMS) for bipolar depression: A systematic review and meta-analysis. J. Affect. Disord. 2021, 279, 250–255. [Google Scholar] [CrossRef]
- Cirillo, P.; Gold, A.K.; Nardi, A.E.; Ornelas, A.C.; Nierenberg, A.A.; Camprodon, J.; Kinrys, G. Transcranial magnetic stimulation in anxiety and trauma-related disorders: A systematic review and meta-analysis. Brain Behav. 2019, 9, e01284. [Google Scholar] [CrossRef] [PubMed]
- Nardone, R.; Versace, V.; Sebastianelli, L.; Brigo, F.; Christova, M.; Scarano, G.I.; Saltuari, L.; Trinka, E.; Hauer, L.; Sellner, J. Transcranial magnetic stimulation in subjects with phantom pain and non-painful phantom sensations: A systematic review. Brain Res. Bull. 2019, 148, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Chen, C.; Wang, T.; Gao, C.; Wang, Y.; Guan, X.; Dong, X. Low-Frequency Repetitive Transcranial Magnetic Stimulation for the Treatment of Chronic Tinnitus: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Biomed. Res. Int. 2020, 2020, 3141278. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Yang, H.; Cheng, G.; Huang, L.; Zhang, T.; Jia, H. Repetitive transcranial magnetic stimulation on chronic tinnitus: A systematic review and meta-analysis. BMC Psychiatry 2020, 20, 547. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.C.; Khalifa, N.; Völlm, B. The effects of repetitive transcranial magnetic stimulation on empathy: A systematic review and meta-analysis. Psychol. Med. 2018, 48, 737–750. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; He, Y.; Wang, Z.; Zou, W.; Liu, X. The effect of repetitive transcranial magnetic stimulation for insomnia: A systematic review and meta-analysis. Sleep Med. 2021, 77, 226–237. [Google Scholar] [CrossRef]
- Nardone, R.; Versace, V.; Sebastianelli, L.; Brigo, F.; Golaszewski, S.; Christova, M.; Saltuari, L.; Trinka, E. Transcranial magnetic stimulation and bladder function: A systematic review. Clin. Neurophysiol. 2019, 130, 2032–2037. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.Q.; Fong, K.N.K.; Ouyang, R.G.; Siu, A.M.H.; Kranz, G.S. Effects of repetitive transcranial magnetic stimulation (rTMS) on craving and substance consumption in patients with substance dependence: A systematic review and meta-analysis. Addiction 2019, 114, 2137–2149. [Google Scholar] [CrossRef] [PubMed]
- Taib, S.; Ory-Magne, F.; Brefel-Courbon, C.; Moreau, Y.; Thalamas, C.; Arbus, C.; Simonetta-Moreau, M. Repetitive transcranial magnetic stimulation for functional tremor: A randomized, double-blind, controlled study. Mov. Disord. 2019, 34, 1210–1219. [Google Scholar] [CrossRef] [PubMed]
- Nardone, R.; Brigo, F.; Höller, Y.; Sebastianelli, L.; Versace, V.; Saltuari, L.; Lochner, P.; Trinka, E. Transcranial magnetic stimulation studies in complex regional pain syndrome type I: A review. Acta Neurol. Scand. 2018, 137, 158–164. [Google Scholar] [CrossRef]
- Attia, M.; McCarthy, D.; Abdelghani, M. Repetitive Transcranial Magnetic Stimulation for Treating Chronic Neuropathic Pain: A Systematic Review. Curr. Pain Headache Rep. 2021, 25, 48. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Yan, W.; Wan, R.; Lin, Y.; Zhu, X.; Song, G.; Zheng, K.; Wang, Y.; Wang, X. Effects of repetitive transcranial magnetic stimulation on neuropathic pain: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2021, 132, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Naro, A.; Milardi, D.; Russo, M.; Terranova, C.; Rizzo, V.; Cacciola, A.; Marino, S.; Calabro, R.S.; Quartarone, A. Non-invasive Brain Stimulation, a Tool to Revert Maladaptive Plasticity in Neuropathic Pain. Front. Hum. Neurosci. 2016, 10, 376. [Google Scholar] [CrossRef]
