The Role of Non-Alcoholic Fatty Liver Disease in Infections
Abstract
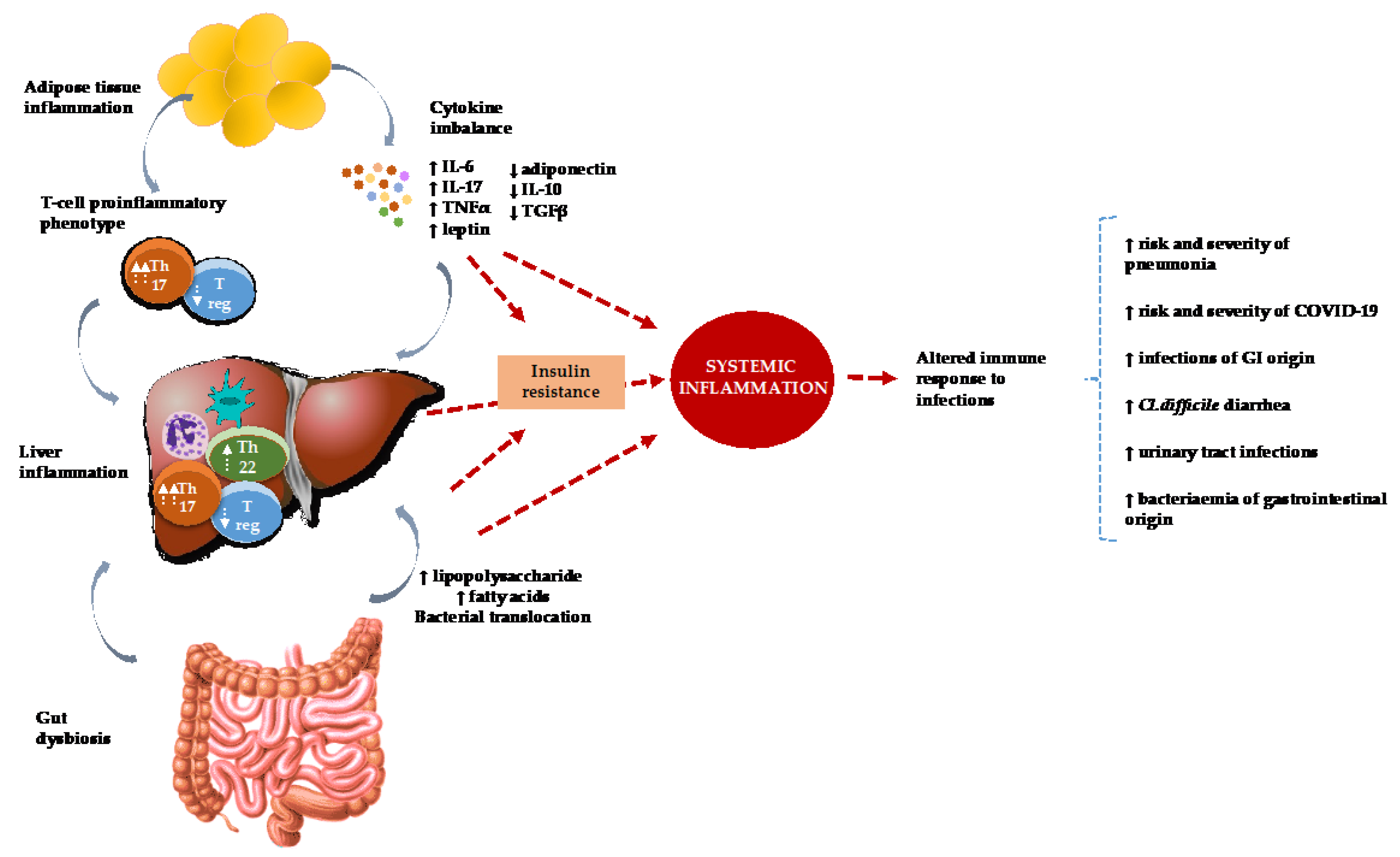
:1. Introduction
1.1. Defining the NAFLD
1.2. The Pathogenesis of NAFLD and Its Relation to Infectious Diseases
2. Association between NAFLD and Infectious Diseases
2.1. NAFLD and Community Acquired Pneumonia
2.2. NAFLD and COVID-19
2.3. NAFLD and H. pylori
2.4. NAFLD and Urinary Tract Infections
2.5. NAFLD and C. difficile
2.6. NAFLD, Bacteremia and Recurring Bacterial Infections
2.7. NAFLD and Hepatitis B, C and HIV
2.8. NAFLD and Periodontitis
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Puri, P.; Sanyal, A.J. Nonalcoholic fatty liver disease: Definitions, risk factors, and workup. Clin. Liver Dis. 2012, 1, 99–103. [Google Scholar] [CrossRef]
- Barbosa, J.V.; Lai, M. Nonalcoholic Fatty Liver Disease Screening in Type 2 Diabetes Mellitus Patients in the Primary Care Setting. Hepatol. Commun. 2020, 5, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Kader, S.M.; El-Den Ashmawy, E.M.S. Non-alcoholic fatty liver disease: The diagnosis and management. World J. Hepatol. 2015, 7, 846–858. [Google Scholar] [CrossRef]
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2017, 15, 11–20. [Google Scholar] [CrossRef] [PubMed]
- DiStefano, J.K. NAFLD and NASH in Postmenopausal Women: Implications for Diagnosis and Treatment. Endocrinology 2020, 161, bqaa134. [Google Scholar] [CrossRef] [PubMed]
- Hamaguchi, M.; Kojima, T.; Ohbora, A.; Takeda, N.; Fukui, M.; Kato, T. Aging is a risk factor of nonalcoholic fatty liver disease in premenopausal women. World J. Gastroenterol. 2012, 18, 237–243. [Google Scholar] [CrossRef]
- Nasiri-Ansari, N.; Androutsakos, T.; Flessa, C.-M.; Kyrou, I.; Siasos, G.; Randeva, H.S.; Kassi, E.; Papavassiliou, A.G. Endothelial Cell Dysfunction and Nonalcoholic Fatty Liver Disease (NAFLD): A Concise Review. Cells 2022, 11, 2511. [Google Scholar] [CrossRef] [PubMed]
- Huby, T.; Gautier, E.L. Immune cell-mediated features of non-alcoholic steatohepatitis. Nat. Rev. Immunol. 2021, 22, 429–443. [Google Scholar] [CrossRef] [PubMed]
- Berger, D.; Desai, V.; Janardhan, S. Con: Liver Biopsy Remains the Gold Standard to Evaluate Fibrosis in Patients with Nonalcoholic Fatty Liver Disease. Clin. Liver Dis. 2019, 13, 114–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yaqub, S.; Ananias, P.; Shah, A.; Luenam, K.; Jose, A.M.; Melo, J.P.; Turkistani, A.; Mohammed, L. Decoding the Pathophysiology of Non-alcoholic Fatty Liver Disease Progressing to Non-alcoholic Steatohepatitis: A Systematic Review. Cureus 2021, 13, e18201. [Google Scholar] [CrossRef]
- Phipps, M.; Wattacheril, J. Non-alcoholic fatty liver disease (NAFLD) in non-obese individuals. Front. Gastroenterol. 2019, 11, 478–483. [Google Scholar] [CrossRef] [PubMed]
- Verwer, B.J.; Scheffer, P.G.; Vermue, R.P.; Pouwels, P.J.; Diamant, M.; Tushuizen, M.E. NAFLD is related to Post-prandial Triglyceride-enrichment of HDL Particles in Association with Endothelial and HDL Dysfunction. Liver Int. 2020, 40, 2439–2444. [Google Scholar] [CrossRef]
