Abstract
(1) Background: Polycystic ovary syndrome (PCOS) is the most common cause of anovulatory infertility and endocrine disorders among women of reproductive age. Previous studies have employed lifestyle interventions to manage anovulatory infertility and endocrine disorders. However, the effect of lifestyle interventions on the metabolic index remains ambiguous; (2) Methods: Data were obtained through a systematic search of the Ovid-Medline, Ovid-EMBASE, and Cochrane Library databases. Two reviewers independently reviewed the literature in two stages. A consensus was achieved through discussions regarding the final selection of the literature; (3) Results: This study observed that the group that underwent lifestyle modifications displayed significant improvement in reproductive function compared to the control group. Combination therapy with diet and exercise resulted in improved fasting insulin levels, compared to monotherapy with diet or exercise. Moreover, moderate weight loss (a minimum of 5%) resulted in an improved metabolic index. The subgroup analysis revealed that the group that underwent lifestyle modifications had a significantly higher number of patients with improved menstrual cycles, compared to the control groups; (4) Conclusions: Lifestyle modification using combination therapy is a promising therapeutic approach that can be employed in the management of PCOS patients with obesity. This scenario warrants further studies with larger sample sizes to develop ideal treatment protocols.
1. Introduction
Polycystic ovary syndrome (PCOS) is the most common cause of anovulatory infertility and endocrine disorders and affects approximately 8–13% of reproductive age women [1,2]. The diagnosis and management of PCOS is a challenging endeavor because it is a mysterious condition with major symptoms that vary with age, and the treatment should be tailored to meet the specific requirements of each patient [3]. The application of the Rotterdam criteria for the diagnosis of adult women with PCOS was approved by international evidence-based guidelines. The diagnosis requires fulfillment of a minimum of two of the following three conditions: oligo-ovulation or anovulation, clinical or biochemical hyperandrogenism, and detection of the radiographic features of polycystic ovaries by means of ultrasonography [2]. The main symptoms of the syndrome include infertility attributable to anovulation, irregular menstrual cycles, and symptoms caused by androgen excess, such as hirsutism [4]. Moreover, the condition can be associated with concurrent chronic metabolic diseases, such as increased insulin resistance, which necessitates appropriate treatment to prevent complications [3].
Obesity is one of the most common concerns among patients with PCOS. In addition, there is a high correlation between obesity and the prevalence of PCOS. The prevalence of PCOS is 4.3% among women with a body mass index (BMI) less than or equal to 25 kg/m2 and 14% among women with a BMI above 30 kg/m2 [5]. Moreover, it has been reported that the risk of obesity is four times higher among patients with PCOS than among healthy controls [6]. Previous studies have shown that a high BMI causes metabolic abnormalities in patients with PCOS, such as increased insulin resistance and exacerbation of hyperandrogenemia [2]. Increased body weight and insulin resistance are the underlying causes of symptoms in PCOS patients with obesity. Hence, international evidence-based guidelines emphasize the importance of pre-pregnancy weight management among PCOS patients [2].
Lifestyle modification is recommended as the primary treatment for weight management in PCOS patients with obesity [3]. A variety of balanced dietary approaches to reduce dietary caloric intake and a gradual increase in physical activity are recommended to accomplish weight loss [2]. Several previous studies have endeavored to improve the lifestyles of patients with PCOS using various methods, such as diet, exercise therapy, and behavioral therapy. Consequently, a previous review reported that lifestyle modification programs were observed to affect weight loss or BMI among PCOS patients with or without obesity [7], and it is necessary to confirm the efficacy of lifestyle modification programs in the management of PCOS patients with obesity who struggle with weight management. Conversely, previous studies have reported ambiguous results regarding the variation in metabolic indicators after lifestyle interventions among patients with PCOS [7]. Furthermore, despite the fact that improvement in reproductive function is an important factor regarding PCOS, the effect of lifestyle interventions on the improvement of reproductive function has not been confirmed to date.
Hence, this study aimed to present an updated paper to confirm the effectiveness of lifestyle modification programs in the management of PCOS patients with obesity and confirm the effect of lifestyle modification programs on improving reproductive function for the first time. In addition, this study performed a subgroup analysis according to the type of intervention and degree of weight loss.
2. Materials and Methods
The research protocol of the present systematic review was registered with the National Research Foundation of Korea prior to the commencement of this research (No. 2020R1F1A1073141). The abovementioned protocol could not be modified arbitrarily, and the pre-selected patient, intervention, comparison, and outcome (PICO) could not be fixed by the researcher, which prevented selection reporting bias (List S1).
2.1. Data Sources and Search Strategy
The current review (Table S1) performed a systematic search of three databases to identify eligible articles, which are stated as follows: Ovid-Medline (1946 to 18 June 2021), Ovid-EMBASE (1974 to 18 June 2021), and Cochrane Library, according to the guidelines of the Cochrane community (https://community.cochrane.org/) (accessed on 18 June 2021). All papers published prior to the commencement of the literature search were analyzed. Moreover, additional documents were identified through hand-searching, and the references of selected documents (reference of reference) were searched. Relevant articles were included to supplement the comprehensiveness of the search. Keywords and Medical Subject Headings (MeSH) were used in combination to identify all relevant articles. The terms pertaining to the participants included “exp body mass index/,” “exp overweight/,” “exp obesity/,” “BMI,” “exp infertility/,” “exp anovulation/,” and “exp polycystic ovary syndrome/.” The terms pertaining to intervention included “lifestyle modification,” “lifestyle intervention,” “lifestyle change,” “lifestyle program,” “exp diet/,” “exp exercise/,” and “exp weight loss/.”
2.2. Eligibility Criteria and Selection Process
The articles included in the current review were selected by evaluating all the papers obtained through a literature search, in accordance with predefined inclusion and exclusion criteria. The inclusion criteria were as follows: (a) studies that involved patients diagnosed with PCOS and obesity, (b) studies that involved a lifestyle intervention program (diet and/or exercise program), and (c) studies that assessed more than one variable of interest (reproductive, anthropometric, androgenic, and metabolic indices). The present study excluded review articles, abstracts, conference posters, articles written in languages other than English, and duplicate publications.
In the current review, two reviewers independently selected and excluded papers in two stages. In the first stage of selection/exclusion, the documents that were deemed irrelevant to the present systematic review were excluded by screening the titles and abstracts according to the predefined criteria. In the second stage, full texts of the documents selected during the first stage were reviewed, and appropriate articles were selected. Consensus was achieved through discussions regarding the final selection of relevant literature. Reliability was assessed using Cohen’s kappa coefficient (κ = 0.83).
2.3. Variables and Data Collection
The reviewers performed data extraction using a prearranged data extraction form and double-checked the same. The variables that were documented are stated as follows: characteristics pertaining to the included participants and the type of intervention employed by the studies were recorded. In the current review, age, definition of obesity, and the criteria used to diagnose PCOS were recorded as the characteristics of the participants. Furthermore, the type of intervention; specific components of the intervention program; and duration, frequency, and time of day with reference to the implementation of intervention were recorded as the characteristics pertaining to the intervention program, that is, lifestyle modification.
In addition, the current review assessed the clinical outcomes of lifestyle modification programs. The variables of interest included reproductive, anthropometric, androgenic, and metabolic indices. The indices used in the studies were as follows: (a) reproductive index: number of patients with regular/irregular menstrual cycle, number of patients with improvement in menstrual cycle, ovarian volume, and number of ovarian follicles; (b) anthropometric index: weight, BMI, and waist circumference (WC); (c) metabolic index: fasting glucose level, fasting insulin level, and homeostatic model assessment for insulin resistance (HOMA-IR); (d) androgenic index: testosterone level, sex-hormone binding globulin (SHBG), and free androgen index (FAI).
