A Pilot Study on the Use of Low Doses of CBD to Control Seizures in Rare and Severe Forms of Drug-Resistant Epilepsy
Abstract
1. Introduction
2. Materials and Methods
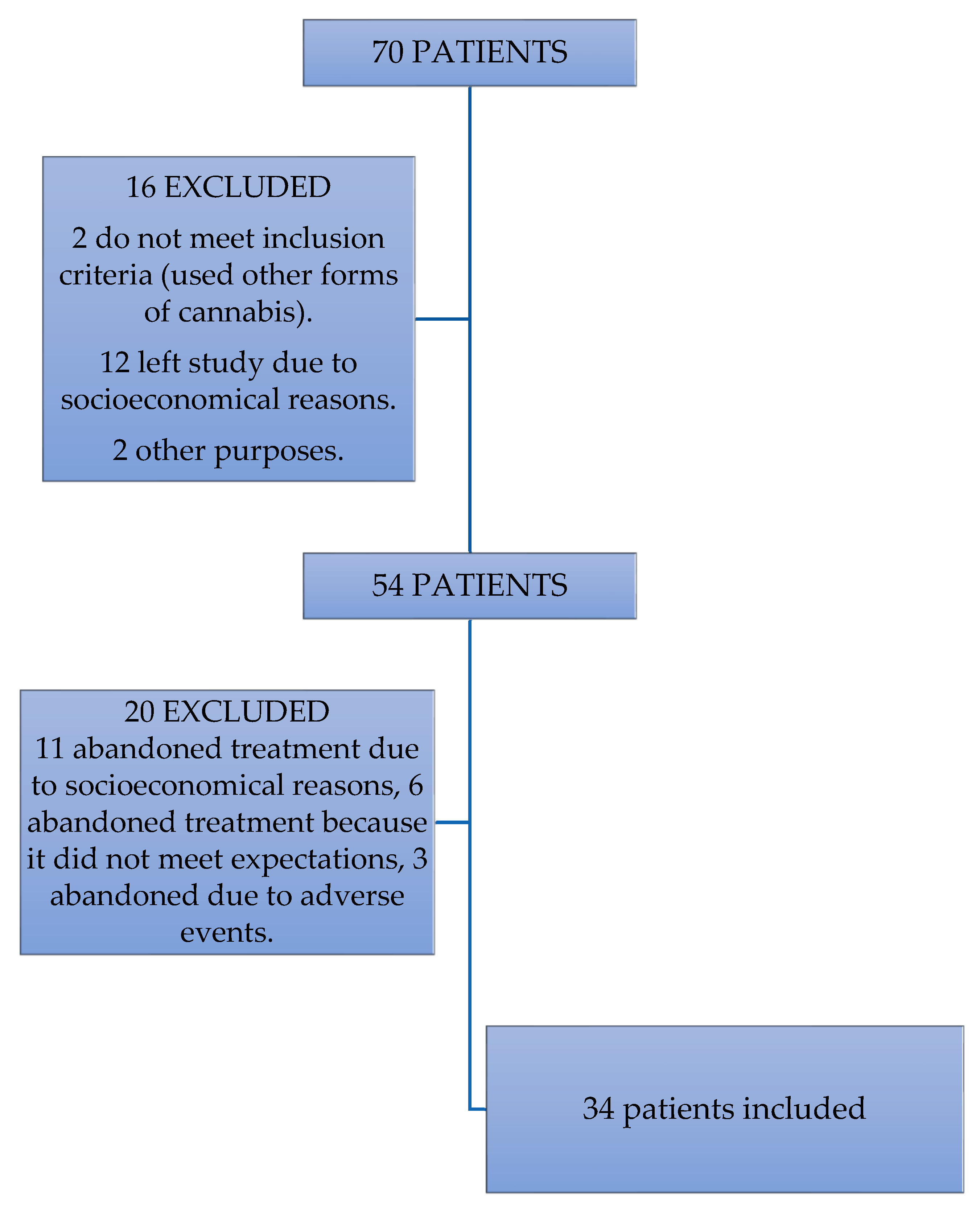
2.1. Study Design
2.2. Population
2.3. Bias
2.4. Data Sources
2.5. Medical Records
2.6. Use of Cannabidiol
2.7. Statistical Analysis
2.8. Ethical Concerns
3. Results
3.1. Frequency of Seizures
3.2. Duration of Seizures
3.3. Type of Seizures
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cilio, M.R.; Thiele, E.A.; Devinsky, O. The case for assessing cannabidiol in epilepsy. Epilepsia 2014, 55, 787–790. [Google Scholar] [CrossRef]
- Koo, C.M.; Kang, H.C. Could Cannabidiol be a Treatment Option for Intractable Childhood and Adolescent Epilepsy? J. Epilepsy Res. 2017, 7, 16–20. [Google Scholar] [CrossRef][Green Version]
- Pesantez, G.; Jimbo, R.; Sánchez, X.; Valencia, C.; Curatolo, P.; Pesantez, G. Esclerosis tuberosa en Ecuador. Reporte de serie de casos. Neurol. Argent 2018, 10, 66–71. [Google Scholar] [CrossRef]
- Wilmshurst, J.M.; Gaillard, W.D.; Vinayan, K.P.; Tsuchida, T.N.; Plouin, P.; Van Bogaert, P.; Carrizosa, J.; Elia, M.; Craiu, D.; Jovic, N.J.; et al. Summary of recommendations for the management of infantile seizures: Task Force Report for the ILAE Commission of Pediatrics. Epilepsia 2015, 56, 1185–1197. [Google Scholar] [CrossRef]
- Welty, T.E.; Luebke, A.; Gidal, B.E. Cannabidiol: Promise and pitfalls. Epilepsy Curr. 2014, 14, 250–252. [Google Scholar] [CrossRef]
- Mori, M.A.; Meyer, E.; Soares, L.M.; Milani, H.; Guimarães, F.S.; de Oliveira, R.M.W. Cannabidiol reduces neuroinflammation and promotes neuroplasticity and functional recovery after brain ischemia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2017, 75, 94–105. [Google Scholar] [CrossRef]
- Rocha, L.; Frías-Soria, C.L.; Ortiz, J.G.; Auzmendi, J.; Lazarowski, A. Is cannabidiol a drug acting on unconventional targets to control drug-resistant epilepsy? Epilepsia Open 2020, 5, 36–49. [Google Scholar] [CrossRef]
- Longo, D.; Friedman, D.; Devinsky, O. Cannabinoids in the Treatment of Epilepsy. N. Engl. J. Med. 2015, 373, 1048–1058. [Google Scholar]
- Porter, B.E.; Jacobson, C. Report of a parent survey of cannabidiol-enriched cannabis use in pediatric treatment-resistant epilepsy. Epilepsy Behav. EB 2013, 29, 574–577. [Google Scholar] [CrossRef]
- Jones, N.A.; Glyn, S.E.; Akiyama, S.; Hill, T.D.M.; Hill, A.J.; Weston, S.E.; Burnett, M.D.; Yamasaki, Y.; Stephens, G.J.; Whalley, B.J.; et al. Cannabidiol exerts anti-convulsant effects in animal models of temporal lobe and partial seizures. Seizure-Eur. J. Epilepsy 2012, 21, 344–352. [Google Scholar] [CrossRef]
