Antibiofilm Activity of Weissella spp. and Bacillus coagulans Isolated from Equine Skin against Staphylococcus aureus
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation of Microorganisms
2.2. Hemolytic Activity Test
2.3. Genotypic Identification of the Isolates
2.4. Antibiotic Susceptibility Testing and MIC Determinations
2.5. Preparation of the Cell-Free Supernatant (CFS) of Skin Isolates
2.6. Organic Acids Production Analysis
2.7. Effect of the CFS of the Skin Isolates on the Growth and Biofilm Formation of Staphylococcus aureus CCM 4223 and MRSA
2.7.1. Effect of the CFS of Skin Isolates on the Dispersion of Biofilm Produced by S. aureus CCM 4223 and MRSA
2.7.2. Effect of Catalase- and Trypsin-Treated nCFS of Weissella spp. on the Growth of S. aureus CCM 4223
2.8. Screening of the Ability to Produce Biosurfactants (BS)
2.8.1. Determination of the Extracellular BS
2.8.2. Determination of the Cell-Bound BS
2.8.3. Screening of the Genes Responsible for the Biosynthesis of the Biosurfactants of Bacillus coagulans
2.9. Statistical Analysis
3. Results
3.1. Genotypic Identification of Isolates
3.2. Antibiotic Susceptibility Testing and MIC Determinations
3.3. Organic Acids Production Analysis
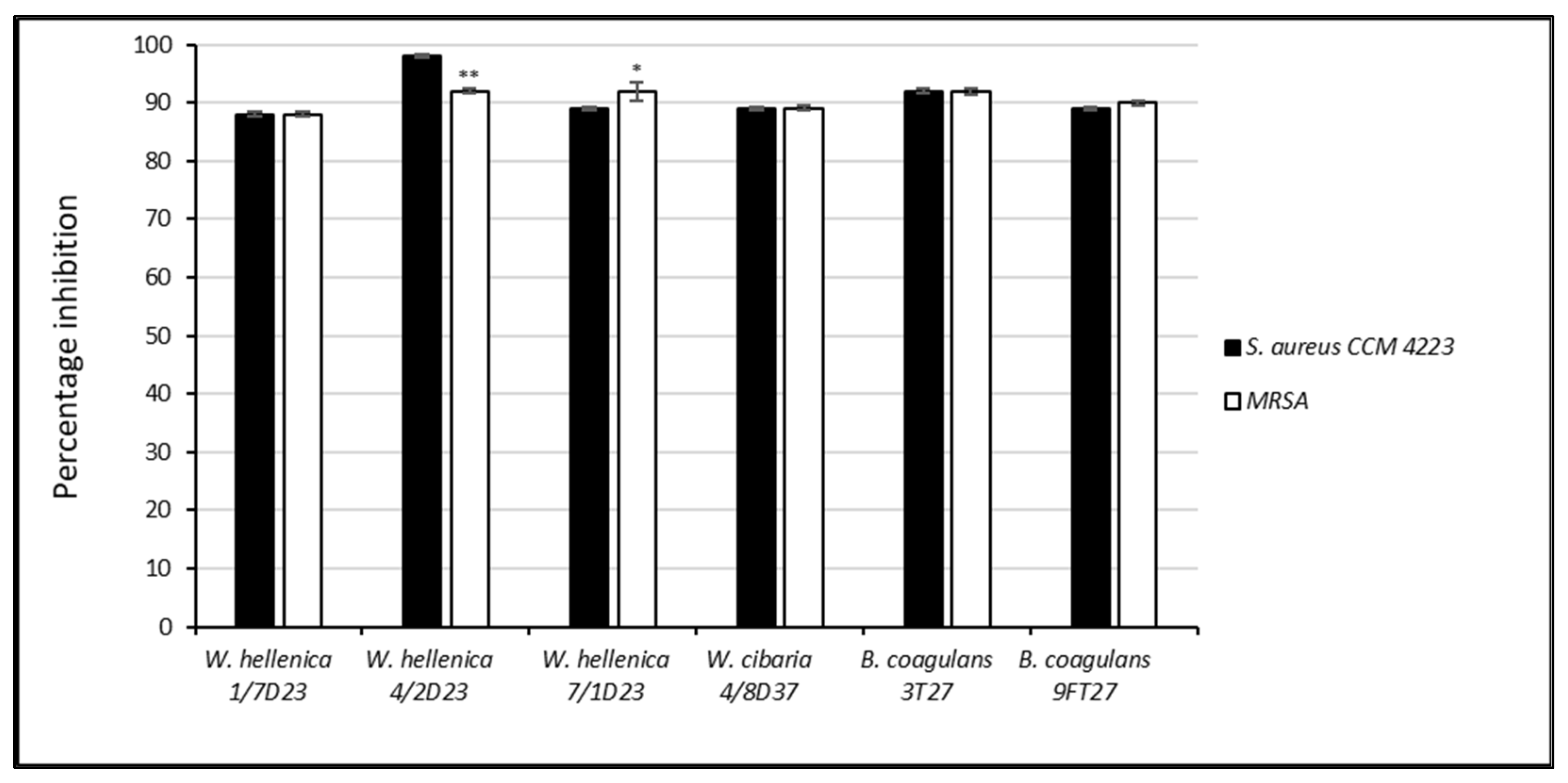
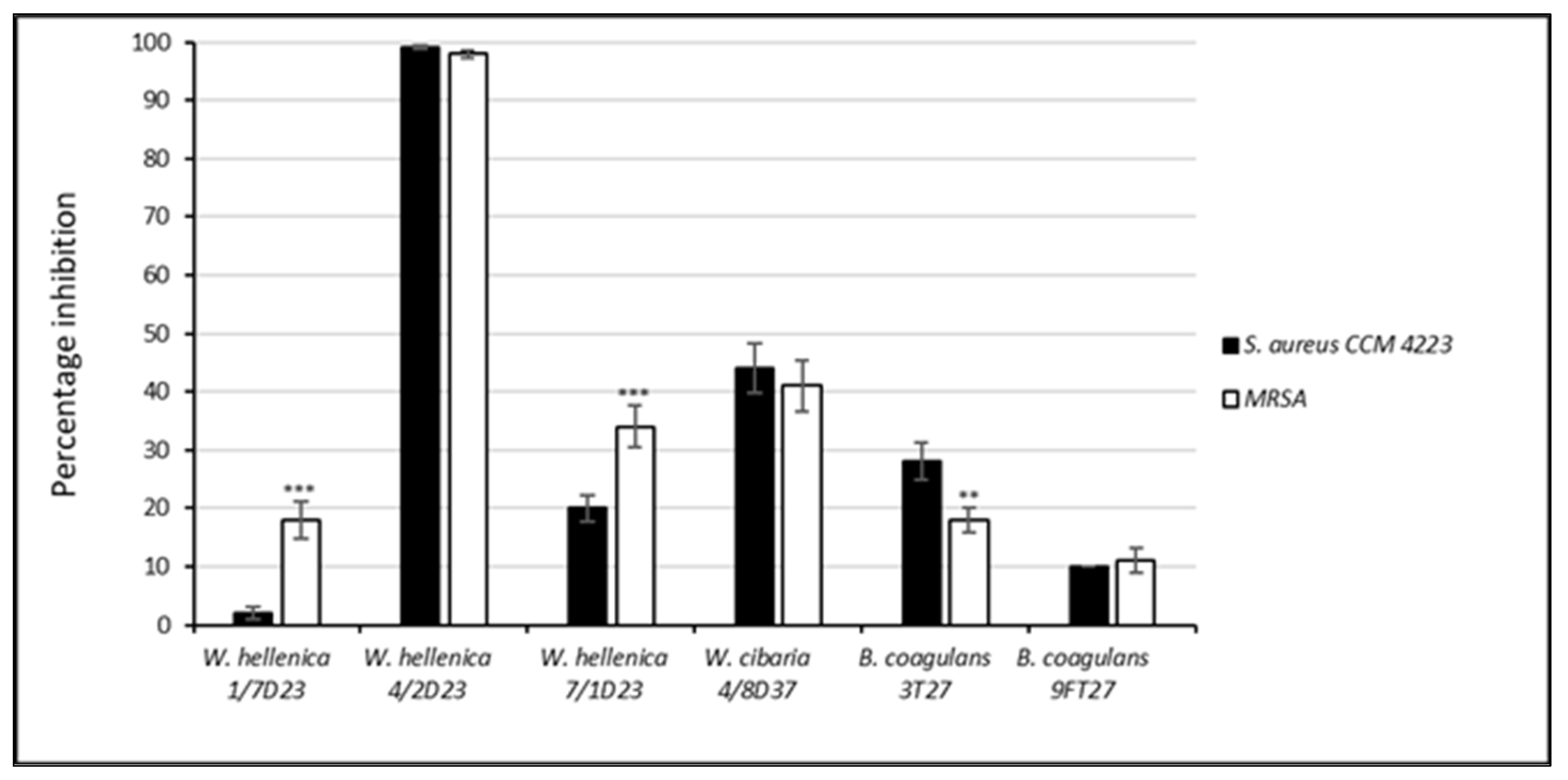
3.4. Effect of the CFS of Skin Isolates on the Growth and Biofilm Formation of S. aureus CCM 4223 and MRSA
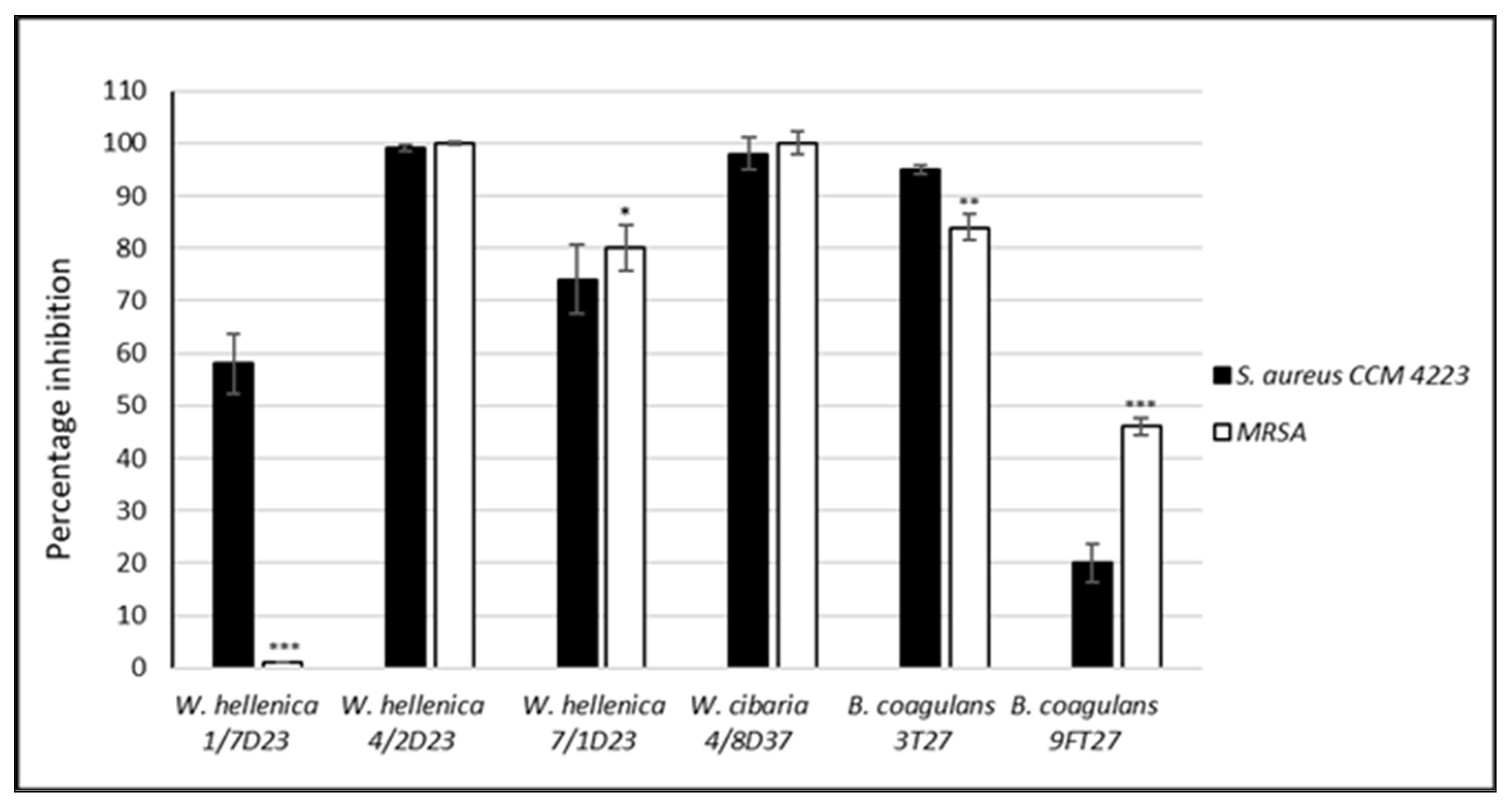
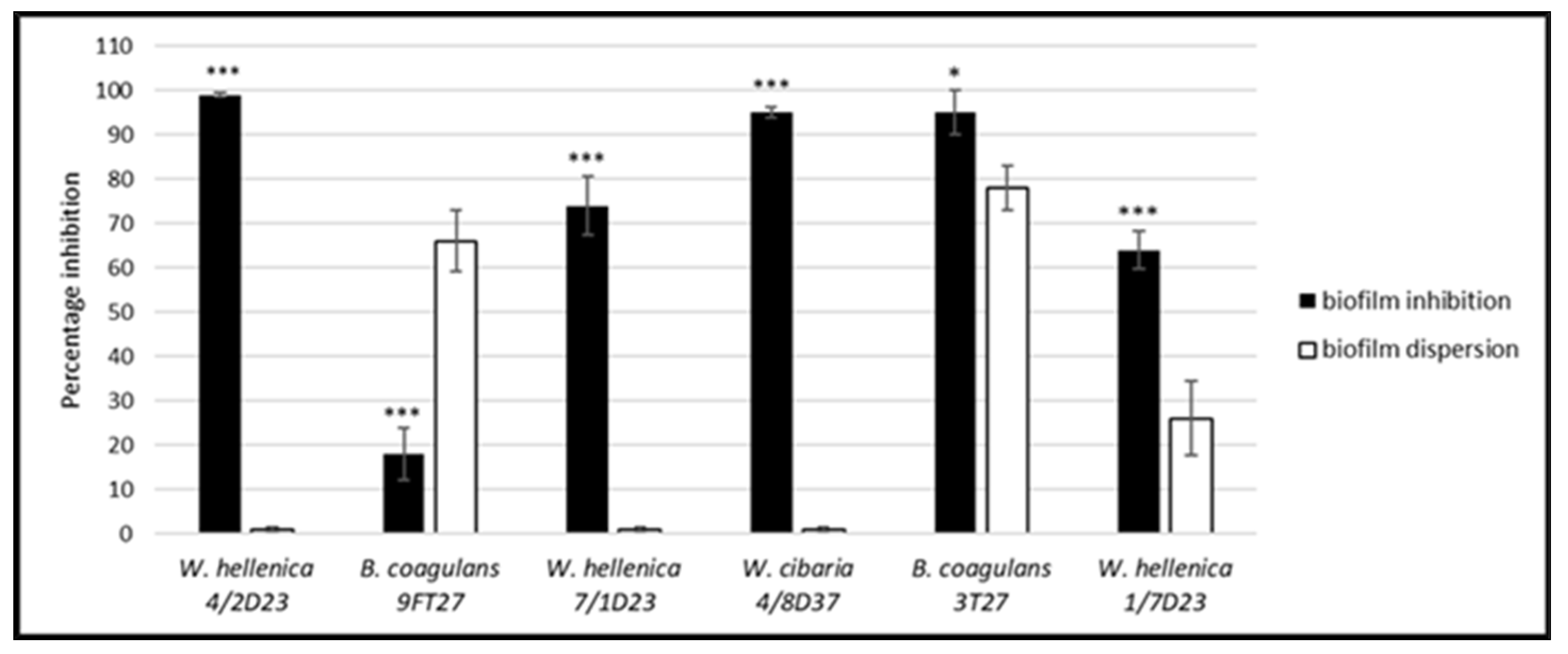
3.5. Effect of the CSF of Skin Isolates on the Dispersion of Biofilm Produced by S. aureus CCM 4223 and MRSA
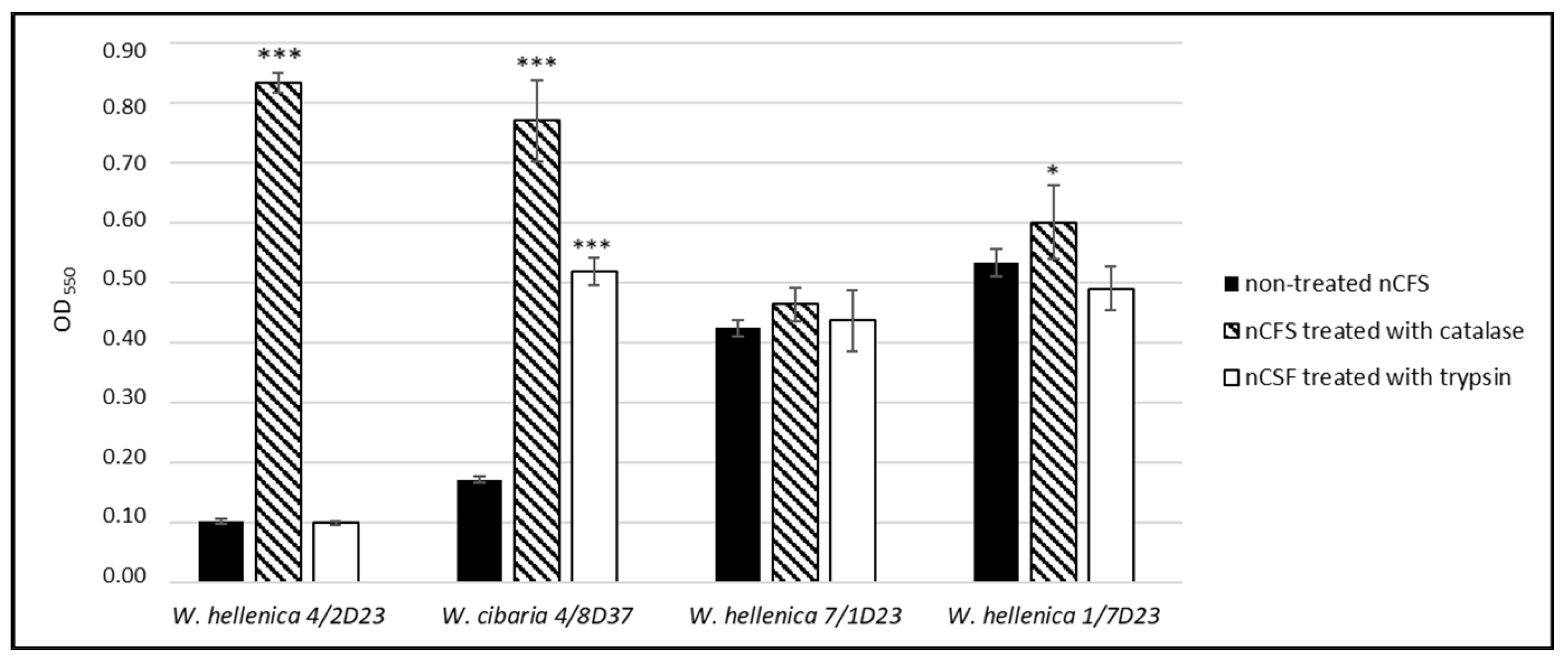
3.6. Antimicrobial Activity of the Treated nCFS of Weissella spp.
3.7. Screening of the Ability to Produce Biosurfactants (BS)
3.8. Screening of the Genes Responsible for the Production of BS in B. coagulans