2.4. Risk of Bias Assessment
In the current review, two independent reviewers assessed the methodological quality of the studies. Disagreements were resolved through consensus meetings. The tools used for the assessment of the risk of bias were contingent upon the study design. This study used the Cochrane Risk of Bias tool (RoB) and the Risk of Bias Assessment Tool for Non-randomized Studies (RoBANS) to evaluate randomized control trials (RCTs) and other studies. Each criterion in the tool was rated as follows: “low,” “high,” or “unclear” risk of bias.
2.5. Analysis
Data management and meta-analysis were performed using the RevMan program (Review Manager 5.4) for items that were reported by two or more studies. The current study computed the combined estimate of the odds ratios (ORs) pertaining to two groups using the Mantel–Haenszel method to assess dichotomous variables. The effect measures of continuous variables were estimated as mean difference (MD) and 95% confidence intervals (CI) using the inverse variance method. The chi-squared test was used to assess statistical heterogeneity among studies, and the significance was set at p < 0.10. Heterogeneity was quantified using the I2 statistic. In the absence of heterogeneity, the current meta-analysis employed a fixed-effects model as the basis for the statistical model. In the current review, a p-value less than 0.05 was considered to be statistically significant. Publication bias could not be evaluated owing to the inadequate number of studies included in the meta-analysis.
This study was approved by the Ethics Review Committee (1044396-202010-HR-192-01).
3. Results
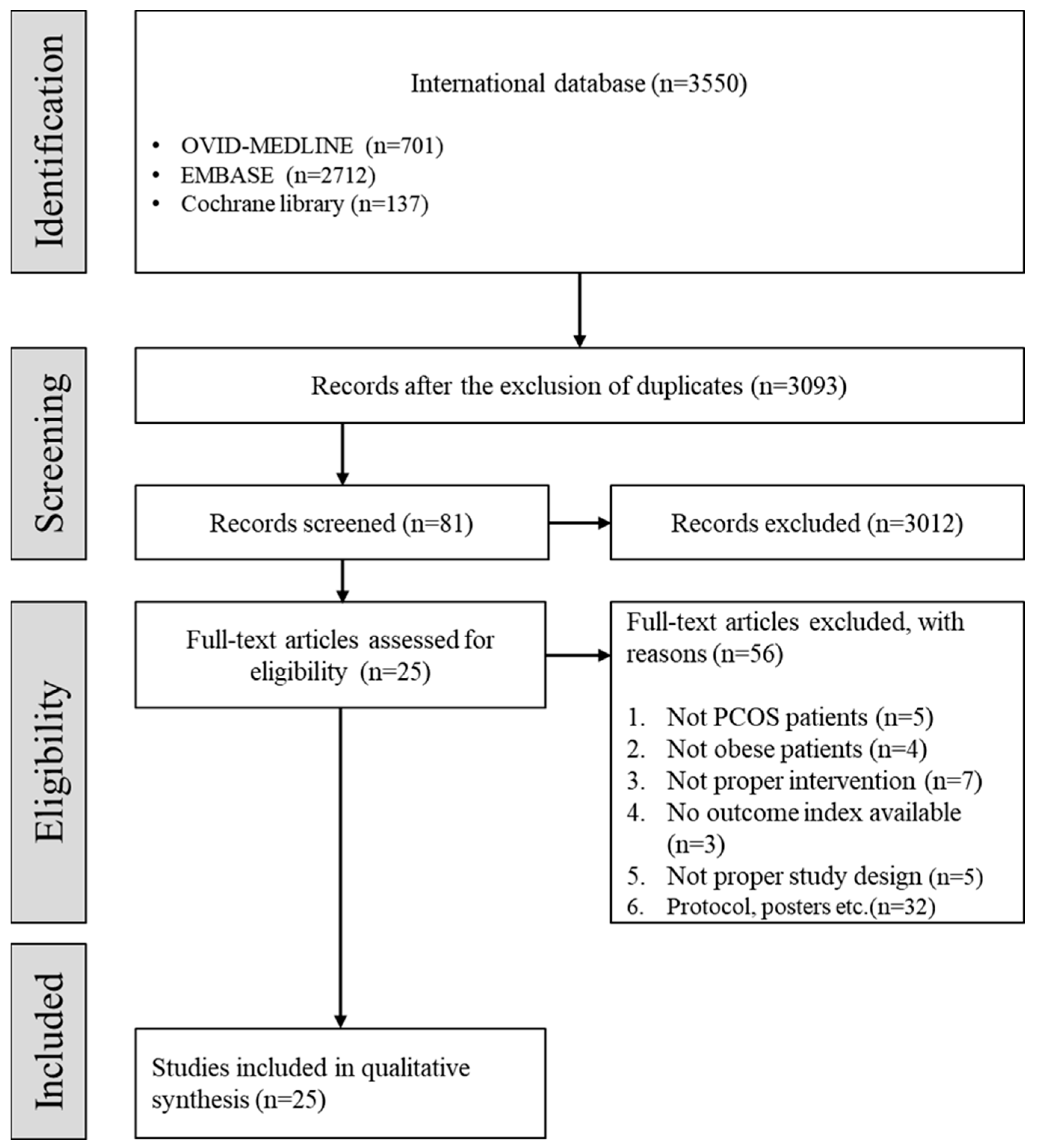
Of the 3093 studies, 3012 studies were excluded by screening the titles and abstracts, followed by 56 studies from the full-text review. A total of 25 studies were included in the present systematic review after the selection process based on the aforementioned inclusion and exclusion criteria (Figure 1).
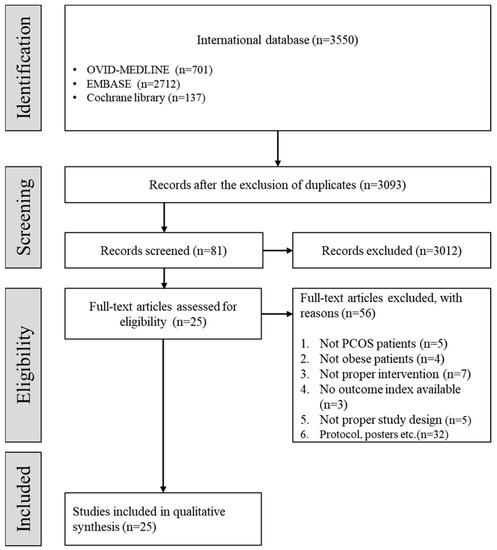
Figure 1.
Flow chart depicting the selection of studies. (PCOS: polycystic ovary syndrome.)
3.1. Study Characteristics
A summary of the characteristics of these studies is presented in Table 1. Among the 25 articles included in the current systematic review, eight were RCTs and 17 were non-randomized clinical trials or observational studies. Considering the geographic distribution, nine studies were published in Europe, seven in North America, five in Oceania, two in Asia, and one each in Africa and the Middle East.

Table 1.
Characteristics of the studies included in the present review.
All studies included in the current review involved overweight or obese PCOS patients. Regarding the inclusion criteria used by the studies, 21 included overweight or obese subjects who were included on the basis of the value of BMI, among which, ten, five, and three studies used BMI values of 25, 27, and 30, respectively. Additionally, one study used a BMI value of 28.6. Conversely, two studies presented data as age-specific percentiles. Moreover, only one study selected subjects with obesity based on body weight, whereas three studies did not state the specific criteria used to identify obesity.