- Silvestro, S.; Mammana, S.; Cavalli, E.; Bramanti, P.; Mazzon, E. Use of Cannabidiol in the Treatment of Epilepsy: Efficacy and Security in Clinical Trials. Molecules 2019, 24, 1459. [Google Scholar] [CrossRef]
- French, J.A.; Koepp, M.; Naegelin, Y.; Vigevano, F.; Auvin, S.; Rho, J.M.; Rosenberg, E.; Devinsky, O.; Olofsson, P.S.; Dichter, M.A. Clinical studies and anti-inflammatory mechanisms of treatments. Epilepsia 2017, 58, 69–82. [Google Scholar] [CrossRef]
- Peng, J.; Fan, M.; An, C.; Ni, F.; Huang, W.; Luo, J. A narrative review of molecular mechanism and therapeutic effect of cannabidiol (CBD). Basic Clin. Pharmacol. Toxicol. 2022, 130, 439–456. [Google Scholar] [CrossRef]
- Specchio, N.; Pietrafusa, N.; Cross, H.J. Source of cannabinoids: What is available, what is used, and where does it come from? Epileptic Disord. 2020, 22, 1–9. [Google Scholar]
- Thiele, E.; Marsh, E.; Mazurkiewicz-Beldzinska, M.; Halford, J.J.; Gunning, B.; Devinsky, O.; Checketts, D.; Roberts, C. Cannabidiol in patients with Lennox-Gastaut syndrome: Interim analysis of an open-label extension study. Epilepsia 2019, 60, 419–428. [Google Scholar] [CrossRef]
- Nabbout, R.; Thiele, E.A. The role of cannabinoids in epilepsy treatment: A critical review of efficacy results from clinical trials. Epileptic Disord 2020, 22, S23–S28. [Google Scholar]
- Gray, R.A.; Whalley, B.J. The proposed mechanisms of action of CBD in epilepsy. Epileptic Disord. 2020, 22, 10–15. [Google Scholar]
- Klein, P.; Friedman, A.; Hameed, M.; Kaminski, R.; Henrik, G.; Mathias, K. Repurposed molecules for antiepileptogenesis: Missing an opportunity to prevent epilepsy? Epilepsia 2020, 61, 359–386. [Google Scholar] [CrossRef]
- Szaflarski, J.; Bebin, E.; Comi, A.; Patel, A.; Joshi, C.; Checketts, D. Long-term safety and treatment effects of cannabidiol in children and adults with treatment-resistant epilepsies: Expanded access program results. Epilepsia 2018, 59, 1–9. [Google Scholar] [CrossRef]
- Devinsky, O.; Marsh, E.; Friedman, D.; Thiele, E.; Laux, L.; Sullivan, J.; Miller, I.; Flamini, R.; Wilfong, A.; Filloux, F.; et al. Cannabidiol in patients with treatment-resistant epilepsy: An open-label interventional trial. Lancet Neurol. 2016, 15, 270–278. [Google Scholar] [CrossRef]
- Devinsky, O.; Patel, A.D.; Cross, J.H.; Villanueva, V.; Wirrell, E.C.; Privitera, M.; Greenwood, S.M.; Roberts, C.; Checketts, D.; VanLandingham, K.E.; et al. Effect of Cannabidiol on Drop Seizures in the Lennox–Gastaut Syndrome. N. Engl. J. Med. 2018, 378, 1888–1897. [Google Scholar] [CrossRef]