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Donlan, R.M.; Costerton, J.W. Biofilms, survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.; Narkowicz, C.; Peterson, G.M.; Jacobson, G.A.; Narayana, A. Treatment of pastern dermatitis with a formulation containing kunzea oil: A randomised controlled trial. Vet. Rec. 2009, 20, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Hughes, G.; Webber, M.A. Novel approaches to the treatment of bacterial biofilm infections. Br. J. Pharmacol. 2017, 174, 2237–2246. [Google Scholar] [CrossRef] [PubMed]
- Cinque, B.; La Torre, C.; Melchiorre, E.; Marchesani, G.; Zoccali, G.; Palumbo, P.; Di Marzio, L.; Masci, A.; Mosca, L.; Mastromarino, P.; et al. Use of probiotics for dermal applications. In Probiotics; Liong, M.T., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 221–241. [Google Scholar]
- Lee, K.W.; Park, J.Y.; Jeong, H.R.; Heo, H.J.; Han, N.S.; Kim, J.H. Probiotic properties of Weissella strains isolated from human faeces. Anaerobe 2012, 18, 96–102. [Google Scholar] [CrossRef]
- Lew, L.C.; Liong, M.T. Bioactives from probiotics for dermal health: Functions and benefits. J. Appl. Microbiol. 2013, 114, 1241–1253. [Google Scholar] [CrossRef]
- Gillor, O.; Etzion, A.; Riley, M.A. The dual role of bacteriocins as anti- and probiotics. Appl. Microbiol. Biotechnol. 2008, 81, 591–606. [Google Scholar] [CrossRef]
- Ouwehand, A.C.; Tiihonen, K.; Lahtinen, S. The potential of probiotics and prebiotics for skin health. In Textbook of Aging Skin; Farage, M.A., Miller, K.W., Maibach, H.I., Eds.; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Fusco, V.; Quero, G.M.; Stea, G.; Morea, M.; Visconti, A. Novel PCR-based identification of Weissella confusa using an AFLP-derived marker. Int. J. Food Microbiol. 2011, 145, 437–443. [Google Scholar] [CrossRef]