Regarding the criteria used to diagnose PCOS, eight studies employed the Rotterdam diagnostic criteria, one study used the National Institutes of Health diagnostic criteria, and one study employed both. Among the studies included in the present review, five diagnosed PCOS on the basis of clinical characteristics, two employed radiographic evaluation by means of ultrasound scans, three studies used a combination of clinical characteristics and ultrasound scans, and five studies did not state the exact diagnostic criteria or methods used for diagnosis, although the subjects were reported to be PCOS patients.
The lifestyle modification program was divided into two groups: monotherapy with diet or exercise and combination therapy involving diet and exercise (hereinafter referred to as combination therapy). Regarding the type of intervention program used by the studies, nine used monotherapy with diet, six employed monotherapy with exercise, and ten studies used combination therapy (Table 2).

Table 2.
Characteristics of the lifestyle modification program.
This study analyzed the reproductive, anthropometric, androgenic, and metabolic indices to confirm the effects of the lifestyle modification program in PCOS patients with obesity. Among the studies included in the present review, five [8,9,12,14,31] compared the data pertaining to the intervention group who received the lifestyle modification program and the control group who received usual or minimal care. Summary estimates of individual studies were synthesized by meta-analysis when the results of two or more studies were reported using the same subindex. Additionally, the statistical significance of the data pertaining to the 20 studies that did not compare the group that underwent the lifestyle intervention program with the control group was confirmed by comparing the results before and after the intervention.
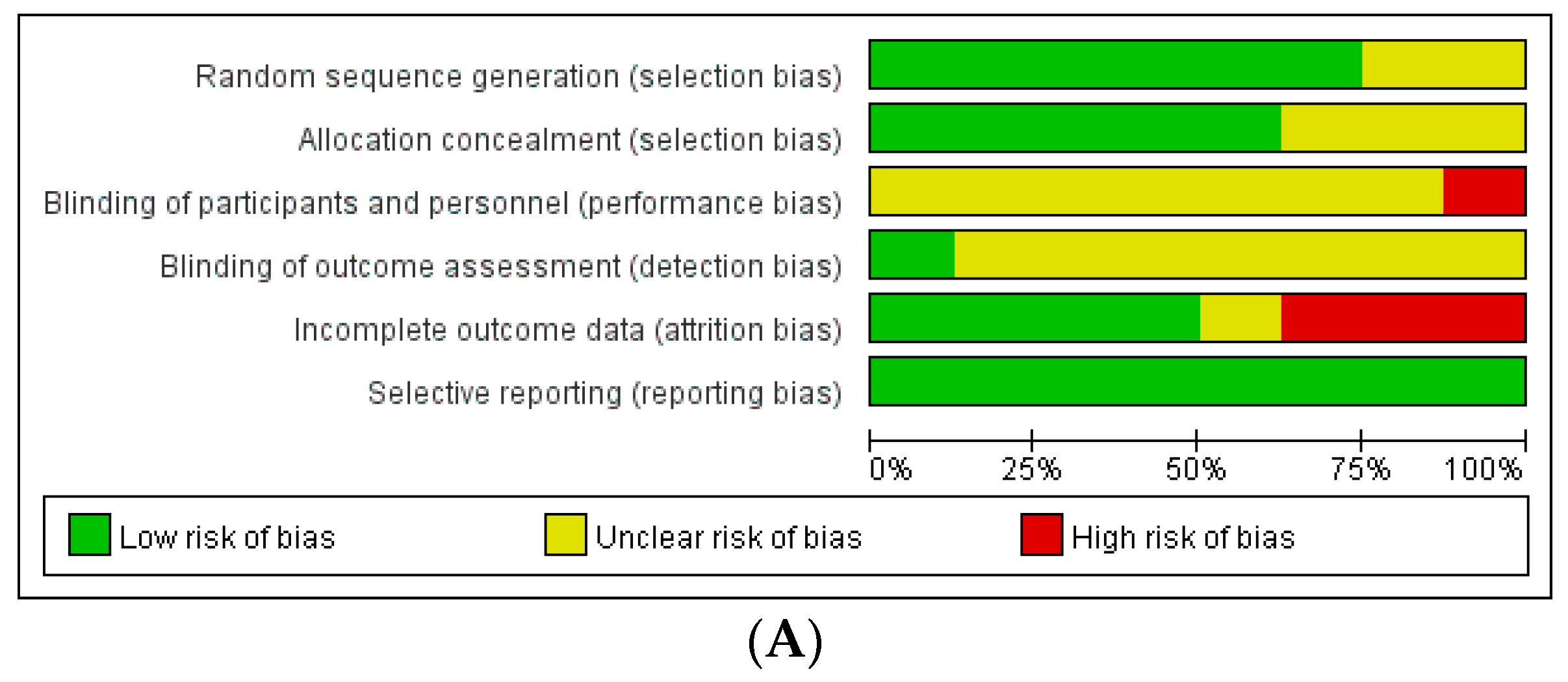
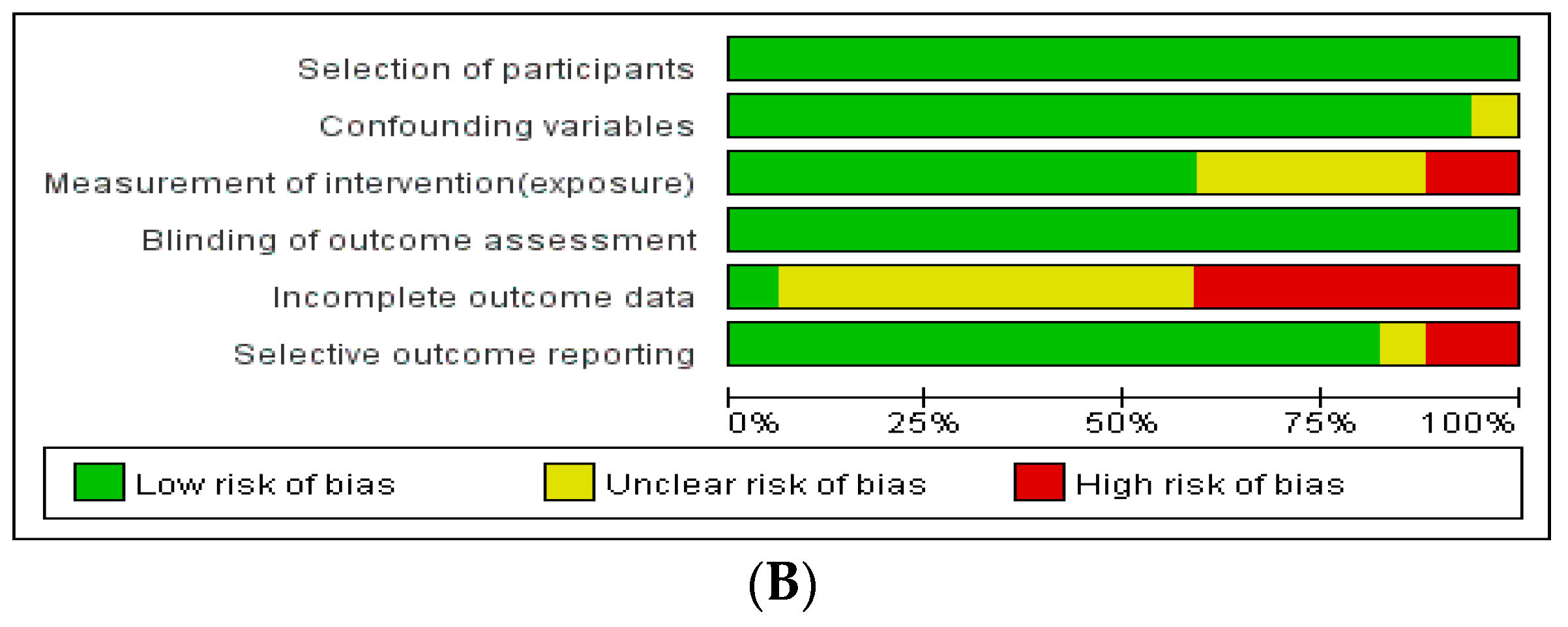
The assessment of the risk of bias in these studies revealed the following results (Figure 2): Among the RCTs, seven studies had a low risk of selection bias, and seven studies reported an unclear risk of blinding of participant, personnel, and outcome assessments. Three out of eight studies had a high risk of attrition bias associated with high drop rates. Among non-randomized studies, seven out of 17 studies had a high risk of attrition bias related to incomplete outcome data.