- Sears, B.; Perry, M. The role of fatty acids in insulin resistance. Lipids Health Dis. 2015, 14, 121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byrne, C.D.; Targher, G. NAFLD: A multisystem disease. J. Hepatol. 2015, 62, S47–S64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du Plessis, J.; Korf, H.; Van Pelt, J.; Windmolders, P.; Vander Elst, I.; Verrijken, A.; Hubens, G.; Van Gaal, L.; Cassiman, D.; Nevens, F.; et al. Pro-Inflammatory Cytokines but Not Endotoxin-Related Parameters Associate with Disease Severity in Patients with NAFLD. PLoS ONE 2016, 11, e0166048. [Google Scholar] [CrossRef] [Green Version]
- Adenote, A.; Dumic, I.; Madrid, C.; Barusya, C.; Nordstrom, C.W.; Prada, L.R. NAFLD and Infection, a Nuanced Relationship. Can. J. Gastroenterol. Hepatol. 2021, 2021, 5556354. [Google Scholar] [CrossRef] [PubMed]
- Lesmana, C.R.A.; Kencana, Y.; Rinaldi, I.; Kurniawan, J.; Hasan, I.; Sulaiman, A.S.; Gani, R.A. Diagnostic Value of Neutrophil to Lymphocyte Ratio in Non-Alcoholic Fatty Liver Disease Evaluated Using Transient Elastography (TE) with Controlled Attenuated Parameter (CAP). Diabetes Metab. Syndr. Obes. Targets Ther. 2022, 15, 15–22. [Google Scholar] [CrossRef]
- Tesmer, L.A.; Lundy, S.; Sarkar, S.; Fox, D.A. Th17 cells in human disease. Immunol. Rev. 2008, 223, 87–113. [Google Scholar] [CrossRef]
- Duan, Y.; Pan, X.; Luo, J.; Xiao, X.; Li, J.; Bestman, P.L.; Luo, M. Association of Inflammatory Cytokines with Non-Alcoholic Fatty Liver Disease. Front. Immunol. 2022, 13, 880298. [Google Scholar] [CrossRef]
- Han, J.; Zhang, X. Complement Component C3: A Novel Biomarker Participating in the Pathogenesis of Non-alcoholic Fatty Liver Disease. Front. Med. 2021, 8, 1230. [Google Scholar] [CrossRef]
- Guicciardi, M.E.; Malhi, H.; Mott, J.L.; Gores, G.J. Apoptosis and Necrosis in the Liver. Compr. Physiol. 2013, 3, 977–1010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Deng, X.; Liu, Y.; Tan, Q.; Huang, G.; Che, Q.; Guo, J.; Su, Z. Kupffer Cells in Non-alcoholic Fatty Liver Disease: Friend or Foe? Int. J. Biol. Sci. 2020, 16, 2367–2378. [Google Scholar] [CrossRef]
- Kawaguchi, K.; Sakai, Y.; Terashima, T.; Shimode, T.; Seki, A.; Orita, N.; Takeshita, Y.; Shimakami, T.; Takatori, H.; Arai, K.; et al. Decline in serum albumin concentration is a predictor of serious events in nonalcoholic fatty liver disease. Medicine 2021, 100, e26835. [Google Scholar] [CrossRef] [PubMed]
