Functional Characterization of the Ryanodine Receptor Gene in Diaphorina citri
Abstract
:1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Full-Length Cloning of DcRyR cDNA
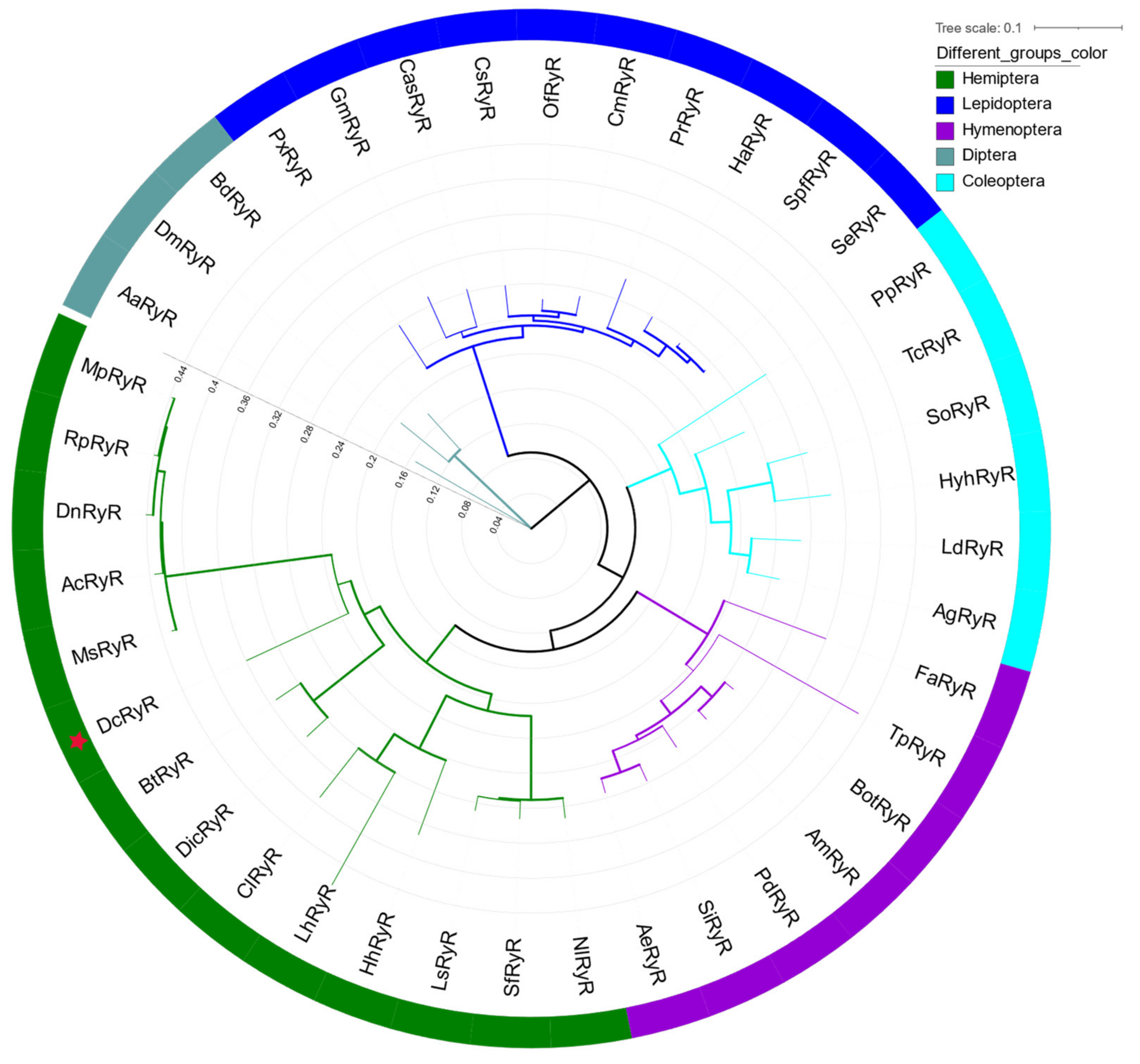
2.3. Sequencing and Phylogenetic Analysis
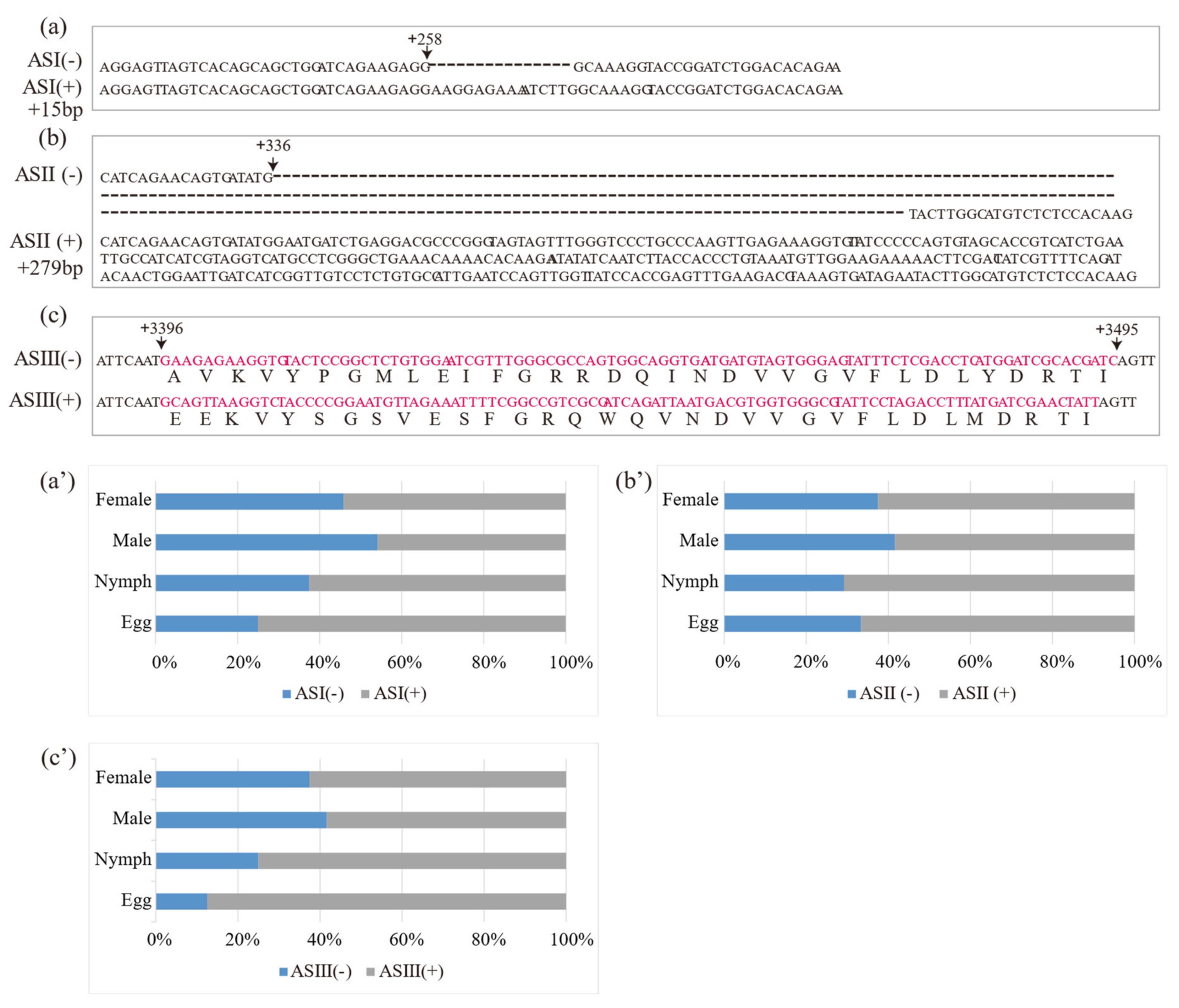
2.4. Detection of Alternative Splice (AS) Sites
2.5. Detection of RyR Mutations in D. citri Populations
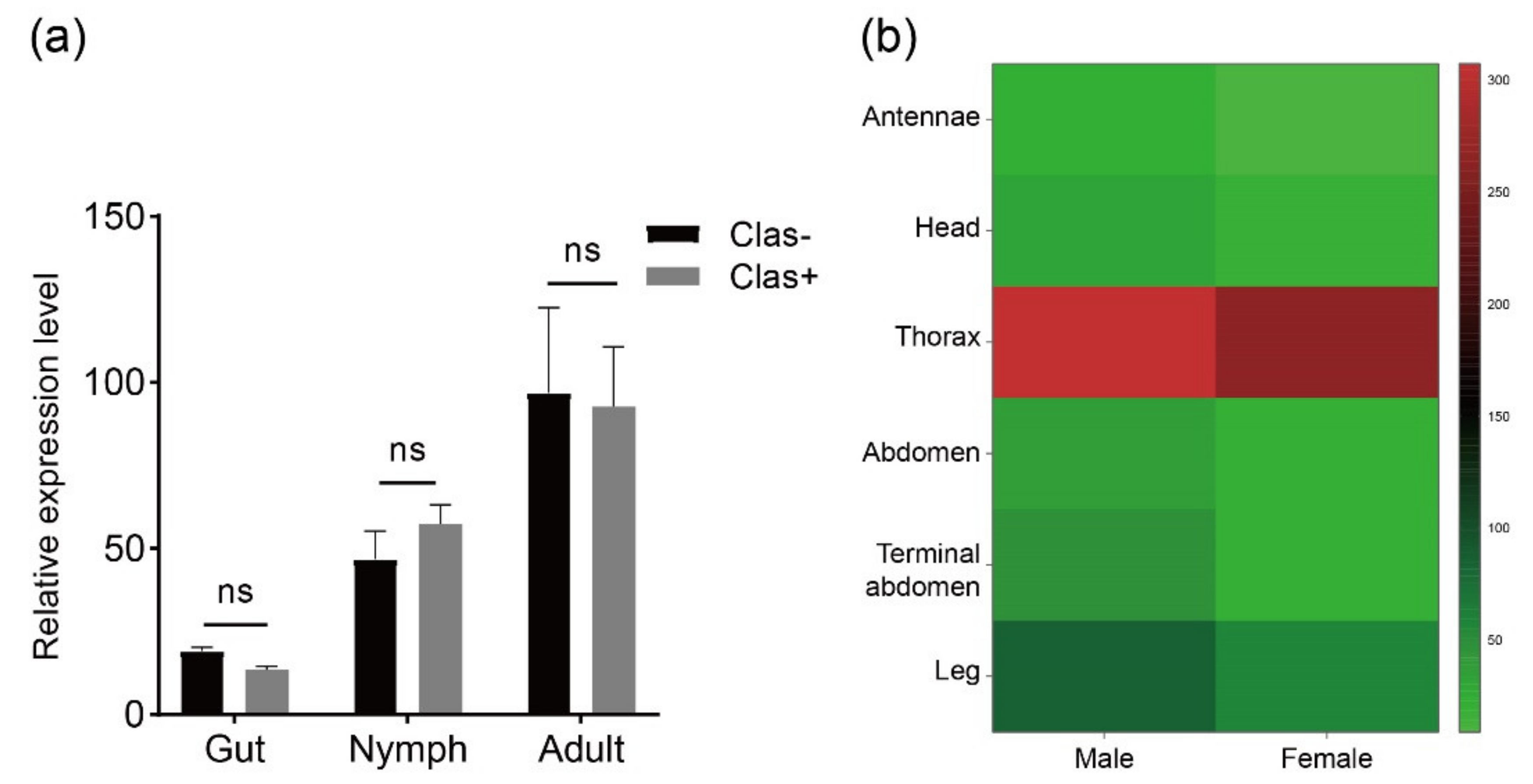
2.6. Data Analysis of DcRyR Expression
3. Results
3.1. Isolation of DcRyR
3.2. Conserved Domain and Phylogenetic Analysis of DcRyR
3.3. Detection of AS Sites of DcRyR
3.4. DcRyR Expression Profiles
3.5. Detection of DcRyR Mutations
4. Discussion
4.1. Isolated and Characterization of DcRyR
4.2. AS Site of DcRyR
4.3. DcRyR Expression Profiles
4.4. Detection of DcRyR Mutations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sparks, T.C.; Crossthwaite, A.J.; Nauen, R.; Banba, S.; Cordova, D.; Earley, F.; Ebbinghaus-Kintscher, U.; Fujioka, S.; Hirao, A.; Karmon, D. Insecticides, biologics and nematicides: Updates to IRAC’s mode of action classification-a tool for resistance management. Pestic. Biochem. Physiol. 2020, 167, 104587. [Google Scholar] [CrossRef] [PubMed]
- Sparks, T.C.; Nauen, R. IRAC: Mode of action classification and insecticide resistance management. Pestic. Biochem. Physiol. 2015, 121, 122–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Usherwood, P. The action of the alkaloid ryanodine on insect skeletal muscle. Comp. Biochem. Physiol. 1962, 6, 181–199. [Google Scholar] [CrossRef]
- Pessah, I.N.; Waterhouse, A.L.; Casida, J.E. The calcium-ryanodine receptor complex of skeletal and cardiac muscle. Biochem. Biophys. Res. Commun. 1985, 128, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Ebbinghaus-Kintscher, U.; Luemmen, P.; Lobitz, N.; Schulte, T.; Funke, C.; Fischer, R.; Masaki, T.; Yasokawa, N.; Tohnishi, M. Phthalic acid diamides activate ryanodine-sensitive Ca2+ release channels in insects. Cell Calcium. 2006, 39, 21–33. [Google Scholar] [CrossRef]
- Bové, J.M. Huanglongbing: A destructive, newly-emerging, century-old disease of citrus. J. Plant Pathol. 2006, 88, 7–37. [Google Scholar] [CrossRef] [Green Version]
- Da Graça, J.V.; Douhan, G.W.; Halbert, S.E.; Keremane, M.L.; Lee, R.F.; Vidalakis, G.; Zhao, H. Huanglongbing: An overview of a complex pathosystem ravaging the world's citrus. J. Integr. Plant Biol. 2016, 58, 373–387. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.; Motghare, M.; Gowda, S. Citrus greening: Overview of the most severe disease of citrus. Adv. Agric. Res. Technol. J. 2018, 2, 83–100. [Google Scholar]