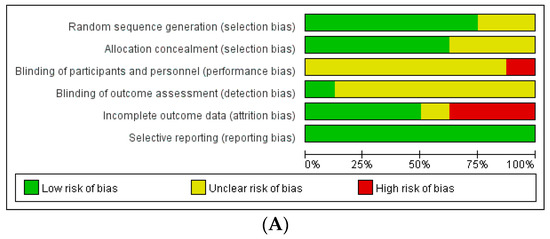
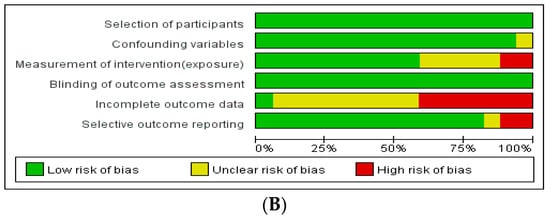
Figure 2.
Assessment of risk of bias. (A) Cochrane Risk of Bias tool (RoB). (B) Risk of Bias Assessment Tool for Non-randomized Studies (RoBANS).
3.2. Effectiveness of Lifestyle Modification
3.2.1. Reproductive Index
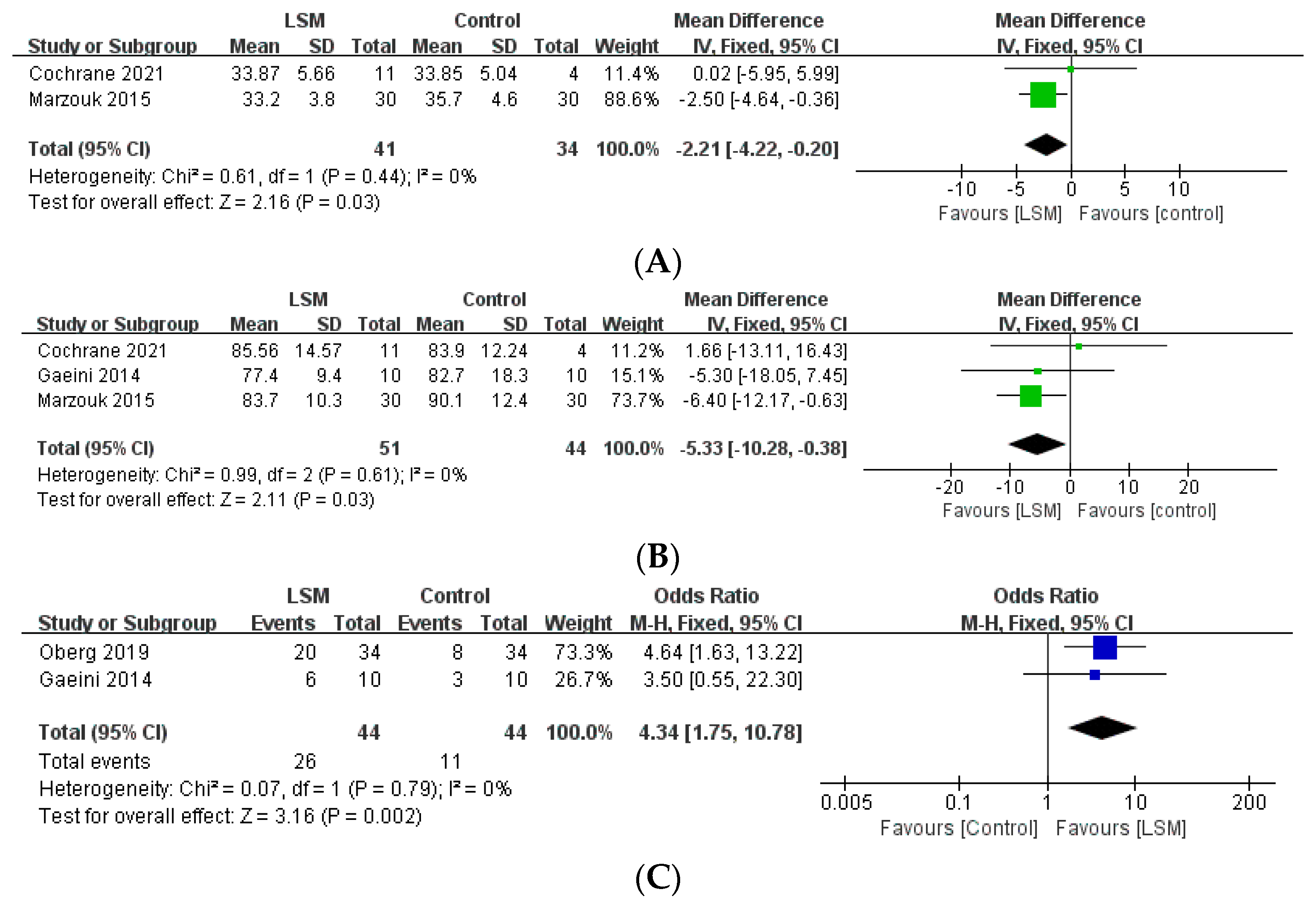
Among the studies included in the present review, reproductive indices were used to assess the outcomes and report the results of 24 studies (Table 3). Among the aforementioned five studies that compared the intervention and control groups, the lifestyle modification group had a significantly higher number of patients with improved menstrual cycles, compared to the control group (OR: 4.34, 95% CI: 1.75–10.78, p = 0.02, I2 = 0%, Figure 3). Moreover, one study [12] reported that the intervention group had a significantly higher number of menstrual episodes than the control group (p = 0.01). In another study [14], the number of ovarian follicles in the intervention group was significantly decreased (from 17 [SD = 2] to 12 [SD = 2] [p < 0.05]), whereas there was no significant difference in the control group. Furthermore, 6 of the 10 patients (60%) in the intervention group showed improved menstrual cycles, while the same was observed in only 3 of the 10 patients (30%) in the control group (p < 0.05). Another study [31] reported that four of the six (66.7%) patients in the intervention group displayed ovulation, whereas the same was observed in only one of the six (16.7%) patients in the control group.

Table 3.
Reproductive effects of the lifestyle modification program.
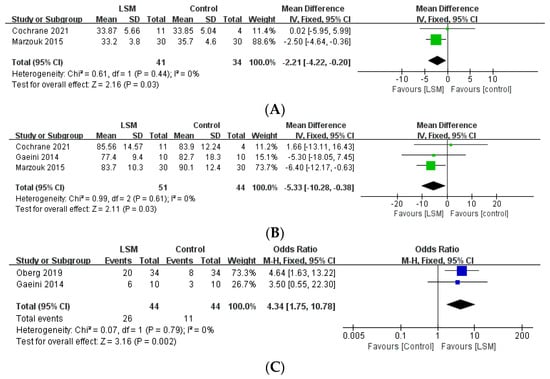
Figure 3.