- Abdhul, K.; Ganesh, M.; Shanmughapriya, S.; Vanithamani, S.; Kanagavel, M.; Anbarasu, K.; Natarajaseenivasan, K. Bacteriocinogenic potential of a probiotic strain Bacillus coagulans [BDU3] from Ngari. Int. J. Biol. Macromol. 2015, 79, 800–806. [Google Scholar] [CrossRef]
- Konuray, G.; Erginkaya, Z. Potential Use of Bacillus coagulans in the food industry. Foods 2018, 7, 92. [Google Scholar] [CrossRef]
- Spigelman, M.; Ross, M. Method of Using Topical Probiotics for the Inhibition of Surface Contamination by a Pathogenic Microorganisms and Composition Therefor. U.S. Patent 0107699 A1, 8 May 2008. [Google Scholar]
- Kang, M.S.; Chung, J.; Kim, S.M.; Yang, K.H.; Oh, J.S. Effect of Weissella cibaria isolates on the formation of Streptococcus mutans biofilm. Caries Res. 2006, 40, 418–425. [Google Scholar] [CrossRef]
- Jang, H.J.; Kang, M.S.; Yi, S.H.; Hong, J.Y.; Hong, S.P. Comparative study on the characteristics of Weissella cibaria CMU and probiotic strains for oral care. Molecules 2016, 21, 1752. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.S.; Yeu, J.E.; Hong, S.P.; Kang, M.S. Characterization of antibacterial cell-free supernatant from oral care probiotic Weissella cibaria, CMU. Molecules 2018, 23, 1984. [Google Scholar] [CrossRef] [PubMed]
- Subsanguan, T.; Khondee, N.; Nawavimarn, P.; Rongsayamanont, W.; Chen, C.Y.; Luepromchai, E. Reuse of immobilized Weissella cibaria PN3 for long-term production of both extracellular and cell-bound glycolipid biosurfactants. Front. Bioeng. Biotechnol. 2020, 8, 751. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.A. Equine Pastern Dermatitis. Vet. Clin. North. Am. Equine Pract. 2013, 29, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Akucewich, L.; Yu, A.A. Equine pastern dermatitis. Compend. Equine Ed. 2007, 2, 214–228. [Google Scholar]
- Kaiser-Thom, S.; Hilty, M.; Axiak, S.; Gerber, V. The skin microbiota in equine pastern dermatitis: A case-control study of horses in Switzerland. Vet Dermatol. 2021, 32, 646–e172. [Google Scholar] [CrossRef]