- Wang, N.; Trivedi, P. Citrus huanglongbing: A newly relevant disease presents unprecedented challenges. Phytopathology 2013, 103, 652–665. [Google Scholar] [CrossRef] [Green Version]
- Gottwald, T.R. Current epidemiological understanding of citrus huanglongbing. Annu. Rev. Phytopathol. 2010, 48, 119–139. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Pierson, E.A.; Setubal, J.C.; Xu, J.; Levy, J.G.; Zhang, Y.; Li, J.; Rangel, L.T.; Martins, J., Jr. The Candidatus Liberibacter–host interface: Insights into pathogenesis mechanisms and disease control. Annu. Rev. Phytopathol. 2017, 55, 451–482. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xu, Y.; Yang, X.; Jia, J.; Zhou, J.; Zeng, J.; Yan, X.; Li, J.; Yue, J.; Guo, J. Novel insight into the distribution and dissemination of Candidatus Liberibacter asiaticus, the causal agent of citrus Huanglongbing. Plant Biotech. J. 2022, 20, 247. [Google Scholar] [CrossRef] [PubMed]
- Wang, N. The citrus huanglongbing crisis and potential solutions. Mol. Plant 2019, 12, 607–609. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.-T.; Clark, K.; Ma, W.; Mulchandani, A. Detection of a secreted protein biomarker for citrus Huanglongbing using a single-walled carbon nanotubes-based chemiresistive biosensor. Biosens. Bioelectron. 2020, 147, 111766. [Google Scholar] [CrossRef]
- Grafton-Cardwell, E.E.; Stelinski, L.L.; Stansly, P.A. Biology and management of Asian citrus psyllid, vector of the huanglongbing pathogens. Annu. Rev. Entomol. 2013, 58, 413–432. [Google Scholar] [CrossRef] [Green Version]
- Clark, K.; Franco, J.Y.; Schwizer, S.; Pang, Z.; Hawara, E.; Liebrand, T.W.; Pagliaccia, D.; Zeng, L.; Gurung, F.B.; Wang, P. An effector from the Huanglongbing-associated pathogen targets citrus proteases. Nat. Commun. 2018, 9, 1718. [Google Scholar] [CrossRef] [Green Version]
- Boina, D.R.; Bloomquist, J.R. Chemical control of the Asian citrus psyllid and of huanglongbing disease in citrus. Pest Manag. Sci. 2015, 71, 808–823. [Google Scholar] [CrossRef]
- Chen, X.D.; Stelinski, L.L. Resistance Management for Asian Citrus Psyllid, Diaphorina citri Kuwayama, in Florida. Insects 2017, 8, 103. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.D.; Neupane, S.; Gill, T.A.; Gossett, H.; Pelz-Stelinski, K.S.; Stelinski, L.L. Comparative transcriptome analysis of thiamethoxam susceptible and resistant Asian citrus psyllid, Diaphorina citri (Hemiptera: Liviidae), using RNA-sequencing. Insect Sci. 2021, 28, 1708–1720. [Google Scholar] [CrossRef]