Forest plot for the meta-analysis of lifestyle modification, compared to controls. (A) BMI. (B) Weight. (C) Improved menstrual cycle. (CI: confidence interval, LSM: lifestyle modification, SD: standard deviation.)
The effect of intervention on the reproductive index reported by the studies that did not involve control groups is stated as follows: Among the nine studies that reported the number of patients with improved menstrual cycles after intervention, the proportion of patients with improved cycles ranged from 19.2% to 70%. Ovulation was reported in six studies, and the proportion of patients with ovulation ranged from 35% to 60%. Moreover, the number of ovarian follicles was reported in six studies, among which five studies reported a significant reduction in the number of ovarian follicles after lifestyle intervention compared to the status prior to the intervention.
3.2.2. Metabolic Index
Among the five studies that compared the group that underwent lifestyle intervention programs with the control group [31], one study assessed and reported fasting insulin levels. The intervention group showed a significant decrease in fasting insulin levels after 12 weeks (from 73.6 [SD = 7.01] 57.1 [SD = 11.5]), whereas no significant difference was observed in the control group.
Furthermore, in reference to the variation in parameters before and after lifestyle modification, four of six studies reported a significant reduction in the fasting glucose level after lifestyle intervention compared to the level prior to the intervention. Seven of thirteen studies reported a significant reduction in fasting insulin levels, and four of five studies reported a significant reduction in HOMA-IR scores.
3.2.3. Anthropometric Index
All 25 studies included in the current review reported the effect of lifestyle intervention programs on anthropometric indices. The five studies that compared the group that underwent the lifestyle intervention program with the control group reported that the intervention group showed a statistically significant decrease in BMI (MD: −2.21, 95% CI: −4.22, −0.20, p = 0.03, I2 = 0%) and weight (MD: −5.33, 95% CI: −10.28 to −0.38, p = 0.03, I2 = 0%), compared to the control group (Figure 3). Furthermore, one study [12] reported that the group that underwent lifestyle modifications had a significantly decreased WC compared to the control group (p = 0.029).
In addition, in reference to the variation in parameters before and after lifestyle modification, 7 of 11 studies reported a significant decrease in BMI after lifestyle intervention compared to the situation before intervention, while four studies did not report any statistically significant difference. Moreover, 9 of the 12 studies reported a significant reduction in body weight after lifestyle intervention. All 12 studies that evaluated WC reported a significant decrease after the lifestyle intervention.
3.3. Subgroup Analysis
3.3.1. Effectiveness of Lifestyle Modification according to the Type of Intervention
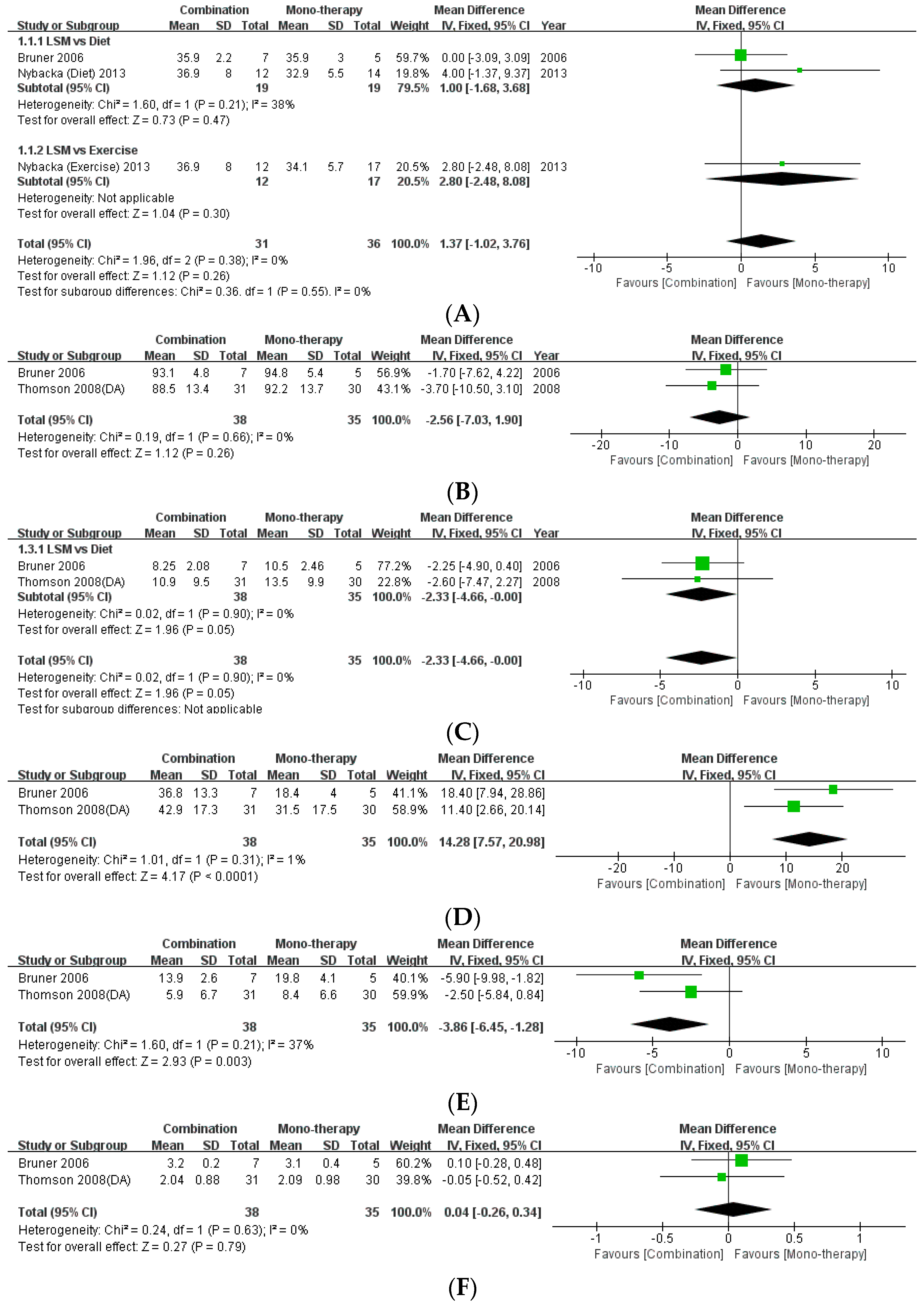
The present review included three studies that compared the effects of monotherapy (diet/exercise) with combination therapy [16,21,24]. This study performed a meta-analysis of the data pertaining to the anthropometric indices of BMI and WC (Figure 4). There was no statistical difference between monotherapy and combination therapy with regard to BMI (MD: 1.37, 95% CI: −1.02 to 3.76, p = 0.26, I2 = 0%) and WC (MD: −2.56, 95% CI: −7.03 to 1.90, p = 0.26, I2 = 0%). Moreover, meta-analysis of the data regarding the levels of fasting insulin as a metabolic index revealed that combination therapy effected a statistically significant decrease in fasting insulin levels compared to monotherapy with diet (MD: −2.33, 95% CI: −4.66, −0.00, p = 0.05, I2 = 0%). Regarding the difference between monotherapy and combination therapy in the androgenic indices, there was no statistically significant difference in the levels of testosterone (MD: 0.04, 95% CI: −0.26 to −0.34, p = 0.79, I2 = 0%). However, combination therapy achieved significant improvement in reference to the levels of SHBG (MD: 14.28, 95% CI: 7.57 to 20.98, p < 0.0001, I2 = 1%) and FAI (MD: −3.86, 95% CI: −6.45, −1.28, p = 0.003, I2 = 37%) compared to monotherapy.