- Babalı, A.; Çakal, E.; Purnak, T.; Bıyıkoğlu, I.; Çakal, B.; Yüksel, O.; Köklü, S. Serum α-fetoprotein levels in liver steatosis. Hepatol. Int. 2009, 3, 551–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valenti, L.; Dongiovanni, P.; Piperno, A.; Fracanzani, A.L.; Maggioni, M.; Rametta, R.; Loria, P.; Casiraghi, M.A.; Suigo, E.; Ceriani, R.; et al. α1-Antitrypsin mutations in NAFLD: High prevalence and association with altered iron metabolism but not with liver damage. Hepatology 2006, 44, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Xue, X.; Zhang, J.; Liang, S.; Xu, C.; Wang, Y.; Zhu, J. Liver Expressed Antimicrobial Peptide 2 is Associated with Steatosis in Mice and Humans. Exp. Clin. Endocrinol. Diabetes 2020, 129, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Kargili, A.; Cipil, H.; Karakurt, F.; Kasapoglu, B.; Koca, C.; Aydn, M.; Uysal, S.; Ikizek, M.; Uz, E.; Balcik, O.S.; et al. Hemostatic alterations in fatty liver disease. Blood Coagul. Fibrinolysis 2010, 21, 325–327. [Google Scholar] [CrossRef]
- Marmur, J.; Beshara, S.; Eggertsen, G.; Onelöv, L.; Albiin, N.; Danielsson, O.; Hultcrantz, R.; Stål, P. Hepcidin levels correlate to liver iron content, but not steatohepatitis, in non-alcoholic fatty liver disease. BMC Gastroenterol. 2018, 18, 78. [Google Scholar] [CrossRef]
- Boutagy, N.E.; McMillan, R.P.; Frisard, M.I.; Hulver, M.W. Metabolic endotoxemia with obesity: Is it real and is it relevant? Biochimie 2015, 124, 11–20. [Google Scholar] [CrossRef] [Green Version]
- Brandl, K.; Schnabl, B. Intestinal microbiota and nonalcoholic steatohepatitis. Curr. Opin. Gastroenterol. 2017, 33, 128–133. [Google Scholar] [CrossRef]
- Arvaniti, V.; D’Amico, G.; Fede, G.; Manousou, P.; Tsochatzis, E.; Pleguezuelo, M.; Burroughs, A.K. Infections in Patients with Cirrhosis Increase Mortality Four-Fold and Should Be Used in Determining Prognosis. Gastroenterology 2010, 139, 1246–1256.e5. [Google Scholar] [CrossRef]
- Yan, J.; Li, S.; Li, S. The Role of the Liver in Sepsis. Int. Rev. Immunol. 2014, 33, 498–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pacifico, L.; Osborn, J.F.; Bonci, E.; Pierimarchi, P.; Chiesa, C. Association between Vitamin D Levels and Nonalcoholic Fatty Liver Disease: Potential Confounding Variables. Mini-Rev. Med. Chem. 2019, 19, 310–332. [Google Scholar] [CrossRef] [PubMed]
- Welte, T.; Köhnlein, T. Global and Local Epidemiology of Community-Acquired Pneumonia: The Experience of the CAPNETZ Network. Semin. Respir. Crit. Care Med. 2009, 30, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Nseir, W.; Artul, S.; Abu Rajab, S.; Mograbi, J.; Nasralla, N.; Mahamid, M. Association between non-alcoholic fatty liver disease and hospitalized patients with community-acquired pneumonia. Isr. Med. Assoc. J. 2017, 19, 198. [Google Scholar] [PubMed]
- Nseir, W.B.; Mograbi, J.M.; Amara, A.E.; Abu Elheja, O.H.; Mahamid, M.N. Non-alcoholic fatty liver disease and 30-day all-cause mortality in adult patients with community-acquired pneumonia. QJM Int. J. Med. 2018, 112, 95–99. [Google Scholar] [CrossRef]
- Falguera, M.; Pifarre, R.; Martin, A.; Sheikh, A.; Moreno, A. Etiology and Outcome of Community-Acquired Pneumonia in Patients with Diabetes Mellitus. Chest 2005, 128, 3233–3239. [Google Scholar] [CrossRef]