- Boina, D.R.; Onagbola, E.O.; Salyani, M.; Stelinski, L.L. Antifeedant and sublethal effects of imidacloprid on Asian citrus psyllid, Diaphorina citri. Pest Manag. Sci. Former. Pestic. Sci. 2009, 65, 870–877. [Google Scholar] [CrossRef]
- Huang, J.; Hu, R.; Pray, C.; Qiao, F.; Rozelle, S. Biotechnology as an alternative to chemical pesticides: A case study of Bt cotton in China. Agric. Econ. 2003, 29, 55–67. [Google Scholar] [CrossRef]
- Tiwari, S.; Killiny, N.; Stelinski, L.L. Dynamic insecticide susceptibility changes in Florida populations of Diaphorina citri (Hemiptera: Psyllidae). J. Econ. Entomol. 2013, 106, 393–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kishk, A.; Hijaz, F.; Anber, H.A.; AbdEl-Raof, T.K.; El-Sherbeni, A.-H.D.; Hamed, S.; Killiny, N. RNA interference of acetylcholinesterase in the Asian citrus psyllid, Diaphorina citri, increases its susceptibility to carbamate and organophosphate insecticides. Pestic. Biochem. Physiol. 2017, 143, 81–89. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, S.; Mann, R.S.; Rogers, M.E.; Stelinski, L.L. Insecticide resistance in field populations of Asian citrus psyllid in Florida. Pest Manag. Sci. 2011, 67, 1258–1268. [Google Scholar] [CrossRef]
- Rao, C.; Shivankar, V.; Deole, S.; David, K.; Dhengre, V. Insecticide resistance in field populations of Asian citrus psyllid, Diaphorina citri Kuwayama (Hemiptera: Psyllidae). Pestic. Res. J. 2014, 26, 42–47. [Google Scholar]
- Naeem, A.; Freed, S.; Jin, F.L.; Akmal, M.; Mehmood, M. Monitoring of insecticide resistance in Diaphorina citri Kuwayama (Hemiptera: Psyllidae) from citrus groves of Punjab, Pakistan. Crop Prot. 2016, 86, 62–68. [Google Scholar] [CrossRef]
- Pardo, S.; Martínez, A.M.; Figueroa, J.I.; Chavarrieta, J.M.; Viñuela, E.; Rebollar-Alviter, Á.; Miranda, M.A.; Valle, J.; Pineda, S. Insecticide resistance of adults and nymphs of Asian citrus psyllid populations from Apatzingán Valley, Mexico. Pest Manag. Sci. 2018, 74, 135–140. [Google Scholar] [CrossRef]
- Tian, F.; Mo, X.; Rizvi, S.A.H.; Li, C.; Zeng, X. Detection and biochemical characterization of insecticide resistance in field populations of Asian citrus psyllid in Guangdong of China. Sci. Rep. 2018, 8, 12587. [Google Scholar] [CrossRef] [Green Version]