- Devinsky, O.; Nabbout, R.; Miller, I.; Laux, L.; Zolnowska, M.; Wright, S.; Roberts, C. Long-term cannabidiol treatment in patients with Dravet syndrome: An open-label extension trial. Epilepsia 2019, 60, 294–302. [Google Scholar] [CrossRef]
- Landmark, C.J.; Brandl, U. Pharmacology and drug interactions of cannabinoids. Epileptic Disord. 2020, 22, 16–22. [Google Scholar]
- Morse, R.; Marsh, E.; Filloux, F.; Clark, G.; Dlugos, D.; Nagaraddi, V. Cannabidiol in patients with seizures associated with Lennox-Gastaut syndrome (GWPCARE4): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2018, 391, 181–196. [Google Scholar]
- Sheikh, S.; Thompson, N.; Bingaman, W.; Gonzalez-Martinez, J.; Najm, I.; Jehi, L. (Re)Defining success in epilepsy surgery: The importancse of relative seizure reduction in patient-reported quality of life. Epilepsia 2019, 60, 278–285. [Google Scholar] [CrossRef]
- Campbell, C.T.; Phillips, M.S.; Manasco, K. Cannabinoids in Pediatrics. J. Pediatr. Pharm. Ther. 2017, 22, 176–185. [Google Scholar] [CrossRef]
- Czornyj, L.; Auzmendi, J.; Lazarowski, A. Transporter hypothesis in pharmacoresistant epilepsies. Is it at the central or peripheral level? Epilepsia Open 2022, 7 (Suppl. S1), S34–S46. [Google Scholar]


| General Description | N = 34 |
|---|---|
| Sex | |
| Female | 15 (44.1%) |
| Male | 19 (55.9%) |
| Ethnicity | |
| Hispanic | 33 (97.05%) |
| Indigenous | 1 (2.9%) |
| Age | |
| Median = 5.3 years | |
| Range = 0.1–40 years | |
| Place of birth | |
| Coastal region | 3(8.82%) |
| Mountainous region | 30 (88.23%) |
| Amazon region | 1 (2.9%) |
| Diagnosis of epilepsy | |
| Focal symptomatic epilepsy | 12 (35.2%) |
| Familial myoclonic epilepsy | 1 (2.9%) |
| Continuous partial epilepsy | 1 (2.9%) |
| Possible Dravet syndrome | 1 (2.9%) |
| Doose syndrome | 1 (2.9%) |
| Lennox–Gastaut syndrome | 12 (35.2%) |
| Ohtahara syndrome | 1 (2.9%) |
| West syndrome | 5 (14.7%) |
| Neuroimaging alterations | |
| Yes | 30 (88%) |
| Median = 2 anomalies | |
| Range = 0–5 anomalies | |
| No | 2 (6%) |
| No results | 2 (6%) |
| Number of pharmacological treatments before and after CBD | |
| Average before CBD (range) | 3 (0–5) |
| Average after CBD (range) | 3 (0–4) |
| Householder | |
| Work that requires higher education | 7 (20.6%) |
| Work that does not require higher education | 22 (64.7%) |
| Does not work | 5 (14.7%) |
| Time Consuming CBD (Months) | Frequency of Daily Seizures (N = 34) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | >0–<1 | 1–5 | 6–10 | 11–20 | 21–30 | >30 | ||||||||
| 0 | 0 | (0%) | 1 | (2.94%) | 12 | (35.29%) | 5 | (14.7%) | 7 | (20.6%) | 1 | (2.9%) | 8 | (23.5%) |