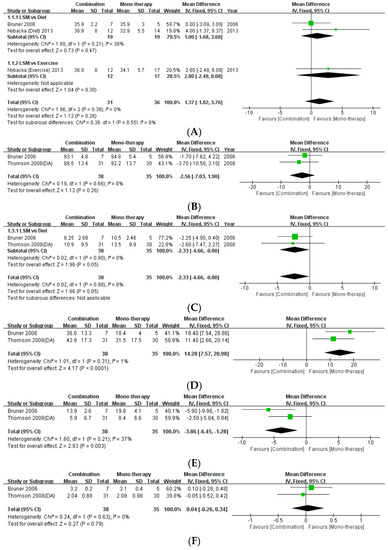
Figure 4.
Forest plot for the meta-analysis of combination therapy, compared to monotherapy. (A) BMI. (B) Waist circumference. (C) Fasting insulin level. (D) Testosterone level. (E) Sex-hormone binding globulin. (F) Free androgen index. (CI: confidence interval, SD: standard deviation.)
3.3.2. Effectiveness of Lifestyle Modification according to the Degree of Weight Loss
This review involved three studies that categorized the subjects into groups according to the degree of weight loss and compared the results [17,25,32]. Among the studies, two studies divided the subjects into groups according to the success or failure of weight loss by 5% of body weight [25,32], while one study divided the subjects on the basis of the reduction in respective BMI SD score by 0.2 or more [17].
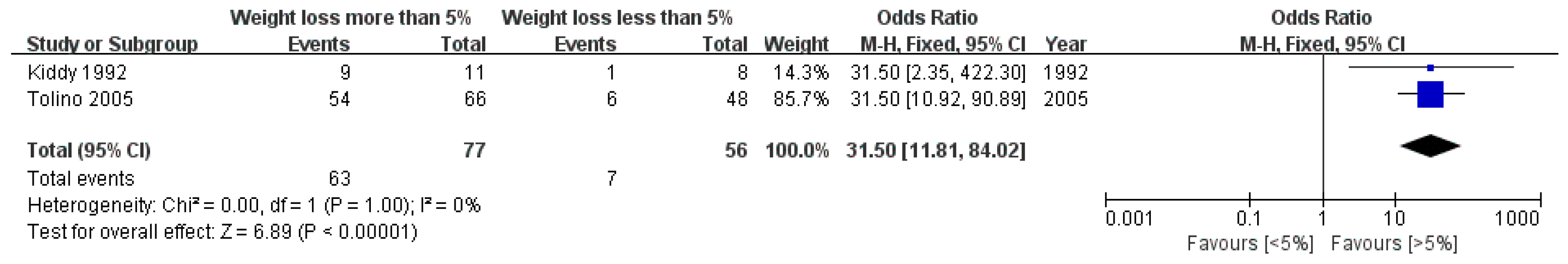
This study performed a meta-analysis of data pertaining to improvements in reproductive function (Figure 5). The number of patients with improved reproductive function was significantly higher in the group that achieved weight loss of 5% or more compared to the subjects who achieved less than 5% weight loss (OR: 31.50, 95% CI: 11.81 to 84.02, p < 0.00001, I2 = 0%).
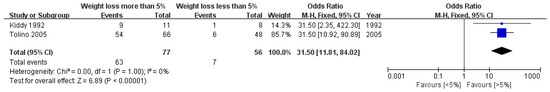
Figure 5.
Forest plot for the meta-analysis of improvements in reproductive function according to the degree of weight loss. (CI: confidence interval, SD: standard deviation.)
Moreover, regarding the metabolic index, two studies [25,32] observed a significant reduction in the fasting insulin level in the group that lost more than 5% of body weight, whereas no significant change was observed in the group that lost less than 5% of weight. In addition, one study [17] reported that the group that successfully attained weight loss displayed a tendency toward lower fasting insulin levels (23 to 17), whereas the group that failed to achieve the target weight showed a significant increase in fasting insulin levels (25 to 33, p < 0.05).
4. Discussion
A systematic review and meta-analysis was performed to confirm the effectiveness of lifestyle modifications in the management of PCOS patients with obesity. To the best of our knowledge, this is the most updated and comprehensive systematic review of this subject. Moreover, this is the first study to report the effects of lifestyle modifications on reproductive function. In addition, the current review performed a subgroup analysis according to the type of intervention and degree of weight loss.
The main finding of the present review is that the group that underwent lifestyle modifications displayed significant improvement in reproductive function compared to the control group. Furthermore, the subgroup analysis revealed that combination therapy with diet and exercise had better effects on metabolic and androgenic parameters than monotherapy. In addition, moderate weight loss by a minimum of 5% of body weight appeared to be effective in improving the metabolic index, that is, fasting insulin levels. The BMI and body weight of the group that underwent lifestyle intervention were significantly lower than those of the control group (BMI: MD −2.21, p = 0.03, weight: MD −5.33, p = 0.03). These results are concurrent with the results reported by previous studies, which observed that lifestyle modification programs have positive effects on anthropometric indices [7].
4.1. Lifestyle Modification Has a Positive Effect on Reproductive Outcomes in PCOS Patients with Obesity
Lifestyle modification plays an important role in the improvement of reproductive outcomes in PCOS patients with obesity. This study performed a meta-analysis of the data pertaining to reproductive function and found that the group that underwent lifestyle modifications had a significantly higher number of patients with improved menstrual cycles compared to the control group (OR: 4.34, p = 0.02). Furthermore, the RCTs reported a significant improvement in menstrual episodes or number of ovarian follicles in the group that underwent lifestyle modifications compared to the control group [12,14]. Several therapeutic approaches, such as combined oral contraceptives, insulin sensitizers, anti-androgenic drugs, and assisted reproductive therapy, have been used in the management of PCOS patients who wish to conceive a child. However, these treatments might be associated with the risk of adverse effects [9]. The current results regarding the effect of lifestyle modification alone, without any additional treatment, on the reproductive index are expected to offer encouragement for patients regarding their chances of conception due to the fact that PCOS patients often have concerns about fertility [33].
4.2. Combination Therapy with Diet and Exercise Rather Than Monotherapy
It is necessary to recommend healthy lifestyle modifications, including dietary interventions to reduce caloric intake and regular exercise, for obese women with PCOS. The results of the present meta-analysis confirmed that combination therapy had better effects on the improvement of fasting insulin levels (MD: −2.33, p = 0.05), SHBG (MD: 14.28, p < 0.0001), and FAI (MD: −3.86, p = 0.003) compared to monotherapy. Moreover, there was no significant difference between the two interventions with regard to weight loss, and both resulted in adequate weight loss.
The aforementioned result is concurrent with the updated international evidence-based guidelines that recommend a healthy lifestyle involving a healthy diet and regular physical activity for the management of patients with PCOS [2]. A calorie-restricted diet has been mainly used to achieve weight loss in PCOS patients with obesity, and several studies have confirmed the effects of symptomatic improvement along with weight loss [7]. In addition, resistance exercise improves insulin sensitivity by increasing muscle mass, and aerobic exercise improves glucose disposal by increasing glycogen synthase activity [34,35]. Considering the fact that PCOS is characterized by hyperinsulinemia and exacerbated by abdominal obesity [21], a combination of diet and exercise can be a potentially effective mode of treatment.
Furthermore, this study attempted to perform a subgroup analysis according to the type of intervention. However, the analysis could not be performed owing to the heterogeneity of the interventions employed in the studies. Regarding dietary intervention, several nutrient-restricted diets, such as a low-carbohydrate diet, low-fat diet, and high-protein diet, have been followed by PCOS patients. However, the effect of nutrient-restricted diets has not been confirmed to date, and only the effect of a low-calorie diet has been reported in the literature [12,19,23,26]. Hence, this scenario warrants the development of an optimal intervention for the management of PCOS patients with obesity.