- Tian, F.; Li, C.; Wang, Z.; Liu, J.; Zeng, X. Identification of detoxification genes in imidacloprid-resistant Asian citrus psyllid (Hemiptera: Lividae) and their expression patterns under stress of eight insecticides. Pest Manag. Sci. 2019, 75, 1400–1410. [Google Scholar] [CrossRef]
- Kanga, L.H.; Eason, J.; Haseeb, M.; Qureshi, J.; Stansly, P. Monitoring for insecticide resistance in Asian citrus psyllid (Hemiptera: Psyllidae) populations in Florida. J. Econ. Entomol. 2016, 109, 832–836. [Google Scholar] [CrossRef]
- Chen, X.D.; Gill, T.A.; Ashfaq, M.; Pelz-Stelinski, K.S.; Stelinski, L.L. Resistance to commonly used insecticides in Asian citrus psyllid: Stability and relationship to gene expression. J. Appl. Entomol. 2018, 142, 967–977. [Google Scholar] [CrossRef]
- Huang, X.; Madan, A. CAP3: A DNA sequence assembly program. Genome Res. 1999, 9, 868–877. [Google Scholar] [CrossRef] [PubMed]
- Madeira, F.; Pearce, M.; Tivey, A.; Basutkar, P.; Lee, J.; Edbali, O.; Madhusoodanan, N.; Kolesnikov, A.; Lopez, R. Search and sequence analysis tools services from EMBL-EBI in 2022. Nucleic Acids Res. 2022, 50, W276–W279. [Google Scholar] [CrossRef]
- Waterhouse, A.M.; Procter, J.B.; Martin, D.M.; Clamp, M.; Barton, G.J. Jalview Version 2—A multiple sequence alignment editor and analysis workbench. Bioinformatics 2009, 25, 1189–1191. [Google Scholar] [CrossRef] [Green Version]
- Lu, S.; Wang, J.; Chitsaz, F.; Derbyshire, M.K.; Geer, R.C.; Gonzales, N.R.; Gwadz, M.; Hurwitz, D.I.; Marchler, G.H.; Song, J.S. CDD/SPARCLE: The conserved domain database in 2020. Nucleic Acids Res. 2020, 48, D265–D268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kyte, J.; Doolittle, R.F. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 1982, 157, 105–132. [Google Scholar] [CrossRef] [Green Version]
- Kozlov, A.M.; Darriba, D.; Flouri, T.; Morel, B.; Stamatakis, A. RAxML-NG: A fast, scalable and user-friendly tool for maximum likelihood phylogenetic inference. Bioinformatics 2019, 35, 4453–4455. [Google Scholar] [CrossRef] [Green Version]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef]
- Kõressaar, T.; Lepamets, M.; Kaplinski, L.; Raime, K.; Andreson, R.; Remm, M. Primer3_masker: Integrating masking of template sequence with primer design software. Bioinformatics 2018, 34, 1937–1938. [Google Scholar] [CrossRef] [Green Version]