- Lory, S. The family Staphylococcaceae. In The Prokaryotes; Rosenberg, E., DeLong, E.F., Stackebrandt, E., Thompson, F., Eds.; Springer: Berlin, Germany, 2014; pp. 363–366. [Google Scholar]
- Wolcott, R.D.; Hanson, J.D.; Rees, E.J.; Koenig, L.D.; Phillips, C.D.; Wolcott, R.A.; Cox, S.B.; White, J.S. Analysis of the chronic wound microbiota of 2963 patients by 16S rDNA pyrosequencing. Wound Repair Regen. 2016, 24, 163–174. [Google Scholar] [CrossRef]
- Dowd, S.E.; Sun, Y.; Secor, P.R.; Rhoads, D.D.; Wolcott, B.M.; James, G.A.; Wolcott, R.D. Survey of bacterial diversity in chronic wounds using Pyrosequencing, DGGE, and full ribosome shotgun sequencing. BMC Microbiol. 2008, 8, 43. [Google Scholar] [CrossRef]
- Chiers, K.; Decostere, A.; Devriese, L.A.; Haesebrouck, F. Bacteriological and mycological findings, and in vitro antibiotic sensitivity of pathogenic staphylococci in equine skin infections. Vet. Rec. 2003, 152, 138–141. [Google Scholar] [CrossRef]
- Vincze, S.; Stamm, I.; Kopp, P.A.; Hermes, J.; Adlhoch, C.; Semmler, T.; Wieler, L.H.; Lübke-Becker, A.; Walther, B. Alarming proportions of methicillin-resistant Staphylococcus aureus (MRSA) in wound samples from companion animals, Germany 2010–2012. PLoS ONE 2014, 9, e85656. [Google Scholar] [CrossRef]
- Frank, J.A.; Reich, C.I.; Sharma, S.; Weisbaum, J.S.; Wilson, B.A.; Olsen, G.J. Critical evaluation of two primers commonly used for amplification of bacterial 16S rRNA genes. Appl. Environ. Microbiol. 2008, 74, 2461–2470. [Google Scholar] [CrossRef] [PubMed]
- ISO 10932:2010 (IDF 223:2010); Milk and Milk Products-Determination of Minimal Inhibitory Concentration (MIC) of Antibiotic Applicable to Bifidobacteria and Non-Enterococcal Lactic Acid Bacteria (LAB). International Organization for Standardization: Geneva, Switzerland, 2010.
- EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP). Guidance on the assessment of bacterial susceptibility to antimicrobials of human and veterinary importance. EFSA J. 2012, 10, 2740. [Google Scholar]
- EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP). Guidance on the characterization of microorganisms used as feed additives or as production organisms. EFSA J. 2018, 16, 5206. [Google Scholar]
- Lin, X.; Chen, X.; Chen, Y.; Jiang, W.; Chen, H. The effect of five probiotic lactobacilli strains on the growth and biofilm formation of Streptococcus mutans. Oral Dis. 2015, 21, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Styková, E.; Nemcová, R.; Gancarčíková, S.; Valocký, I.; Lauková, A. Bovine vaginal lactobacilli and their adherence to mucus in different phases of the estrous cycle. Afr. J. Microbiol. Res. 2014, 8, 3017–3024. [Google Scholar] [CrossRef]
- Englerová, K.; Bedlovičová, Z.; Nemcová, R.; Király, J.; Maďar, M.; Hajdučková, V.; Styková, E.; Mucha, R.; Reiffová, K. Bacillus amyloliquefaciens-derived lipopeptide biosurfactants inhibit biofilm formation and expression of biofilm-related genes of Staphylococcus aureus. Antibiotics 2021, 10, 1252. [Google Scholar] [CrossRef]
- O’ Toole, G.A.; Pratt, L.A.; Watnick, P.I.; Newman, D.K.; Weaver, V.B.; Kolter, R. Genetic approaches to study of biofilms. Methods Enzymol. 1999, 310, 91–109. [Google Scholar] [CrossRef]
- Kaur, S.; Sharma, P.; Kalia, N.; Singh, J.; Kaur, S. Anti-biofilm properties of the fecal probiotic lactobacilli against Vibrio spp. Front. Cell. Infect. Microbiol. 2018, 8, 120. [Google Scholar] [CrossRef]
- Wasfi, R.; Abd El-Rahman, O.A.; Zafer, M.M.; Ashour, H.M. Probiotic Lactobacillus sp. inhibit growth, biofilm formation and gene expression of caries-inducing Streptococcus mutans. J. Cell. Mol. Med. 2018, 22, 1972–1983. [Google Scholar] [CrossRef]
- Morikawa, M.; Hirata, Y.; Imanaka, T. A study on the structure-function relationship of lipopeptide biosurfactants. Biochim. Biophys. Acta. 2000, 1488, 211–218. [Google Scholar] [CrossRef]
- Ghasemi, A.; Moosavi-Nasab, M.; Setoodeh, P.; Mesbahi, G.; Yousefi, G. Biosurfactant production by lactic acid bacterium Pediococcus dextrinicus SHU1593 grown on different carbon sources: Strain screening followed by product characterization. Sci. Rep. 2019, 9, 5287. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.; Kong, H.; Buyer, J.S.; Lakshman, D.K.; Lydon, J.; Kim, S.D.; Roberts, D.P. Isolation and partial characterization of Bacillus subtilis ME488 for suppression of soilborne pathogens of cucumber and pepper. Appl. Microbiol. Biotechnol. 2008, 80, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Amruta, N.; Prasanna Kumar, M.K.; Puneeth, M.E.; Sarika, G.; Kandikattu, H.K.; Vishwanath, K.; Narayanaswamy, S. Exploring the potentiality of novel rhizospheric bacterial strains against the rice blast fungus Magnaporthe oryzae. Plant Pathol. J. 2018, 34, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Citron, D.M.; Appleman, M.D. In vitro activities of daptomycin, ciprofloxacin, and other antimicrobial agents against the cells and spores of clinical isolates of Bacillus species. J. Clin. Microbiol. 2006, 44, 3814–3818. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cecchini, M.; Langer, J.; Slawomirski, L. Antimicrobial Resistance in G7 Countries and Beyond: Economic Issues, Policies and Options for Action; OECD Report; OECD: Paris, France, 2015. [Google Scholar]
- Nakamura, Y.; Daya, M. Use of appropriate antimicrobials in wound management. Emerg. Med. Clin. N. Am. 2007, 25, 159–176. [Google Scholar] [CrossRef] [PubMed]
- Mihai, M.M.; Preda, M.; Lungu, I.; Gestal, M.C.; Popa, M.I.; Holban, A.M. Nanocoatings for chronic wound repairmodulation of microbial colonization and biofilm formation. Int. J. Mol. Sci. 2018, 19, 1179. [Google Scholar] [CrossRef]
- Walker, W.A. Mechanisms of action of probiotics. Clin. Infect. Dis. 2008, 46, 87–91. [Google Scholar] [CrossRef]
- Lister, J.L.; Horswill, A.R. Staphylococcus aureus biofilms: Recent developments in biofilm dispersal. Front. Cell Infect. Microbiol. 2014, 4, 178. [Google Scholar] [CrossRef]
- Idrees, M.; Sawant, S.; Karodia, N.; Rahman, A. Staphylococcus aureus biofilm: Morphology, genetics, pathogenesis and treatment strategies. Int. J. Environ. Res. Public Health 2021, 18, 7602. [Google Scholar] [CrossRef]
- Zhang, Z.; Peng, X.; Zhang, N.; Liu, L.; Wang, Y.; Ou, S. Cytotoxicity comparison of quercetin and its metabolites from in vitro fermentation of several gut bacteria. Food Funct. 2014, 5, 2152–2156. [Google Scholar] [CrossRef]
- Cai, Y.; Benno, Y.; Nakase, T.; Oh, T.K. Specific probiotic characterization of Weissella hellenica DS-12 isolated from flounder intestine. J. Gen. Appl. Microbiol. 1998, 44, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.Y.; Kim, K.P.; Bae, C.H.; Choi, A.J.; Paik, H.D.; Kim, I.H. Evaluation of Weissella cibaria JW15 probiotic derived from fermented korean vegetable product supplementation in diet on performance characteristics in adult beagle dog. Animals 2019, 9, 581. [Google Scholar] [CrossRef] [PubMed]