4.3. Metabolic Index Improved When Moderate Weight Loss Was Achieved
The results reported by previous studies regarding the effects of lifestyle modifications on the metabolic index have been controversial. In addition, recent reviews have reported that the effect of lifestyle interventions on an oral glucose tolerance test is uncertain [7,36]. Furthermore, in this review, the analysis of results concerning the metabolic index was challenging owing to the lack of a sufficient number of studies. Hence, the current study performed a subgroup analysis according to the improvement in fasting insulin level or lack of the same.
In this review, 7 of 13 studies showed improvement in fasting insulin levels after intervention, and six did not report any improvement. Among the 13 studies, 5 compared the body weight before and after the intervention. Studies that involved subjects who attained weight loss by a minimum of 5% of body weight reported an improvement in insulin levels. Nonetheless, subjects who lost less than 5% of their body weight did not display any significant difference in fasting insulin levels after the intervention. Among the studies that divided the subjects into groups according to the degree of weight loss and compared the results, two studies [25,32] observed a significant reduction in insulin levels in the group that lost more than 5% of body weight. No significant change was observed in the group that lost less than 5% of body weight. Considering the abovementioned results, it is assumed that moderate weight loss (minimum of 5%) might be effective in improving the metabolic index.
Insulin resistance is a major cause of the increased severity of PCOS, and PCOS patients with obesity are relatively more vulnerable compared to PCOS patients with normal body weight [37]. Thus, understanding and verifying the metabolic effects of lifestyle modification among PCOS patients with obesity is important from the perspective of management. Because a meta-analysis was not possible due to the lack of a sufficient number of studies, further studies are required to improve the level of evidence on the subject.
4.4. Strengths and Limitations
The present systematic review studied the efficacy of lifestyle modification in the management of obese PCOS patients using the most updated data. Moreover, the current study performed subgroup analyses based on the type of intervention and degree of weight loss in order to identify the ideal intervention in such patients.
The current study has certain limitations. First, the sample size of the studies included in the review was not adequate. The scenario warrants high-quality studies with large sample sizes in order to improve the level of evidence. Second, owing to the heterogeneity concerning the study design, it was difficult to conduct a meta-analysis. Comparative analysis in accordance with the type of intervention requires the establishment of a standardized study design. Finally, further studies that include the assessment of pregnancy and ovulation rates as reproductive functions are required to confirm the effect of lifestyle modification among patients with PCOS.
5. Conclusions
This review identified evidence supporting the effectiveness of lifestyle modifications in PCOS patients with obesity. Lifestyle modification as a first-line treatment of obese women with PCOS may effect outcomes, and accompanying moderate weight loss is also expected to improve the metabolic index. Lifestyle modification using combination therapy is a promising therapeutic approach that can be employed in the management of PCOS patients with obesity.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/life12020308/s1, Table S1: PRISMA 2020 checklist; List S1: Study protocol.
Author Contributions
Conceptualization, S.-H.L.; methodology, S.-H.L.; software, C.-H.K.; formal analysis, S.-H.L. and C.-H.K.; data curation, S.-H.L. and C.-H.K.; writing—original draft preparation, C.-H.K.; writing—review and editing, S.-H.L.; supervision, S.-H.L.; project administration, S.-H.L.; funding acquisition, S.-H.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Research Foundation of Korea (NRF) funded by the Korean government (MSIT) (No. 2020R1F1A1073141).
Institutional Review Board Statement
This study was approved by the Ethics Review Committee of Gachon university (1044396-202010-HR-192-01).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Patten, R.; Boyle, R.A.; Moholdt, T.; Kiel, I.; Hopkins, W.G.; Harrison, C.L.; Stepto, N.K. Exercise interventions in polycystic ovary syndrome: A systematic review and meta-analysis. Front. Physiol. 2020, 11, 606. [Google Scholar] [CrossRef] [PubMed]
- Teede, H.J.; Misso, M.L.; Costello, M.F.; Dokras, A.; Laven, J.; Moran, L.; Piltonen, T.; Norman, R.J. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum. Reprod. 2018, 33, 1602–1618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoeger, K.M.; Dokras, A.; Piltonen, T. Update on PCOS: Consequences, challenges, and guiding treatment. J. Clin. Endocrinol. Metab. 2020, 106, e1071–e1083. [Google Scholar] [CrossRef] [PubMed]
- Acién, P.; Quereda, F.; Matallıín, P.; Villarroya, E.; López-Fernández, J.A.; Acién, M.; Mauri, M.; Alfayate, R. Insulin, androgens, and obesity in women with and without polycystic ovary syndrome: A heterogeneous group of disorders. Fertil. Steril. 1999, 72, 32–40. [Google Scholar] [CrossRef]
- Teede, H.J.; Joham, A.E.; Paul, E.; Moran, L.; Loxton, D.; Jolley, D.; Lombard, C. Longitudinal weight gain in women identified with polycystic ovary syndrome: Results of an observational study in young women. Obesity 2013, 21, 1526–1532. [Google Scholar] [CrossRef]
- Lim, S.; Davies, M.; Norman, R.; Moran, L. Overweight, obesity and central obesity in women with polycystic ovary syndrome: A systematic review and meta-analysis. Hum. Reprod. Update 2012, 18, 618–637. [Google Scholar] [CrossRef]
- Lim, S.S.; Hutchison, S.K.; Van Ryswyk, E.; Norman, R.J.; Teede, H.J.; Moran, L.J. Lifestyle changes in women with polycystic ovary syndrome. Cochrane Database Syst. Rev. 2019. [Google Scholar] [CrossRef]
- Cochrane, T.; Tengku-Kamalden, T.F.; Davey, R.; Dev, R.D.O. Effect of Exercise and Weight Loss in Polycystic Ovarian Syndrome among Obese Women. Pertanika J. Soc. Sci. Hum. 2021, 29, 119–134. [Google Scholar]
- Oberg, E.; Gidlöf, S.; Jakson, I.; Mitsell, M.; Egnell, P.T.; Hirschberg, A.L. Improved menstrual function in obese women with polycystic ovary syndrome after behavioural modification intervention—A randomized controlled trial. Clin. Endocrinol. 2019, 90, 468–478. [Google Scholar] [CrossRef]
- Kirubamani, H.; Abraham, M. Effect of aerobic exercise (self-help strategy) on the common endocrine problem (PCOS) in late adolescent & young women & impact on their quality of life. Int. J. Res. Pharm. Sci. 2018, 9, 1238–1242. [Google Scholar]
- Deepthi, G.; Sankarakumaran, P.; Jerome, A.; Kalirathinam, D.; Raj, N.B.; Us, M.R. Effect of aerobic exercise in improving the quality of life in polycystic ovarian disease. Res. J. Pharm. Technol. 2017, 10, 1788–1790. [Google Scholar] [CrossRef]