- Du, G.G.; MacLennan, D.H. Functional consequences of mutations of conserved, polar amino acids in transmembrane sequences of the Ca2+ release channel (ryanodine receptor) of rabbit skeletal muscle sarcoplasmic reticulum. J. Biol. Chem. 1998, 273, 31867–31872. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.W.; Ebisawa, K.; Li, X.; Zhang, L. Molecular identification of the ryanodine receptor Ca2+ sensor. J. Biol. Chem. 1998, 273, 14675–14678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, H.; Feng, X.; Gao, L.; Xu, L.; Pasek, D.A.; Seok, J.-H.; Meissner, G. Identification of a two EF-hand Ca2+ binding domain in lobster skeletal muscle ryanodine receptor/Ca2+ release channel. Biochemistry 1998, 37, 4804–4814. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Li, P.; Li, X.; Zhang, L.; Winkfein, R.J.; Chen, S.W. Molecular identification of the ryanodine receptor pore-forming segment. J. Biol. Chem. 1999, 274, 25971–25974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, L.; Balshaw, D.; Xu, L.; Tripathy, A.; Xin, C.; Meissner, G. Evidence for a role of the lumenal M3-M4 loop in skeletal muscle Ca2+ release channel (ryanodine receptor) activity and conductance. Biophys. J. 2000, 79, 828–840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosmani, P.S.; Flores-Gonzalez, M.; Shippy, T.; Vosburg, C.; Massimino, C.; Tank, W.; Reynolds, M.; Tamayo, B.; Miller, S.; Norus, J. Chromosomal length reference assembly for Diaphorina citri using single-molecule sequencing and Hi-C proximity ligation with manually curated genes in developmental, structural and immune pathways. Biorxiv 2019, 869685. [Google Scholar] [CrossRef] [Green Version]
- Nauen, R. Insecticide mode of action: Return of the ryanodine receptor. Pest Manag. Sci. Former. Pestic. Sci. 2006, 62, 690–692. [Google Scholar] [CrossRef] [PubMed]
- Lahm, G.P.; Stevenson, T.M.; Selby, T.P.; Freudenberger, J.H.; Cordova, D.; Flexner, L.; Bellin, C.A.; Dubas, C.M.; Smith, B.K.; Hughes, K.A. Rynaxypyr™: A new insecticidal anthranilic diamide that acts as a potent and selective ryanodine receptor activator. Bioorganic Med. Chem. Lett. 2007, 17, 6274–6279. [Google Scholar] [CrossRef]
- Guo, L.; Tang, B.; Dong, W.; Liang, P.; Gao, X. Cloning, characterisation and expression profiling of the cDNA encoding the ryanodine receptor in diamondback moth, Plutella xylostella (L.) (Lepidoptera: Plutellidae). Pest Manag. Sci. 2012, 68, 1605–1614. [Google Scholar] [CrossRef]
- Sun, L.; Cui, L.; Rui, C.; Yan, X.; Yang, D.; Yuan, H. Modulation of the expression of ryanodine receptor mRNA from Plutella xylostella as a result of diamide insecticide application. Gene 2012, 511, 265–273. [Google Scholar] [CrossRef]