- Elshaghabee, F.M.F.; Rokana, N.; Gulhane, R.D.; Sharma, C.; Panwar, H. Bacillus as potential probiotics: Status, concerns, and future perspectives. Front. Microbiol. 2017, 8, 1490. [Google Scholar] [CrossRef] [PubMed]
- Haldar, L.; Gandhi, D.N. Effect of oral administration of Bacillus coagulans B37 and Bacillus pumilus B9 strains on fecal coliforms, Lactobacillus and Bacillus spp. in rat animal model. Vet. World. 2016, 9, 766–772. [Google Scholar] [CrossRef] [PubMed]
- Harakeh, S.M.; Khan, I.; Kumosani, T.; Barbour, E.; Almasaudi, S.B.; Bahijri, S.M.; Alfadul, S.M.; Ajabnoor, G.M.A.; Azhar, E.I. Gut microbiota: A contributing factor to obesity. Front. Cell. Infect. Microbiol. 2016, 6, 95. [Google Scholar] [CrossRef]
- Jensen, G.S.; Cash, H.A.; Farmer, S.; Keller, D. Inactivated probiotic Bacillus coagulans GBI-30 induces complex immune activating, anti-inflammatory, and regenerative markers in vitro. J. Inflamm. Res. 2017, 10, 107–117. [Google Scholar] [CrossRef]
- Flórez, A.B.; Campedelli, I.; Delgado, S.; Alegría, Á.; Salvetti, E.; Felis, G.E.; Mayo, B.; Torriani, S. Antibiotic susceptibility profiles of dairy Leuconostoc, analysis of the genetic basis of atypical resistances and transfer of genes in vitro and in a food matrix. PLoS ONE 2016, 11, e0145203. [Google Scholar] [CrossRef]
- Abriouel, H.; Lerma, L.L.; Casado Muñoz, M.d.C.; Montoro, B.P.; Kabisch, J.; Pichner, R.; Cho, G.S.; Neve, H.; Fusco, V.; Franz, C.M.; et al. The controversial nature of the Weissella genus: Technological and functional aspects versus whole genome analysis-based pathogenic potential for their application in food and health. Front. Microbiol. 2015, 6, 1197. [Google Scholar] [CrossRef]
- Ogier, J.C.; Casalta, E.; Farrokh, C.; Saïhi, A. Safety assessment of dairy microorganisms: The Leuconostoc genus. Int. J. Food Microbiol. 2008, 126, 286–290. [Google Scholar] [CrossRef]
- Hemme, D.; Foucaud-Scheunemann, C. Leuconostoc, characteristics, use in dairy technology and prospects in functional foods. Int. Dairy J. 2004, 14, 467–494. [Google Scholar] [CrossRef]
- Endres, J.R.; Clewell, A.; Jade, K.A.; Farber, T.; Hauswirth, J.; Schauss, A.G. Safety assessment of a proprietary preparation of a novel probiotic, Bacillus coagulans, as a food ingredient. Food Chem. Toxicol. 2009, 47, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Lawley, R.; Curtis, L.; Davis, J. Use of extracellular extracts of lactic acid bacteria and bifidobacteria for the inhibition of dermatological pathogen Staphylococcus aureus. In The Food Safety Hazard Guidebook; Hor, Y.Y., Liong, M.T., Eds.; RSC Publishing: Cambridge, UK, 2008; pp. 70–74. [Google Scholar]
- Aween, M.M.; Hassan, Z.; Muhialdin, B.J.; Noor, H.M.; Eljamel, Y.A. Evaluation on antibacterial activity of Lactobacillus acidophilus strains isolated from honey. Am. J. Appl. Sci. 2012, 9, 807–817. [Google Scholar] [CrossRef]
- Nagoba, B.; Wadher, B.; Kulkarni, P.; Kolhe, S. Acetic acid treatment of pseudomonas wound infections. Eur. J. Gen. Med. 2008, 5, 104–106. [Google Scholar] [CrossRef]
- Hirshfield, I.N.; Terzulli, S.; O’Byrne, C. Weak organic acids: A panoply of effects on bacteria. Sci. Prog. 2003, 86, 245–269. [Google Scholar] [CrossRef]
- Brul, S.; Coote, P. Preservative agents in foods. Mode of action and microbial resistance mechanisms. Int. J. Food Microbiol. 1999, 50, 1–17. [Google Scholar] [CrossRef]
- Bjarnsholt, T.; Alhede, M.; Jensen, P.Ø.; Nielsen, A.K.; Johansen, H.K.; Homøe, P.; Høiby, N.; Givskov, M.; Kirketerp-Møller, K. Antibiofilm properties of acetic acid. Adv. Wound Care 2015, 4, 363–372. [Google Scholar] [CrossRef]
- Halstead, F.D.; Rauf, M.; Moiemen, N.S.; Bamford, A.; Wearn, C.M.; Fraise, A.P.; Lund, P.A. The antibacterial activity of acetic acid against biofilm-producing pathogens of relevance to burns patients. PLoS ONE 2015, 10, e0136190. [Google Scholar] [CrossRef]
- Juturu, V.; Wu, J.C. Microbial production of lactic acid: The latest development. Crit. Rev. Biotechol. 2016, 36, 967–977. [Google Scholar] [CrossRef]
- Patel, A.; Falck, P.; Shah, N.; Immerzeel, P.; Adlercreutz, P.; Stålbrand, H.; Prajapati, J.B.; Holst, O.; Nordberg Karlsson, E. Evidence for xylooligosaccharide utilization in Weissella strains isolated from Indian fermented foods and vegetables. FEMS Microbiol. Lett. 2013, 346, 20–28. [Google Scholar] [CrossRef]
- Pelyuntha, W.; Chaiyasut, C.; Kantachote, D.; Sirilun, S. Organic acids and 2,4-Di-tert-butylphenol: Major compounds of Weissella confusa WM36 cell-free supernatant against growth, survival and virulence of Salmonella Typhi. PeerJ. 2020, 8, e8410. [Google Scholar] [CrossRef]