- Wang, H.; Lee, C.-C.; Chou, E.H.; Hsu, W.-T.; Robinson, R.D.; Su, K.-Y.; Kirby, J.J.; Hassani, D. Mortality association between obesity and pneumonia using a dual restricted cohort model. Obes. Res. Clin. Pract. 2020, 14, 350–359. [Google Scholar] [CrossRef]
- Fisher-Hoch, S.P.; Mathews, C.E.; McCormick, J.B. Obesity, diabetes and pneumonia: The menacing interface of non-communicable and infectious diseases. Trop. Med. Int. Health 2013, 18, 1510–1519. [Google Scholar] [CrossRef]
- Mancuso, P. Obesity and respiratory infections: Does excess adiposity weigh down host defense? Pulm. Pharmacol. Ther. 2013, 26, 412–419. [Google Scholar] [CrossRef]
- Nie, W.; Zhang, Y.; Jee, S.H.; Jung, K.J.; Li, B.; Xiu, Q. Obesity survival paradox in pneumonia: A meta-analysis. BMC Med. 2014, 12, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bourgonje, A.R.; Abdulle, A.E.; Timens, W.; Hillebrands, J.L.; Navis, G.J.; Gordijn, S.J.; Bolling, M.C.; Dijkstra, G.; Voors, A.A.; Osterhaus, A.D.; et al. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV -2 and the pathophysiology of coronavirus disease 2019 (COVID-19). J. Pathol. 2020, 251, 228–248. [Google Scholar] [CrossRef] [PubMed]
- Goyal, A.; Nimmakayala, K.R.; Zonszein, J. Is There a Paradox in Obesity? Cardiol. Rev. 2014, 22, 163–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fondevila, M.F.; Mercado-Gómez, M.; Rodríguez, A.; Gonzalez-Rellan, M.J.; Iruzubieta, P.; Valentí, V.; Escalada, J.; Schwaninger, M.; Prevot, V.; Dieguez, C.; et al. Obese patients with NASH have increased hepatic expression of SARS-CoV-2 critical entry points. J. Hepatol. 2020, 74, 469–471. [Google Scholar] [CrossRef]
- Anirvan, P.; Singh, S.P.; Giammarino, A.; Satapathy, S.K. Association of non-alcoholic fatty liver disease and COVID-19: A literature review of current evidence. World J. Hepatol. 2021, 13, 916–925. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Stepanova, M.; Lam, B.; Cable, R.; Felix, S.; Jeffers, T.; Younossi, E.; Pham, H.; Srishord, M.; Austin, P.; et al. Independent Predictors of Mortality Among Patients with NAFLD Hospitalized With COVID-19 Infection. Hepatol. Commun. 2021, 6, 3062–3072. [Google Scholar] [CrossRef]
- Forlano, R.; Mullish, B.H.; Mukherjee, S.K.; Nathwani, R.; Harlow, C.; Crook, P.; Judge, R.; Soubieres, A.; Middleton, P.; Daunt, A.; et al. In-hospital mortality is associated with inflammatory response in NAFLD patients admitted for COVID-19. PLoS ONE 2020, 15, e0240400. [Google Scholar] [CrossRef]
- Ji, D.; Qin, E.; Xu, J.; Zhang, D.; Cheng, G.; Wang, Y.; Lau, G. Non-alcoholic fatty liver diseases in patients with COVID-19: A retrospective study. J. Hepatol. 2020, 73, 451–453. [Google Scholar] [CrossRef]
- Bramante, C.; Tignanelli, C.J.; Dutta, N.; Jones, E.; Tamariz, L.; Clark, J.M.; Usher, M.; Metlon-Meaux, G.; Ikramuddin, S. Non-alcoholic fatty liver disease (NAFLD) and risk of hospitalization for Covid-19. medRxiv 2020. [CrossRef]