- Liu, Y.; Li, C.; Gao, J.; Wang, W.; Huang, L.; Guo, X.; Li, B.; Wang, J. Comparative characterization of two intracellular Ca2+-release channels from the red flour beetle, Tribolium castaneum. Sci. Rep. 2014, 4, 6702. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Li, K.; Xu, W.; Liang, N.; Chu, D.; Guo, L. Characterization of the ryanodine receptor gene in Encarsia formosa (Gahan) and its expression profile in response to diamide insecticides. Pestic. Biochem. Physiol. 2021, 178, 104921. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wan, P.-J.; Hu, X.-X.; Li, G.-Q. RNAi mediated knockdown of the ryanodine receptor gene decreases chlorantraniliprole susceptibility in Sogatella furcifera. Pestic. Biochem. Physiol. 2014, 108, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.-R.; Shi, W.-Z.; Yang, W.-J.; Jiang, X.-Z.; Dou, W.; Wang, J.-J. Molecular characteristics, mRNA expression, and alternative splicing of a ryanodine receptor gene in the oriental fruit fly, Bactrocera dorsalis (Hendel). PLoS ONE 2014, 9, e95199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Xie, Z.; Gao, J.; Liu, Y.; Wang, W.; Huang, L.; Wang, J. Molecular cloning and characterization of a ryanodine receptor gene in brown planthopper (BPH), Nilaparvata lugens (Stål). Pest Manag. Sci. 2014, 70, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Wan, P.-J.; Guo, W.-Y.; Yang, Y.; Lü, F.-G.; Lu, W.-P.; Li, G.-Q. RNAi suppression of the ryanodine receptor gene results in decreased susceptibility to chlorantraniliprole in Colorado potato beetle Leptinotarsa decemlineata. J. Insect Physiol. 2014, 63, 48–55. [Google Scholar] [CrossRef]
- Sattelle, D.B.; Cordova, D.; Cheek, T.R. Insect ryanodine receptors: Molecular targets for novel pest control chemicals. Invertebr. Neurosci. 2008, 8, 107–119. [Google Scholar] [CrossRef]
- Samurkas, A.; Fan, X.; Ma, D.; Sundarraj, R.; Lin, L.; Yao, L.; Ma, R.; Jiang, H.; Cao, P.; Gao, Q. Discovery of potential species-specific green insecticides targeting the lepidopteran ryanodine receptor. J. Agric. Food Chem. 2020, 68, 4528–4537. [Google Scholar] [CrossRef]
- Lee, Y.; Rio, D.C. Mechanisms and regulation of alternative pre-mRNA splicing. Annu. Rev. Biochem. 2015, 84, 291. [Google Scholar] [CrossRef] [Green Version]