| 1–3 | 7 | (20.59%) | 5 | (14.71%) | 15 | (44.1%) | 3 | (8.8%) | 1 | (2.9%) | 1 | (2.9%) | 0 | (0%) |
| 6–9 | 14 | (41.18%) | 5 | (14.71%) | 11 | (32.4%) | 3 | (8.8%) | 1 | (2.9%) | 0 | (0%) | 0 | (0%) |
| 12 | 16 | (47.06%) | 4 | (11.76%) | 7 | (20.6%) | 4 | (11.8%) | 0 | (0%) | 0 | (0%) | 0 | (0%) |
| Duration of Seizures | Time Consuming CBD (Months) | |||||
|---|---|---|---|---|---|---|
| 0 | 6 | 12 | ||||
| No seizures | 0 | (0%) | 12 | (35.29%) | 16 | (47.06%) |
| <1 | 13 | (38.24%) | 14 | (38.24%) | 11 | (35.29%) |
| 1–5 | 18 | (52.94%) | 5 | (14.71%) | 4 | (14.71%) |
| >5 | 3 | (8.82%) | 1 | (2.94%) | 0 | (0%) |
| Combination of Drugs | Number of Patients |
|---|---|
| NONE | 4 |
| LEV/CLB/CBZ | 1 |
| LEV/CLB/VPA/TPM | 1 |
| LEV/CLB/TPM | 1 |
| LEV/CLB/VPA | 1 |
| CLB/VPA | 1 |
| LEV | 4 |
| LEV/CLB | 1 |
| LEV/TPM | 1 |
| LEV/CLB/VPA | 2 |
| LEV/CLB | 1 |
| LEV/CLB/GBP | 1 |
| LEV/CLB/TPM | 1 |
| LEV/TPM | 1 |
| LEV/CLB/TPM/LCM | 1 |
| LEV/CLB/VPA/TPM | 1 |
| LEV/TPM/VPA | 1 |
| LEV/CLB/TPM | 1 |
| LEV/CLB/TPM | 1 |
| VPA/TPM/LTG | 1 |
| VPA | 2 |
| VPA/CBZ/TPM | 1 |
| VPA/CBZ/TPM | 1 |
| LEV/CLB/VPA | 1 |
| LEV/CLB/VPA/TPM | 1 |
| VPA/LTG | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pesántez Ríos, G.; Armijos Acurio, L.; Jimbo Sotomayor, R.; Cueva, V.; Pesántez Ríos, X.; Navarrete Zambrano, H.; Pascual, S.; Pesántez Cuesta, G. A Pilot Study on the Use of Low Doses of CBD to Control Seizures in Rare and Severe Forms of Drug-Resistant Epilepsy. Life 2022, 12, 2065. https://doi.org/10.3390/life12122065
Pesántez Ríos G, Armijos Acurio L, Jimbo Sotomayor R, Cueva V, Pesántez Ríos X, Navarrete Zambrano H, Pascual S, Pesántez Cuesta G. A Pilot Study on the Use of Low Doses of CBD to Control Seizures in Rare and Severe Forms of Drug-Resistant Epilepsy. Life. 2022; 12(12):2065. https://doi.org/10.3390/life12122065
Chicago/Turabian StylePesántez Ríos, Gabriela, Luciana Armijos Acurio, Ruth Jimbo Sotomayor, Victor Cueva, Ximena Pesántez Ríos, Hugo Navarrete Zambrano, Samuel Pascual, and Galo Pesántez Cuesta. 2022. "A Pilot Study on the Use of Low Doses of CBD to Control Seizures in Rare and Severe Forms of Drug-Resistant Epilepsy" Life 12, no. 12: 2065. https://doi.org/10.3390/life12122065
APA StylePesántez Ríos, G., Armijos Acurio, L., Jimbo Sotomayor, R., Cueva, V., Pesántez Ríos, X., Navarrete Zambrano, H., Pascual, S., & Pesántez Cuesta, G. (2022). A Pilot Study on the Use of Low Doses of CBD to Control Seizures in Rare and Severe Forms of Drug-Resistant Epilepsy. Life, 12(12), 2065. https://doi.org/10.3390/life12122065