- Vrsaljko, N.; Samadan, L.; Viskovic, K.; Mehmedović, A.; Budimir, J.; Vince, A.; Papic, N. Association of Nonalcoholic Fatty Liver Disease With COVID-19 Severity and Pulmonary Thrombosis: CovidFAT, a Prospective, Observational Cohort Study. Open Forum Infect. Dis. 2022, 9, ofac073. [Google Scholar] [CrossRef]
- Kariyawasam, J.C.; Jayarajah, U.; Abeysuriya, V.; Riza, R.; Seneviratne, S.L. Involvement of the Liver in COVID-19: A Systematic Review. Am. J. Trop. Med. Hyg. 2022, 106, 1026–1041. [Google Scholar] [CrossRef] [PubMed]
- Norman, B.H. Drug Induced Liver Injury (DILI). Mechanisms and Medicinal Chemistry Avoidance/Mitigation Strategies. J. Med. Chem. 2020, 63, 11397–11419. [Google Scholar] [CrossRef] [PubMed]
- Baeg, M.K.; Yoon, S.K.; Ko, S.-H.; Noh, Y.-S.; Lee, I.-S.; Choi, M.-G. Helicobacter pylori infection is not associated with nonalcoholic fatty liver disease. World J. Gastroenterol. 2016, 22, 2592–2600. [Google Scholar] [CrossRef] [PubMed]
- Abo-Amer, Y.E.-E.; Sabal, A.; Ahmed, R.; Hasan, N.F.E.; Refaie, R.; Mostafa, S.M.; Mohamed, A.A.; Khalil, M.; Elagawy, W.; Abd-Elsalam, S. Relationship Between Helicobacter pylori Infection and Nonalcoholic Fatty Liver Disease (NAFLD) in a Developing Country: A Cross-Sectional Study. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 619–625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nseir, W.; Amara, A.; Farah, R.; Ahmad, H.S.; Mograbi, J.; Mahamid, M. Non-alcoholic Fatty Liver Disease is As-sociated with Recurrent Urinary Tract Infection in Premenopausal Women Independent of Metabolic Syndrome. Isr. Med. Assoc. J. 2019, 21, 386–389. [Google Scholar]
- Wijarnpreecha, K.; Lou, S.; Panjawatanan, P.; Sanguankeo, A.; Pungpapong, S.; Lukens, F.J.; Ungprasert, P. Nonalcoholic fatty liver disease and urolithiasis: A systematic review and meta-analysis. J. Gastrointest. Liver Dis. 2018, 27, 427–432. [Google Scholar] [CrossRef]
- Yang, J.; Chen, G.; Wang, D.; Chen, M.; Xing, C.; Wang, B. Low serum 25-hydroxyvitamin D level and risk of urinary tract infection in infants. Medicine 2016, 95, e4137. [Google Scholar] [CrossRef]
- Saliba, W.; Barnett-Griness, O.; Rennert, G. The association between obesity and urinary tract infection. Eur. J. Intern. Med. 2013, 24, 127–131. [Google Scholar] [CrossRef]
- Samarkos, M.; Mastrogianni, E.; Kampouropoulou, O. The role of gut microbiota in Clostridium difficile infection. Eur. J. Intern. Med. 2018, 50, 28–32. [Google Scholar] [CrossRef]
- Papić, N.; Jelovčić, F.; Karlović, M.; Marić, L.S.; Vince, A. Nonalcoholic fatty liver disease as a risk factor for Clostridioides difficile infection. Eur. J. Clin. Microbiol. 2019, 39, 569–574. [Google Scholar] [CrossRef]
- Nseir, W.B.; Hussein, S.H.H.; Farah, R.; Mahamid, M.N.; Khatib, H.H.; Mograbi, J.M.; Peretz, A.; Amara, A.E. Nonalcoholic fatty liver disease as a risk factor for Clostridium difficile-associated diarrhea. QJM Int. J. Med. 2019, 113, 320–323. [Google Scholar] [CrossRef] [PubMed]