- Puente, E.; Suner, M.-M.; Evans, A.D.; McCaffery, A.R.; Windass, J.D. Identification of a polymorphic ryanodine receptor gene from Heliothis virescens (Lepidoptera: Noctuidae). Insect. Biochem. Mol. Biol. 2000, 30, 335–347. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Han, Z.; Zhu, Y.; Xie, Z.; Wang, J.; Liu, Y.; Li, X. Molecular characterization of a ryanodine receptor gene in the rice leaffolder, Cnaphalocrocis medinalis (Guenée). PLoS ONE 2012, 7, e36623. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Liu, Y.; Gao, J.; Xie, Z.; Huang, L.; Wang, W.; Wang, J. Molecular cloning and mRNA expression of a ryanodine receptor gene in the cotton bollworm, Helicoverpa armigera. Pestic. Biochem. Physiol. 2013, 107, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Yang, D.; Yan, X.; Rui, C.; Wang, Z.; Yuan, H. Molecular cloning, characterization and expression profiling of a ryanodine receptor gene in Asian corn borer, Ostrinia furnacalis (Guenée). PLoS ONE 2013, 8, e75825. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.-Y.; Jiang, X.-Z.; Yuan, G.-R.; Shang, F.; Wang, J.-J. Molecular characterization, mRNA expression and alternative splicing of ryanodine receptor gene in the Brown Citrus aphid, Toxoptera citricida (Kirkaldy). Int. J. Mol. Sci. 2015, 16, 15220–15234. [Google Scholar] [CrossRef] [Green Version]
- Takeshima, H.; Nishi, M.; Iwabe, N.; Miyata, T.; Hosoya, T.; Masai, I.; Hotta, Y. Isolation and characterization of a gene for a ryanodine receptor/calcium release channel in Drosophila melanogaster. FEBS Lett. 1994, 337, 81–87. [Google Scholar] [CrossRef] [Green Version]
- D'Cruz, A.A.; Babon, J.J.; Norton, R.S.; Nicola, N.A.; Nicholson, S.E. Structure and function of the SPRY/B30. 2 domain proteins involved in innate immunity. Protein Sci. 2013, 22, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berridge, M.J. Inositol trisphosphate and calcium signalling. Nature 1993, 361, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Wang, F.; Huang, J.; Fang, Q.; Shen, Z.; Ye, G. Molecular and cellular analyses of a ryanodine receptor from hemocytes of Pieris rapae. Dev. Comp. Immunol. 2013, 41, 1–10. [Google Scholar] [CrossRef]
- Wang, X.; Wu, S.; Yang, Y.; Wu, Y. Molecular cloning, characterization and mRNA expression of a ryanodine receptor gene from diamondback moth, Plutella xylostella. Pestic. Biochem. Physiol. 2012, 102, 204–212. [Google Scholar] [CrossRef]
- Troczka, B.; Zimmer, C.T.; Elias, J.; Schorn, C.; Bass, C.; Davies, T.E.; Field, L.M.; Williamson, M.S.; Slater, R.; Nauen, R. Resistance to diamide insecticides in diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae) is associated with a mutation in the membrane-spanning domain of the ryanodine receptor. Insect Biochem. Mol. Biol. 2012, 42, 873–880. [Google Scholar] [CrossRef]