- Salazar, A.P.S.; Vaz, P.G.; Marchese, R.R.; Stein, C.; Pinto, C.; Pagnussat, A.S. Noninvasive Brain Stimulation Improves Hemispatial Neglect After Stroke: A Systematic Review and Meta-Analysis. Arch. Phys. Med. Rehabil. 2018, 99, 355–366. [Google Scholar] [CrossRef]
- Cooper, Y.A.; Pianka, S.T.; Alotaibi, N.M.; Babayan, D.; Salavati, B.; Weil, A.G.; Ibrahim, G.M.; Wang, A.C.; Fallah, A. Repetitive transcranial magnetic stimulation for the treatment of drug-resistant epilepsy: A systematic review and individual participant data meta-analysis of real-world evidence. Epilepsia Open 2017, 3, 55–65. [Google Scholar] [CrossRef]
- Liang, K.; Li, H.; Bu, X.; Li, X.; Cao, L.; Liu, J.; Gao, Y.; Li, B.; Qiu, C.; Bao, W.; et al. Efficacy and tolerability of repetitive transcranial magnetic stimulation for the treatment of obsessive-compulsive disorder in adults: A systematic review and network meta-analysis. Transl. Psychiatry 2021, 11, 332. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Yan, L.; Huang, L.; Guan, X.; Dong, C.; Tao, H.; Wang, T.; Qin, X.; Wan, Q. Repetitive transcranial magnetic stimulation for the treatment of Alzheimer’s disease: A systematic review and meta-analysis of randomized controlled trials. PLoS ONE 2018, 13, e0205704. [Google Scholar] [CrossRef]
- Prikryl, R. Repetitive transcranial magnetic stimulation and treatment of negative symptoms of schizophrenia. Neuro Endocrinol. Lett. 2011, 32, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liang, W.; Yang, S.; Dai, P.; Shen, L.; Wang, C. Repetitive transcranial magnetic stimulation for hallucination in schizophrenia spectrum disorders: A meta-analysis. Neural Regen. Res. 2013, 8, 2666–2676. [Google Scholar] [CrossRef]
- Hsu, C.W.; Wang, L.J.; Lin, P.Y. Efficacy of repetitive transcranial magnetic stimulation for Tourette syndrome: A systematic review and meta-analysis. Brain Stimul. 2018, 11, 1110–1118. [Google Scholar] [CrossRef] [PubMed]
- Chou, Y.H.; Hickey, P.T.; Sundman, M.; Song, A.W.; Chen, N.K. Effects of repetitive transcranial magnetic stimulation on motor symptoms in Parkinson disease: A systematic review and meta-analysis. JAMA Neurol. 2015, 72, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Lepping, P.; Schönfeldt-Lecuona, C.; Sambhi, R.S.; Lanka, S.V.; Lane, S.; Whittington, R.; Leucht, S.; Poole, R. A systematic review of the clinical relevance of repetitive transcranial magnetic stimulation. Acta Psychiatr. Scand. 2014, 130, 326–341. [Google Scholar] [CrossRef]
- Fitzgerald, P.B.; Daskalakis, Z.J. A practical guide to the use of repetitive transcranial magnetic stimulation in the treatment of depression. Brain Stimul. 2012, 5, 287–296. [Google Scholar] [CrossRef]
- Machii, K.; Cohen, D.; Ramos-Estebanez, C.; Pascual-Leone, A. Safety of rTMS to non-motor cortical areas in healthy participants and patients. Clin. Neurophysiol. 2006, 117, 455–471. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Hallett, M.; Rossini, P.M.; Pascual-Leone, A.; Safety of TMS Consensus Group. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin. Neurophysiol. 2009, 120, 2008–2039. [Google Scholar] [CrossRef] [PubMed]