- Šamadan, L.; Jeličić, M.; Vince, A.; Papić, N. Nonalcoholic Fatty Liver Disease—A Novel Risk Factor for Recurrent Clostridioides difficile Infection. Antibiotics 2021, 10, 780. [Google Scholar] [CrossRef] [PubMed]
- Nseir, W.; Taha, H.; Khateeb, J.; Grosovski, M.; Assy, N. Fatty Liver Is Associated with Recurrent Bacterial Infections Independent of Metabolic Syndrome. Am. J. Dig. Dis. 2011, 56, 3328–3334. [Google Scholar] [CrossRef] [PubMed]
- De Munck, T.J.I.; Xu, P.; Verwijs, H.J.A.; Masclee, A.A.M.; Jonkers, D.; Verbeek, J.; Koek, G.H. Intestinal permeability in human nonalcoholic fatty liver disease: A systematic review and meta-analysis. Liver Int. 2020, 40, 2906–2916. [Google Scholar] [CrossRef]
- Hu, H.; Lin, A.; Kong, M.; Yao, X.; Yin, M.; Xia, H.; Ma, J.; Liu, H. Intestinal microbiome and NAFLD: Molecular insights and therapeutic perspectives. J. Gastroenterol. 2019, 55, 142–158. [Google Scholar] [CrossRef] [Green Version]
- Nseir, W.; Artul, S.; Nasrallah, N.; Mahamid, M. The association between primary bacteremia of presumed gastrointestinal origin and nonalcoholic fatty liver disease. Dig. Liver Dis. 2015, 48, 343–344. [Google Scholar] [CrossRef]
- Gjurašin, B.; Butić, I.; Vince, A.; Papić, N. Non-Alcoholic Fatty Liver Disease is Associated with an Increased Mortality in Adult Patients with Group B Streptococcus Invasive Disease. Infektološki Glas 2021, 40, 124–128. [Google Scholar] [CrossRef]
- Adinolfi, L.E.; Rinaldi, L.; Guerrera, B.; Restivo, L.; Marrone, A.; Giordano, M.; Zampino, R. NAFLD and NASH in HCV Infection: Prevalence and Significance in Hepatic and Extrahepatic Manifestations. Int. J. Mol. Sci. 2016, 17, 803. [Google Scholar] [CrossRef] [Green Version]
- Maurice, J.; Garvey, L.; Tsochatzis, E.A.; Wiltshire, M.; Cooke, G.; Guppy, N.; McDonald, J.; Marchesi, J.; Nelson, M.; Kelleher, P.; et al. Monocyte-macrophage activation is associated with nonalcoholic fatty liver disease and liver fibrosis in HIV monoinfection independently of the gut microbiome and bacterial translocation. Aids 2019, 33, 805–814. [Google Scholar] [CrossRef]
- Joo, E.-J.; Chang, Y.; Yeom, J.-S.; Ryu, S. Hepatitis B virus infection and decreased risk of nonalcoholic fatty liver disease: A cohort study. Hepatology 2017, 65, 828–835. [Google Scholar] [CrossRef] [Green Version]
- Rafiei, M.; Kiani, F.; Sayehmiri, F.; Sayehmiri, K.; Sheikhi, A.; Azodi, M.Z. Study of Porphyromonas gingivalis in periodontal diseases: A systematic review and meta-analysis. Med. J. Islam. Repub. Iran 2017, 31, 62. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, M.; Naka, S.; Nakano, K.; Wada, K.; Endo, H.; Mawatari, H.; Imajo, K.; Nomura, R.; Hokamura, K.; Ono, M.; et al. Involvement of a periodontal pathogen, Porphyromonas gingivalis on the pathogenesis of non-alcoholic fatty liver disease. BMC Gastroenterol. 2012, 12, 16. [Google Scholar] [CrossRef] [PubMed]
| Acute Phase Proteins | Biological Modifiers | Immune Function | Role in NAFLD |
|---|---|---|---|