- Majeed, M.; Nagabhushanam, K.; Natarajan, S.; Sivakumar, A.; Eshuis-de Ruiter, T.; Booij-Veurink, J.; de Vries, Y.P.; Ali, F. Evaluation of genetic and phenotypic consistency of Bacillus coagulans MTCC 5856: A commercial probiotic strain. World J. Microbiol. Biotechnol. 2016, 32, 60. [Google Scholar] [CrossRef] [PubMed]
- Sui, L.; Zhu, X.; Wu, D.; Ma, T.; Tuo, Y.; Jiang, S.; Qian, F.; Mu, G. In vitro assessment of probiotic and functional properties of Bacillus coagulans T242. Food Biosci. 2020, 36, 100675. [Google Scholar] [CrossRef]
- Konuray, G.; Erginkaya, Z. Identification and characterization of Bacillus coagulans strains for probiotic activity and safety. LWT 2021, 151, 112233. [Google Scholar] [CrossRef]
- Masuda, Y.; Zendo, T.; Sawa, N.; Honrada Perez, R.; Nakayama, J.; Sonomoto, K. Characterization and identification of weissellicin Y and weissellicin M, novel bacteriocins produced by Weissella hellenica QU 13. J. Appl. Microbiol. 2012, 112, 99–108. [Google Scholar] [CrossRef]
- Leong, K.-H.; Chen, Y.-S.; Lin, Y.-H.; Pan, S.-F.; Yu, B.; Wu, H.-C.; Yanagida, F. Weissellicin L, a novel bacteriocin from sian-sianzih-isolated Weissella hellenica 4-7. J. Appl. Microbiol. 2013, 115, 70–76. [Google Scholar] [CrossRef]
- Srionnual, S.; Yanagida, F.; Li-Hsiu, L.; Hsiao, K.N.; Yi-sheng, C. Weissellicin 110, a newly discovered bacteriocin from Weissella cibaria 110, isolated from plaa-som, a fermented fish product from Thailand. Appl. Environ. Microb. 2007, 73, 2247–2250. [Google Scholar] [CrossRef]
- Chen, C.; Chen, X.; Jiang, M.; Rui, X.; Li, W.; Dong, M. A newly discovered bacteriocin from Weissella hellenica D1501 associated with Chinese Dong fermented meat (Nanx Wudl.). Food Control. 2014, 42, 116–124. [Google Scholar] [CrossRef]
- Endo, A.; Futagawa-Endo, Y.; Kawasaki, S.; Dicks, L.M.; Niimura, Y.; Okada, S. Sodium acetate enhances hydrogen peroxide production in Weissella cibaria. Lett. Appl. Microbiol. 2009, 49, 136–141. [Google Scholar] [CrossRef]
- Lee, Y. Characterization of Weissella kimchii PL9023 as a potential probiotic for women. FEMS Microbiol. Lett. 2005, 250, 157–162. [Google Scholar] [CrossRef]
- Hyronimus, B.; Le Marrec, C.; Urdaci, M.C. Coagulin, a bacteriocin-like inhibitory substance produced by Bacillus coagulans I4. J. Appl. Microbiol. 1998, 85, 42–50. [Google Scholar] [CrossRef]
- Fu, L.; Wang, C.; Ruan, X.; Li, G.; Zhao, Y.; Wang, Y. Preservation of large yellow croaker (Pseudosciaena crocea) by Coagulin L1208, a novel bacteriocin produced by Bacillus coagulans L1208. Int. J. Food Microbiol. 2018, 266, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Tsang, S.T.J.; Gwynne, P.J.; Gallagher, M.P.; Simpson, A.H.R.W. The biofilm eradication activity of acetic acid in the management of periprosthetic joint infection. Bone Joint Res. 2018, 7, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Lund, P.; Tramonti, A.; De Biase, D. Coping with low pH: Molecular strategies in neutralophilic bacteria. FEMS Microbiol. Rev. 2014, 38, 1091–1125. [Google Scholar] [CrossRef] [PubMed]
- Peetermans, A.; Foulquié–Moreno, M.R.; Thevelein, J.M. Mechanisms underlying lactic acid tolerance and its influence on lactic acid production in Saccharomyces cerevisiae. Microbial Cell 2021, 8, 111–130. [Google Scholar] [CrossRef] [PubMed]
- Piard, J.C.; Desmazeaud, M. Inhibiting factors produced by lactic acid bacteria. Lait 1991, 71, 525–541. [Google Scholar] [CrossRef]
- Uppuluri, P.; Chaturvedi, A.K.; Srinivasan, A.; Banerjee, M.; Ramasubramaniam, A.K.; Köhler, J.R.; Kadosh, D.; Lopez-Ribot, J.L. Dispersion as an important step in the Candida albicans biofilm developmental cycle. PLoS Pathog. 2010, 6, e1000828. [Google Scholar] [CrossRef]
- Davies, D.G.; Marques, C.N. A fatty acid messenger is responsible for inducing dispersion in microbial biofilms. J. Bacteriol. 2009, 191, 1393–1403. [Google Scholar] [CrossRef]
- Rahmani-Badi, A.; Sepehr, S.; Babaie-Naiej, H. A combination of cis-2-decenoic acid and chlorhexidine removes dental plaque. Arch. Oral Biol. 2015, 60, 1655–1661. [Google Scholar] [CrossRef]
- Banat, I.M.; De Rienzo, M.A.; Quinn, G.A. Microbial biofilms: Biosurfactants as antibiofilm agents. Appl. Microbiol. Biotechnol. 2014, 98, 9915–9929. [Google Scholar] [CrossRef]
- Mouafo, H.T.; Sokamte, A.T.; Mbawala, A.; Ndjouenkeu, R.; Devappa, S. Biosurfactants from lactic acid bacteria: A critical review on production, extraction, structural characterization and food application. Food Biosci. 2022, 46, 101598. [Google Scholar] [CrossRef]
- Huszcza, E.; Burczyk, B. Biosurfactant production by Bacillus coagulans. J. Surfact. Deterg. 2003, 6, 61–64. [Google Scholar] [CrossRef]
- Cortés-Camargo, S.; Pérez-Rodríguez, N.; Pinheiro de Souza Oliveira, R.; Barragán Huerta, B.E.; Domínguez, J.M. Production of biosurfactants from vine-trimming shoots using the halotolerant strain Bacillus tequilensis ZSB10. Ind. Crops Prod. 2016, 79, 258–266. [Google Scholar] [CrossRef]