- Loo, C.K.; McFarquhar, T.F.; Mitchell, P.B. A review of the safety of repetitive transcranial magnetic stimulation as a clinical treatment for depression. Int. J. Neuropsychopharmacol. 2008, 11, 131–147. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Leone, A.; Gates, J.R.; Dhuna, A. Induction of speech arrest and counting errors with rapid-rate transcranial magnetic stimulation. Neurology 1991, 41, 697–702. [Google Scholar] [CrossRef]
- Hömberg, V.; Netz, J. Generalised seizures induced by transcranial magnetic stimulation of motor cortex. Lancet 1989, 2, 1223. [Google Scholar] [CrossRef]
- Dhuna, A.; Gates, J.; Pascual-Leone, A. Transcranial magnetic stimulation in patients with epilepsy. Neurology 1991, 41, 1067–1071. [Google Scholar] [CrossRef]
- Schrader, L.M.; Stern, J.M.; Koski, L.; Nuwer, M.R.; Engel, J., Jr. Seizure incidence during single- and paired-pulse transcranial magnetic stimulation (TMS) in individuals with epilepsy. Clin. Neurophysiol. 2004, 115, 2728–2737. [Google Scholar] [CrossRef]
- Tarapore, P.E.; Picht, T.; Bulubas, L.; Shin, Y.; Kulchytska, N.; Meyer, B.; Berger, M.S.; Nagarajan, S.S.; Krieg, S.M. Safety and tolerability of navigated TMS for preoperative mapping in neurosurgical patients. Clin. Neurophysiol. 2016, 127, 1895–1900. [Google Scholar] [CrossRef] [PubMed]
- Theodore, W.H. Transcranial Magnetic Stimulation in Epilepsy. Epilepsy Curr. 2003, 3, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Tergau, F.; Neumann, D.; Rosenow, F.; Nitsche, M.A.; Paulus, W.; Steinhoff, B. Can epilepsies be improved by repetitive transcranial magnetic stimulation?—Interim analysis of a controlled study. Suppl. Clin. Neurophysiol. 2003, 56, 400–405. [Google Scholar] [CrossRef]
- Fregni, F.; Otachi, P.T.; Do Valle, A.; Boggio, P.S.; Thut, G.; Rigonatti, S.P.; Pascual-Leone, A.; Valente, K.D. A randomized clinical trial of repetitive transcranial magnetic stimulation in patients with refractory epilepsy. Ann. Neurol. 2006, 60, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Cantello, R.; Rossi, S.; Varrasi, C.; Ulivelli, M.; Civardi, C.; Bartalini, S.; Vatti, G.; Cincotta, M.; Borgheresi, A.; Zaccara, G.; et al. Slow repetitive TMS for drug-resistant epilepsy: Clinical and EEG findings of a placebo-controlled trial. Epilepsia 2007, 48, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Joo, E.Y.; Han, S.J.; Chung, S.H.; Cho, J.W.; Seo, D.W.; Hong, S.B. Antiepileptic effects of low-frequency repetitive transcranial magnetic stimulation by different stimulation durations and locations. Clin. Neurophysiol. 2007, 118, 702–708. [Google Scholar] [CrossRef]
- Santiago-Rodríguez, E.; Cárdenas-Morales, L.; Harmony, T.; Fernández-Bouzas, A.; Porras-Kattz, E.; Hernández, A. Repetitive transcranial magnetic stimulation decreases the number of seizures in patients with focal neocortical epilepsy. Seizure 2008, 17, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, C.; Brihmat, N.; Castel-Lacanal, E.; Le Friec, A.; Barbieux-Guillot, M.; Raposo, N.; Pariente, J.; Viguier, A.; Simonetta-Moreau, M.; Albucher, J.F.; et al. Post-stroke remodeling processes in animal models and humans. J. Cereb. Blood Flow Metab. 2020, 40, 3–22. [Google Scholar] [CrossRef]
- Yuan, X.; Yang, Y.; Cao, N.; Jiang, C. Promotion of Poststroke Motor-Function Recovery with Repetitive Transcranial Magnetic Stimulation by Regulating the Interhemispheric Imbalance. Brain Sci. 2020, 10, 648. [Google Scholar] [CrossRef] [PubMed]