| Inflammatory proteins | IL-6, lipopolysaccharide-binding protein, and secreted phospholipase A2, C-reactivee protein, manose binding lectin, collectin liver 1, ficolin-2, serum amyloid P, serum amyloid A | Enhance pro-inflammatory signals and potentiate acute response; secreted pathogen recognition receptors; activation of complement enhanced phagocytosis | Increased levels of CRP, IL1β, IL-6, TNF-α, and ICAM-1 are strongly linked to an increased risk of developing NAFLD [19]. |
| Complement proteins | C3, C4, C9, C4b-binding protein, mannose-binding lectin, C1 esterase inhibitor | Enhanced phagocytosis, chemo attractants, neutrophil degranulation; bacterial cell wall lysis; vascular permeability | The production of C3a and C5a because of complement system activation has been linked to both insulin resistance and pro-inflammatory activity. By controlling the hepatic inflammatory response, the complement system also contributes to NASH [20]. |
| Negative acute phase proteins | Albumin, transferrin, transthyretin, retinol-binding protein, antithrombin, transcortin, insulin like growth factor 1, A2 HS glycoprotein, alpha-fetoprotein, factor XII | Homeostasis, metabolism, transporter proteins | A reduction in serum albumin correlates with a NAFLD severity [23]. As the degree of hepatic steatosis increases, AFP levels rise [24]. |
| Protease inhibitors | α 2 macroglobulin, α 1 antitrypsin, α 1 antichymotrypsin | Anti-inflammatory role | Hyperferritinemia and sinusoidal iron accumulation are linked to α 1 antitrypsin mutations in NAFLD [25]. |
| Antimicrobial proteins | Liver expressed antimicrobial peptide 2, hepcidin | Antimicrobial activity | LEAP2 is linked to steatosis and the lipolytic/lipogenic pathway [26]. |
| Clotting factors | Fibrinogen, plasminogen, protein S, prothrombin, factor VIII, factor IX and Von Willenbrand factor, vitronectin | Coagulation and fibrinolysis | Some changes in hemostatic parameters are anticipated in all forms of liver disease, including NAFLD [27]. |
| Iron-binding proteins | Haptoglobin, hemopexin, ferritin, hepcidin | Reduction of free iron in the serum; antimicrobial functions | The amount of body iron corresponds with serum hepcidin in the liver but not with the severity of steatohepatitis or lipid status [28]. |
| Study Design | Study Methodology | Main Findings | Comment | |
|---|---|---|---|---|
| Bacterial pneumonia | ||||
| Nseir et al. [35] | case-control retrospective study | 141 patients hospitalized for the treatment of CAP |
|
|
| Nseir et al. [36] | retrospective cohort study | 561 patients with CAP |
|
|
| COVID-19 | ||||
| Younossi et al. [46] | retrospective | 553 patients hospitalized with COVID-19 and NAFLD and a baseline Elixhauser comorbidity score of 13.6 |
|
|
| Forlano et al. [47] | cohort study | 193 patients with COVID |
|
|
| Ji et al. [48] | cohort study | 202 consecutive patients with confirmed COVID-19 and information relating NAFLD status |
|
|