- Plaza, G.; Chojniak, J.; Rudnicka, K.; Paraszkiewicz, K.; Bernat, P. Detection of biosurfactants in Bacillus species: Genes and products identification. J. Appl. Microbiol. 2015, 119, 1023–1034. [Google Scholar] [CrossRef] [PubMed]
- Gerayeli, N.; Baghaee-Ravari, S.; Tarighi, S. Evaluation of the antagonistic potential of Bacillus strains against Pectobacterium carotovorum subsp. carotovorum and their role in the induction of resistance to potato soft rot infection. Eur. J. Plant Pathol. 2017, 150, 1049–1063. [Google Scholar]
- Mora, I.; Cabrefiga, J.; Montesinos, E. Cyclic lipopeptide biosynthetic genes and products, and inhibitory activity of plant-associated Bacillus against phytopathogenic bacteria. PLoS ONE 2015, 10, e0127738. [Google Scholar] [CrossRef]
- Xu, Z.; Shao, J.; Li, B.; Yan, X.; Shen, Q.; Zhang, R. Contribution of bacillomycin D in Bacillus amyloliquefaciens SQR9 to antifungal activity and biofilm formation. Appl. Environ. Microbiol. 2013, 79, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Rivardo, F.; Turner, R.J.; Allegrone, G.; Ceri, H.; Martinotti, M.G. Anti-adhesion activity of two biosurfactants produced by Bacillus spp. prevents biofilm formation of human bacterial pathogens. Appl. Microbiol. Biotechnol. 2009, 83, 541–553. [Google Scholar] [CrossRef]




| Gene | Sequence | PCR Product [bp] | Annealing Temperature [°C] |
|---|---|---|---|
| surfactin sfp | F-5′-ATGAAGATTTACGGAATTTA-3′ R-5′-TTATAAAAGCTCTTCGTACG-3′ | 675 | 50 |
| srfAA | F-5′-TCGGGACAGGAAGACATCAT-3′ R-5′-CCACTCAAACGGATAATCCTGA-3′ | 201 | 60 |
| fengycin fenB | F-5′-CCTGGAGAAAGAATATACCGTACCY-3′ R-5′-GCTGGTTCAGTTKGATCACAT-3′ | 670 | 57 |
| fenD | F-5′-GCTGGTTCAGTTKGATCACAT-3′ R-5′-GTCATGCTGACGAGAGCAAA-3′ | 269 | 61 |
| iturin ituD | F-5′-TTGAAYGTCAGYGCSCCTTT-3′ R-5′-TGCGMAAATAATGGSGTCGT-3′ | 482 | 57 |
| ituC | F-5′-GGCTGCTGCAGATGCTTTAT-3′ R-5′-TCGCAGATAATCGCAGTGAG-3′ | 423 | 60 |
| ATB | W. hellenica 1/7 D23 | W. hellenica 4/2 D23 | W. hellenica 7/1 D23 | W. cibaria 4/8 D37 | B. coagulans 3T27 | B. coagulans 9FT27 |
|---|---|---|---|---|---|---|
| Gm | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Km | 2 | 4 | 2 | 8 | 2 | 2 |
| Sm | 4 | 16 | 16 | 16 | 0.5 | 0.5 |
| Nm | 0.5 | 2 | 1 | 2 | 0.5 | 0.5 |
| Tc | 4 | 4 | 4 | 4 | 0.5 | 0.5 |
| Em | 0.12 | 0.12 | 0.25 | 0.5 | 0.5 | 0.12 |
| Cl | 0.03 | 0.5 | 0.03 | 0.25 | 0.12 | 0.12 |
| Cm | 2 | 4 | 2 | 2 | 4 | 8 |
| Am | 0.06 | 2 | 2 | 0.25 | 0.12 | 16 |
| Pc | 0.12 | 1 | 1 | 1 | 0.06 | 16 |
| Va | 128 | 128 | 128 | 16 | 0.25 | 0.5 |
| Vi | 0.5 | 1 | 0.5 | 1 | 1 | 0.5 |
| Lz | 1 | 1 | 1 | 2 | 1 | 2 |
| Tm | 2 | 8 | 8 | 8 | 0.25 | 1 |
| Ci | 8 | 4 | 8 | 16 | 0.25 | 0.5 |
| Ri | 2 | 2 | 4 | 4 | 0.12 | 1 |
| Strain | Acid | ||||
|---|---|---|---|---|---|
| Formic | Lactic | Acetic | Succinic | Acetoacetic | |
| W. hellenica 1/7D23 | 1.68 ± 0.38 | 14.93 ± 0.80 | 25.13 ± 1.66 | 7.41 ± 0.99 | 8.82 ± 0.33 |
| W. hellenica 4/2D23 | 5.70 ± 0.52 | 88.69 ± 1.28 **,* | 101.95 ± 3.19 *** | 8.25 ± 0.23 | 47.75 ± 2.73 *** |
| W. hellenica 7/1D23 | 2.41 ± 0.37 | 20.63 ± 0.71 | 25.67 ± 1.66 | 6.84 ± 0.57 | 8.28 ± 0.33 |
| W. cibaria 4/8D37 | 2.08 ± 1.88 | 119.77 ± 2.47 ***,** | 96.61 ± 3.59 *** | 8.69 ± 0.52 | 35.07 ± 1.66 *** |
| B. coagulans 3T27 | 6.61 ± 3.84 | 52.75 ± 15.75 * | 21.07 ± 1.99 | 8.05 ± 0.19 | 9.36 ± 0.04 |
| B. coagulans 9FT27 | 6.78 ± 1.66 | 61.15 ± 4.74 **,* | 84.02 ± 4.11 *** | 7.55 ± 0.18 | 37.82 ± 2.01 *** |
| Strains | Extracellular BS | Cell-Bound BS |
|---|---|---|
| W. hellenica 1/7D23 | 20 ± 0.2 | negative |
| W. hellenica 4/2D23 | 8 ± 0.1 | negative |
| W. hellenica 7/1D23 | 8 ± 0.1 | negative |
| W. cibaria 4/8D37 | 16 ± 0.2 | negative |
| B. coagulans 3T27 | negative | 15 ± 0.2 |
| B. coagulans 9FT27 | 8 ± 0.1 | 12 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Styková, E.; Nemcová, R.; Maďar, M.; Bujňáková, D.; Mucha, R.; Gancarčíková, S.; Requena Domenech, F. Antibiofilm Activity of Weissella spp. and Bacillus coagulans Isolated from Equine Skin against Staphylococcus aureus. Life 2022, 12, 2135. https://doi.org/10.3390/life12122135
Styková E, Nemcová R, Maďar M, Bujňáková D, Mucha R, Gancarčíková S, Requena Domenech F. Antibiofilm Activity of Weissella spp. and Bacillus coagulans Isolated from Equine Skin against Staphylococcus aureus. Life. 2022; 12(12):2135. https://doi.org/10.3390/life12122135
Chicago/Turabian StyleStyková, Eva, Radomíra Nemcová, Marián Maďar, Dobroslava Bujňáková, Rastislav Mucha, Soňa Gancarčíková, and Francisco Requena Domenech. 2022. "Antibiofilm Activity of Weissella spp. and Bacillus coagulans Isolated from Equine Skin against Staphylococcus aureus" Life 12, no. 12: 2135. https://doi.org/10.3390/life12122135
APA StyleStyková, E., Nemcová, R., Maďar, M., Bujňáková, D., Mucha, R., Gancarčíková, S., & Requena Domenech, F. (2022). Antibiofilm Activity of Weissella spp. and Bacillus coagulans Isolated from Equine Skin against Staphylococcus aureus. Life, 12(12), 2135. https://doi.org/10.3390/life12122135