- Niimi, M.; Hashimoto, K.; Kakuda, W.; Miyano, S.; Momosaki, R.; Ishima, T.; Abo, M. Role of Brain-Derived Neurotrophic Factor in Beneficial Effects of Repetitive Transcranial Magnetic Stimulation for Upper Limb Hemiparesis after Stroke. PLoS ONE 2016, 11, e0152241. [Google Scholar] [CrossRef] [PubMed]
- Dionísio, A.; Duarte, I.C.; Patrício, M.; Castelo-Branco, M. The Use of Repetitive Transcranial Magnetic Stimulation for Stroke Rehabilitation: A Systematic Review. J. Stroke Cerebrovasc. Dis. 2018, 27, 1–31. [Google Scholar] [CrossRef] [PubMed]
- van Lieshout, E.C.C.; van der Worp, H.B.; Visser-Meily, J.M.A.; Dijkhuizen, R.M. Timing of Repetitive Transcranial Magnetic Stimulation Onset for Upper Limb Function After Stroke: A Systematic Review and Meta-Analysis. Front. Neurol. 2019, 10, 1269. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Zhao, H.; Shen, C.; Liu, F.; Qiu, L.; Fu, L. Low-Frequency Repetitive Transcranial Magnetic Stimulation in Patients with Poststroke Aphasia: Systematic Review and Meta-Analysis of Its Effect Upon Communication. J. Speech Lang. Hear. Res. 2020, 63, 3801–3815. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, N.; Tada, T.; Toshima, M.; Matsuo, Y.; Ikoma, K. Repetitive transcranial magnetic stimulation over bilateral hemispheres enhances motor function and training effect of paretic hand in patients after stroke. J. Rehabil. Med. 2009, 41, 1049–1054. [Google Scholar] [CrossRef] [PubMed]
- Barwood, C.H.; Murdoch, B.E.; Whelan, B.M.; Lloyd, D.; Riek, S.; O’ Sullivan, J.D.; Coulthard, A.; Wong, A. Improved language performance subsequent to low-frequency rTMS in patients with chronic non-fluent aphasia post-stroke. Eur. J. Neurol. 2011, 18, 935–943. [Google Scholar] [CrossRef]
- Takeuchi, N.; Izumi, S. Noninvasive brain stimulation for motor recovery after stroke: Mechanisms and future views. Stroke Res. Treat. 2012, 2012, 584727. [Google Scholar] [CrossRef]
- Kakuda, W.; Abo, M.; Sasanuma, J.; Shimizu, M.; Okamoto, T.; Kimura, C.; Kakita, K.; Hara, H. Combination Protocol of Low-Frequency rTMS and Intensive Occupational Therapy for Post-stroke Upper Limb Hemiparesis: A 6-year Experience of More Than 1700 Japanese Patients. Transl. Stroke Res. 2016, 7, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Caglayan, A.B.; Beker, M.; Caglayan, B.; Yalcin, E.; Caglayan, A.; Yulug, B.; Hanoglu, L.; Kutlu, S.; Doeppner, T.R.; Hermann, D.M.; et al. Acute and Post-acute Neuromodulation Induces Stroke Recovery by Promoting Survival Signaling, Neurogenesis, and Pyramidal Tract Plasticity. Front. Cell Neurosci. 2019, 13, 144. [Google Scholar] [CrossRef]
- Ille, S.; Kelm, A.; Schroeder, A.; Albers, L.E.; Negwer, C.; Butenschoen, V.M.; Sollmann, N.; Picht, T.; Vajkoczy, P.; Meyer, B.; et al. Navigated repetitive transcranial magnetic stimulation improves the outcome of postsurgical paresis in glioma patients—A randomized, double-blinded trial. Brain Stimul. 2021, 14, 780–787. [Google Scholar] [CrossRef] [PubMed]
- Ridding, M.C.; Rothwell, J.C. Is there a future for therapeutic use of transcranial magnetic stimulation? Nature reviews. Neuroscience 2007, 8, 559–567. [Google Scholar] [CrossRef]
- Duffau, H. Dynamic Interplay between Lower-Grade Glioma Instability and Brain Metaplasticity: Proposal of an Original Model to Guide the Therapeutic Strategy. Cancers 2021, 13, 4759. [Google Scholar] [CrossRef] [PubMed]