- Marzouk, T.M.; Ahmed, W.A.S. Effect of dietary weight loss on menstrual regularity in obese young adult women with polycystic ovary syndrome. J. Pediatr. Adolesc. Gynecol. 2015, 28, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, D. Lifestyle modification intervention among infertile overweight and obese women with polycystic ovary syndrome. J. Am. Assoc. Nurse Pract. 2014, 26, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Gaeini, A.; Satarifard, S.; Mohamadi, F.; Choobineh, S. The effect of 12 weeks aerobic exercise on DHEAso4, 17OH-Progestron concentrations, number of follicles and menstrual condition of women with PCOS. Hormozgan Med. J. 2014, 18, 298–305. [Google Scholar]
- Roessler, K.K.; Birkebaek, C.; Ravn, P.; Andersen, M.S.; Glintborg, D. Effects of exercise and group counselling on body composition and VO 2max in overweight women with polycystic ovary syndrome. Acta Obstet. Gynecol. Scand. 2013, 92, 272–277. [Google Scholar] [CrossRef]
- Nybacka, Å.; Carlström, K.; Fabri, F.; Hellström, P.M.; Hirschberg, A.L. Serum antimüllerian hormone in response to dietary management and/or physical exercise in overweight/obese women with polycystic ovary syndrome: Secondary analysis of a randomized controlled trial. Fertil. Steril. 2013, 100, 1096–1102. [Google Scholar]
- Lass, N.; Kleber, M.; Winkel, K.; Wunsch, R.; Reinehr, T. Effect of lifestyle intervention on features of polycystic ovarian syndrome, metabolic syndrome, and intima-media thickness in obese adolescent girls. J. Clin. Endocrinol. Metab. 2011, 96, 3533–3540. [Google Scholar] [CrossRef] [Green Version]
- Redman, L.M.; Elkind-Hirsch, K.; Ravussin, E. Aerobic exercise in women with polycystic ovary syndrome improves ovarian morphology independent of changes in body composition. Fertil. Steril. 2011, 95, 2696–2699. [Google Scholar] [CrossRef] [Green Version]
- Ornstein, R.M.; Copperman, N.M.; Jacobson, M.S. Effect of weight loss on menstrual function in adolescents with polycystic ovary syndrome. J. Pediatr. Adolesc. Gynecol. 2011, 24, 161–165. [Google Scholar] [CrossRef]
- Thomson, R.; Buckley, J.; Moran, L.; Noakes, M.; Clifton, P.; Norman, R.; Brinkworth, G. The effect of weight loss on anti-Mullerian hormone levels in overweight and obese women with polycystic ovary syndrome and reproductive impairment. Hum. Reprod. 2009, 24, 1976–1981. [Google Scholar] [CrossRef] [Green Version]
- Thomson, R.; Buckley, J.; Noakes, M.; Clifton, P.; Norman, R.; Brinkworth, G. The effect of a hypocaloric diet with and without exercise training on body composition, cardiometabolic risk profile, and reproductive function in overweight and obese women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2008, 93, 3373–3380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palomba, S.; Giallauria, F.; Falbo, A.; Russo, T.; Oppedisano, R.; Tolino, A.; Colao, A.; Vigorito, C.; Zullo, F.; Orio, F. Structured exercise training programme versus hypocaloric hyperproteic diet in obese polycystic ovary syndrome patients with anovulatory infertility: A 24-week pilot study. Hum. Reprod. 2007, 23, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Moran, L.J.; Noakes, M.; Clifton, P.; Wittert, G.A.; Williams, G.; Norman, R. Short-term meal replacements followed by dietary macronutrient restriction enhance weight loss in polycystic ovary syndrome. Am. J. Clin. Nutr. 2006, 84, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Bruner, B.; Chad, K.; Chizen, D. Effects of exercise and nutritional counseling in women with polycystic ovary syndrome. Appl. Physiol. Nutr. Metab. 2006, 31, 384–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tolino, A.; Gambardella, V.; Caccavale, C.; D’Ettore, A.; Giannotti, F.; D’Antò, V.; De Falco, C. Evaluation of ovarian functionality after a dietary treatment in obese women with polycystic ovary syndrome. Eur. J. Obstet. Gynecol. Reprod. Biol. 2005, 119, 87–93. [Google Scholar] [CrossRef]
- Stamets, K.; Taylor, D.S.; Kunselman, A.; Demers, L.M.; Pelkman, C.L.; Legro, R.S. A randomized trial of the effects of two types of short-term hypocaloric diets on weight loss in women with polycystic ovary syndrome. Fertil. Steril. 2004, 81, 630–637. [Google Scholar] [CrossRef]
- Van Dam, E.W.; Roelfsema, F.; Veldhuis, J.D.; Hogendoorn, S.; Westenberg, J.; Helmerhorst, F.M.; Frolich, M.; Krans, H.M.J.; Meinders, E.; Pijl, H. Retention of estradiol negative feedback relationship to LH predicts ovulation in response to caloric restriction and weight loss in obese patients with polycystic ovary syndrome. Am. J. Physiol. Endocrinol. Metab. 2004, 286, E615–E620. [Google Scholar] [CrossRef] [Green Version]
- Crosignani, P.G.; Colombo, M.; Vegetti, W.; Somigliana, E.; Gessati, A.; Ragni, G. Overweight and obese anovulatory patients with polycystic ovaries: Parallel improvements in anthropometric indices, ovarian physiology and fertility rate induced by diet. Hum. Reprod. 2003, 18, 1928–1932. [Google Scholar] [CrossRef] [Green Version]
- Moran, L.J.; Noakes, M.; Clifton, P.M.; Tomlinson, L.; Norman, R. Dietary composition in restoring reproductive and metabolic physiology in overweight women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2003, 88, 812–819. [Google Scholar] [CrossRef] [Green Version]
- Huber-Buchholz, M.-M.; Carey, D.; Norman, R. Restoration of reproductive potential by lifestyle modification in obese polycystic ovary syndrome: Role of insulin sensitivity and luteinizing hormone. J. Clin. Endocrinol. Metab. 1999, 84, 1470–1474. [Google Scholar] [CrossRef]
- Guzick, D.S.; Wing, R.; Smith, D.; Berga, S.L.; Winters, S.J. Endocrine consequences of weight loss in obese, hyperandrogenic, anovulatory women. Fertil. Steril. 1994, 61, 598–604. [Google Scholar] [CrossRef]
- Kiddy, D.S.; Hamilton-Fairley, D.; Bush, A.; Short, F.; Anyaoku, V.; Reed, M.J.; Franks, S. Improvement in endocrine and ovarian function during dietary treatment of obese women with polycystic ovary syndrome. Clin. Endocrinol. 1992, 36, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Holton, S.; Hammarberg, K.; Johnson, L. Fertility concerns and related information needs and preferences of women with PCOS. Hum. Reprod. Open 2018, 2018, hoy019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cauza, E.; Hanusch-Enserer, U.; Strasser, B.; Ludvik, B.; Metz-Schimmerl, S.; Pacini, G.; Wagner, O.; Georg, P.; Prager, R.; Kostner, K.; et al. The relative benefits of endurance and strength training on the metabolic factors and muscle function of people with type 2 diabetes mellitus. Arch. Phys. Med. Rehabil. 2005, 86, 1527–1533. [Google Scholar] [CrossRef] [PubMed]
- Ivy, J.L. Role of exercise training in the prevention and treatment of insulin resistance and non-insulin-dependent diabetes mellitus. Sports Med. 1997, 24, 321–336. [Google Scholar] [CrossRef] [PubMed]
- Domecq, J.P.; Prutsky, G.; Mullan, R.J.; Hazem, A.; Sundaresh, V.; Elamin, M.B.; Phung, O.J.; Wang, A.; Hoeger, K.; Pasquali, R.; et al. Lifestyle modification programs in polycystic ovary syndrome: Systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2013, 98, 4655–4663. [Google Scholar] [CrossRef] [Green Version]
- Legro, R.S. (Ed.) Obesity and PCOS: Implications for diagnosis and treatment. In Seminars in Reproductive Medicine; Thieme Medical Publishers: New York, NY, USA, 2012. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).