| Bramante et al. [49] | retrospective analysis | 6700 adults with a positive SARS-CoV-2 PCR had assessed odds of hospital admission |
|
|
| Vrsaljko et al. [50] | prospective observational study | 216 adult patients hospitalized with severe COVID were also assessed for NAFLD |
| patients with NAFLD typically required noninvasive ventilation or high-flow nasal cannulas more frequently, spent longer time in hospitals |
| H. pylori | ||||
| Baeg et al. [53] | retrospective | 3663 people were analyzed, 1636 (44.7%) were H. pylori positive |
|
|
| Abo-Amer et al. [54] | cross-sectional study | Of 646 patients; H. pylori infection was found to be present in 538 patients (83.3%). |
|
|
| UTI | ||||
| Nseir et al. [55] | retrospective case-control study | 186 participants with rUTI and 186 controls without a history of rUTI |
|
|
| C. difficile | ||||
| Papić et al. [60] | retrospective cohort study | 314 patients ≥ 65 years, treated with antimicrobial therapy ≥ 24 h, and hospitalized ≥ 72 h in a 36-month period |
|
|
| Nseir et al. [61] | retrospective | 115 patients with CAD vs. 115 patients without |
|
|
| Šamadan et al. [62] | retrospective cohort study | 329 patients ≥ 60 years hospitalized with CAD, outcome: rCAD within 3 months of hospital discharge |
|
|
| Bacteremia and recurrent bacterial infections | ||||
| Nseir et al. [63] | retrospective | 247 patients with NAFLD hospitalized with bacterial infection vs. 100 patients without NAFLD |
|
|
| Nseir et al. [66] | retrospective | 71 patients with PB and hepatic ultrasonography were included |
|
|
| Gjurašin et al. [67] | retrospective cohort study | 102 patients with invasive GBS |
|
|
| Hepatitis B, C, HIV | ||||
| Adinolfi et al. [68] | review paper |
|
| |
| Maurice et al. [69] | prospective study | patients with NAFLD and HIV monoinfection matched to HIV-positive and HIV-negative controls. |
|
|
| Joo et al. [70] | cohort study | 83,339 participants without NAFLD underwent serologic testing HBsAg between 2002 and 2006 and were followed annually or biannually until December 2014. |
|
|
| Periodontitis | ||||
| Yoneda et al. [72] | retrospective observational study | the detection frequencies of periodontal bacteria in oral samples collected from 150 biopsy proven NAFLD patients and 60 non-NAFLD control subjects were determined |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krznarić, J.; Vince, A. The Role of Non-Alcoholic Fatty Liver Disease in Infections. Life 2022, 12, 2052. https://doi.org/10.3390/life12122052
Krznarić J, Vince A. The Role of Non-Alcoholic Fatty Liver Disease in Infections. Life. 2022; 12(12):2052. https://doi.org/10.3390/life12122052
Chicago/Turabian StyleKrznarić, Juraj, and Adriana Vince. 2022. "The Role of Non-Alcoholic Fatty Liver Disease in Infections" Life 12, no. 12: 2052. https://doi.org/10.3390/life12122052
APA StyleKrznarić, J., & Vince, A. (2022). The Role of Non-Alcoholic Fatty Liver Disease in Infections. Life, 12(12), 2052. https://doi.org/10.3390/life12122052