- Barcia, J.A.; Sanz, A.; González-Hidalgo, M.; de Las Heras, C.; Alonso-Lera, P.; Díaz, P.; Pascual-Leone, A.; Oliviero, A.; Ortiz, T. rTMS stimulation to induce plastic changes at the language motor area in a patient with a left recidivant brain tumor affecting Broca’s area. Neurocase 2012, 18, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Rivera, P.A.; Rios-Lago, M.; Sanchez-Casarrubios, S.; Salazar, O.; Yus, M.; González-Hidalgo, M.; Sanz, A.; Avecillas-Chasin, J.; Alvarez-Linera, J.; Pascual-Leone, A.; et al. Cortical plasticity catalyzed by prehabilitation enables extensive resection of brain tumors in eloquent areas. J. Neurosurg. 2017, 126, 1323–1333. [Google Scholar] [CrossRef]
- Barcia, J.A.; Sanz, A.; Balugo, P.; Alonso-Lera, P.; Brin, J.R.; Yus, M.; Gonzalez-Hidalgo, M.; Acedo, V.M.; Oliviero, A. High-frequency cortical subdural stimulation enhanced plasticity in surgery of a tumor in Broca’s area. Neuroreport 2012, 23, 304–309. [Google Scholar] [CrossRef]
- Serrano-Castro, P.J.; Ros-López, B.; Fernández-Sánchez, V.E.; García-Casares, N.; Muñoz-Becerra, L.; Cabezudo-Garcia, P.; Aguilar-Castillo, M.J.; Vidal-Denis, M.; Cruz-Andreotti, E.; Postigo-Pozo, M.J.; et al. Neuroplasticity and Epilepsy Surgery in Brain Eloquent Areas: Case Report. Front. Neurol. 2020, 11, 698. [Google Scholar] [CrossRef] [PubMed]
- Dell’osso, B.; Camuri, G.; Castellano, F.; Vecchi, V.; Benedetti, M.; Bortolussi, S.; Altamura, A.C. Meta-Review of Metanalytic Studies with Repetitive Transcranial Magnetic Stimulation (rTMS) for the Treatment of Major Depression. Clin. Pract. Epidemiol. Ment. Health 2011, 7, 167–177. [Google Scholar] [CrossRef]
- Zhang, L.; Xing, G.; Shuai, S.; Guo, Z.; Chen, H.; McClure, M.A.; Chen, X.; Mu, Q. Low-Frequency Repetitive Transcranial Magnetic Stimulation for Stroke-Induced Upper Limb Motor Deficit: A Meta-Analysis. Neural. Plast. 2017, 2758097. [Google Scholar] [CrossRef] [PubMed]
- Andoh, J.; Artiges, E.; Pallier, C.; Rivière, D.; Mangin, J.F.; Paillère-Martinot, M.L.; Martinot, J.L. Priming frequencies of transcranial magnetic stimulation over Wernicke’s area modulate word detection. Cereb. Cortex 2008, 18, 210–216. [Google Scholar] [CrossRef][Green Version]
- Klomjai, W.; Katz, R.; Lackmy-Vallée, A. Basic principles of transcranial magnetic stimulation (TMS) and repetitive TMS (rTMS). Ann. Phys. Rehabil. Med. 2015, 58, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Magown, P.; Andrade, R.A.; Soroceanu, A.; Kiss, Z.H.T. Deep brain stimulation parameters for dystonia: A systematic review. Parkinsonism Relat. Disord. 2018, 54, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Carron, R.; Chaillet, A.; Filipchuk, A.; Pasillas-Lépine, W.; Hammond, C. Closing the loop of deep brain stimulation. Front. Syst. Neurosci. 2013, 7, 112. [Google Scholar] [CrossRef]
- Jakobs, M.; Fomenko, A.; Lozano, A.M.; Kiening, K.L. Cellular, molecular, and clinical mechanisms of action of deep brain stimulation-a systematic review on established indications and outlook on future developments. EMBO Mol. Med. 2019, 11, e9575. [Google Scholar] [CrossRef]
- Abalkhail, T.M.; MacDonald, D.B.; AlThubaiti, I.; AlOtaibi, F.A.; Stigsby, B.; Mokeem, A.A.; AlHamoud, I.A.; Hassounah, M.I.; Baz, S.M.; AlSemari, A.; et al. Intraoperative direct cortical stimulation motor evoked potentials: Stimulus parameter recommendations based on rheobase and chronaxie. Clin. Neurophysiol. 2017, 128, 2300–2308. [Google Scholar] [CrossRef] [PubMed]
- Tarapore, P.; Berger, M. Outlook on the potential of nTMS in neurosurgery. In Navigated Transcranial Magnetic Stimulation in Neurosurgery, 1st ed.; Krieg, S., Ed.; Springer: Cham, Switzerland, 2017; pp. 287–299. [Google Scholar]
- Rösler, J.; Niraula, B.; Strack, V.; Zdunczyk, A.; Schilt, S.; Savolainen, P.; Lioumis, P.; Mäkelä, J.; Vajkoczy, P.; Frey, D.; et al. Language mapping in healthy volunteers and brain tumor patients with a novel navigated TMS system: Evidence of tumor-induced plasticity. Clin. Neurophysiol. 2014, 125, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Conway, N.; Wildschuetz, N.; Moser, T.; Bulubas, L.; Sollmann, N.; Tanigawa, N.; Meyer, B.; Krieg, S.M. Cortical plasticity of motor-eloquent areas measured by navigated transcranial magnetic stimulation in patients with glioma. J. Neurosurg. 2017, 127, 981–991. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ille, S.; Sogerer, L.; Schwendner, M.; Schröder, A.; Meyer, B.; Wiestler, B.; Krieg, S.M. Elucidating the structural-functional connectome of language in glioma-induced aphasia using nTMS and DTI. Hum. Brain Mapp. 2021. advance online publication. [Google Scholar] [CrossRef] [PubMed]
| Barcia et al. [183] | Barcia et al. [185] | Rivera-Rivera et al. [184] | Serrano-Castro [186] | ||||
|---|---|---|---|---|---|---|---|
| Patient (s) | 59 y/o F | 27 y/o M | 52 y/o F | 34 y/o F | 51 y/o M | 41 y/o M | 17 y/o M |
| Tumour | Oligodendroglioma (WHO II) | Anaplastic astrocytoma (WHO III) | Oligodendroglioma (WHO II) | Anaplastic oligodendroglioma (WHO III) | Anaplastic astrocytoma (WHO III) | Oligodendroglioma (WHO II) | Neuroepithelial dysembryoblastic tumour (WHO I) |
| Anatomy | Adjacent to left IFG | Left IFG | Left IFG, MFG, SFG | Left STG, MTG, ITG | Left PrCG | Left IFG, MFG, SFG | Left temporoparietal region |
| Presenting symptoms | Dysnomia | Speech impairment | Language function | Language production and function | Movement of right hand and shoulder | Movement of hand, language production | Focal motor seizures (right lower limb), aphasia without awareness |
| Pre-op imaging | fMRI: left dominant speech with partial right-side activation | fMRI: left dominant speech with activation within tumour | fMRI: left dominant bilingual speech | fMRI: left dominant speech | fMRI: right hand activation within tumour | fMRI: left dominant speech and motor function within tumour | fMRI: Overlap of Wernicke’s area and tumour, language reorganisation in homologous contralateral hemisphere |
| MEG: left dominant speech | |||||||
| Revision surgery (Y/N) | Y; initial 0.9 yrs prior | Y; initial 4.8 yrs prior | Y; initial 6.2 years prior | Y; initial 4.7 years prior | N | Y; initial 7.8 years prior | Y; initial approx. 11 years prior |
| Technique | Theta-burst rTMS | Extraoperative direct cortical stimulation | |||||
| Power/Intensity | 60% | 0.5–10 V (incremental) | |||||
| Frequency | 45 Hz | 130 Hz | |||||
| Pulses | 3 | 200 | |||||
| Bursts/pulse width (ms) | 5 | 1 ms | |||||
| Cycle duration (s) | 1 s | Continuous, 24 h p/day | |||||
| Number of cycles | 40 | n/a | |||||
| Burst frequency (Hz) | 5 | n/a | |||||
| Cognitive assessment | Boston Diagnostic Aphasia Examination (BDAE) | Mini-mental State examination, Boston Diagnostic Aphasia Examination, Token test, F-A-S Test (subset of Neurosensory Center Comprehensive Examination for Aphasia) | Object naming, repetition, pseudowords and phrases, understanding simple and complex orders, verbal fluency | Object naming, repetition, pseudowords and phrases, understanding simple and complex orders, verbal fluency | Right shoulder movements (elevation, abduction, and flexion), right elbow movements (flexion, extension, pronation, and supination), and right-hand fine motor movements (finger tapping, flexion and extension, abduction and adduction) | object naming, repetition, pseudowords and phrases, understanding simple and complex orders, verbal fluency. Right shoulder movements (elevation, abduction, and flexion), right elbow movements (flexion, extension, pronation, and supination), and right-hand fine motor movements (finger tapping, flexion and extension, abduction and adduction) | Boston Diagnostic Aphasia Examination (BDAE) |
| Length of Prehab (days) | 13 | 25 | 16 | 16 | 22 | 15 | 6 |
| Post-prehab imaging | fMRI: left dominant speech with partial right-side activation | fMRI: new language activation at ipsilateral and contralateral hemisphere | fMRI: reorganization of languages at basal aspect of left inferior gyrus | fMRI: new language activation at contralateral hemisphere | fMRI: displacement of motor function to the depth of the central sulcus | fMRI: reorganization of language and motor hand area | fMRI: decreased activation in left dominant hemisphere, greater activation in right homologous area of Wernicke’s |
| MEG: greater bilateralization | Electrode array: disparity from original mapping | Electrode array: all contacts negative for Spanish, Romanian still present | Electrode array: 9 contacts originally provoking dysnomia and alexia no longer did so | Electrode array: all sites originally positive for motor activation were negative | Electrode array: 9/11 sites originally producing speech disturbances were negative, remaining pair producing phonological aphasia | Electrode array: no residual language over tumour region | |
| Prehab complications | Nil | Focal seizures, osteomylitis of bone flap | Nil | Epidural abscess associated with worsening neurology | Intermittent myoclonus right index finger | Pre-prehab subdural hematoma, subsequent removal and re-implantation of subdural electrode | Nil |
| Neurologic status following surgery | Transient language deficit. BDAE lower than pre-surgery. | Transient dysarthria. Attention and speech function (BDAE) improved. | No new or worsening neurologic deficit | Long term language deterioration | Transient shoulder elevation difficulty | Slight motor aphasia, long term language deterioration | Nil |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamer, R.P.; Yeo, T.T. Current Status of Neuromodulation-Induced Cortical Prehabilitation and Considerations for Treatment Pathways in Lower-Grade Glioma Surgery. Life 2022, 12, 466. https://doi.org/10.3390/life12040466
Hamer RP, Yeo TT. Current Status of Neuromodulation-Induced Cortical Prehabilitation and Considerations for Treatment Pathways in Lower-Grade Glioma Surgery. Life. 2022; 12(4):466. https://doi.org/10.3390/life12040466
Chicago/Turabian StyleHamer, Ryan P., and Tseng Tsai Yeo. 2022. "Current Status of Neuromodulation-Induced Cortical Prehabilitation and Considerations for Treatment Pathways in Lower-Grade Glioma Surgery" Life 12, no. 4: 466. https://doi.org/10.3390/life12040466
APA StyleHamer, R. P., & Yeo, T. T. (2022). Current Status of Neuromodulation-Induced Cortical Prehabilitation and Considerations for Treatment Pathways in Lower-Grade Glioma Surgery. Life, 12(4), 466. https://doi.org/10.3390/life12040466