- Guo, L.; Liang, P.; Zhou, X.; Gao, X. Novel mutations and mutation combinations of ryanodine receptor in a chlorantraniliprole resistant population of Plutella xylostella (L.). Sci. Rep. 2014, 4, 6924. [Google Scholar] [CrossRef] [Green Version]
- Jouraku, A.; Kuwazaki, S.; Miyamoto, K.; Uchiyama, M.; Kurokawa, T.; Mori, E.; Mori, M.X.; Mori, Y.; Sonoda, S. Ryanodine receptor mutations (G4946E and I4790K) differentially responsible for diamide insecticide resistance in diamondback moth, Plutella xylostella L. Insect Biochem. Mol. Biol. 2020, 118, 103308. [Google Scholar] [CrossRef] [PubMed]
- Roditakis, E.; Steinbach, D.; Moritz, G.; Vasakis, E.; Stavrakaki, M.; Ilias, A.; García-Vidal, L.; del Rosario Martinez-Aguirre, M.; Bielza, P.; Morou, E. Ryanodine receptor point mutations confer diamide insecticide resistance in tomato leafminer, Tuta absoluta (Lepidoptera: Gelechiidae). Insect Biochem. Mol. Biol. 2017, 80, 11–20. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, L.; Chen, Q.; Qin, W.; Huang, S.; Jiang, Y.; Qin, H. Chlorantraniliprole resistance and its biochemical and new molecular target mechanisms in laboratory and field strains of Chilo suppressalis (Walker). Pest Manag. Sci. 2018, 74, 1416–1423. [Google Scholar] [CrossRef]
- Yao, R.; Zhao, D.D.; Zhang, S.; Zhou, L.Q.; Wang, X.; Gao, C.F.; Wu, S.F. Monitoring and mechanisms of insecticide resistance in Chilo suppressalis (Lepidoptera: Crambidae), with special reference to diamides. Pest Manag. Sci. 2017, 73, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, T.; Ozawa, A. Rapid development of resistance to diamide insecticides in the smaller tea tortrix, Adoxophyes honmai (Lepidoptera: Tortricidae), in the tea fields of Shizuoka Prefecture, Japan. Appl. Entomol. Zool. 2014, 49, 529–534. [Google Scholar] [CrossRef]
- Kim, J.; Nam, H.Y.; Kwon, M.; Choi, J.H.; Cho, S.R.; Kim, G.-H. Novel diamide resistance-linked mutation in Korean Spodoptera exigua and a LAMP assay based on a mutation-associated intronic InDel. J. Pest Sci. 2021, 94, 1017–1029. [Google Scholar] [CrossRef]
- Zuo, Y.Y.; Ma, H.H.; Lu, W.J.; Wang, X.L.; Wu, S.W.; Nauen, R.; Wu, Y.D.; Yang, Y.H. Identification of the ryanodine receptor mutation I4743M and its contribution to diamide insecticide resistance in Spodoptera exigua (Lepidoptera: Noctuidae). Insect Sci. 2020, 27, 791–800. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.-S.; Sun, X.-L.; Bin, M.-L.; Yi, G.-J.; Zhang, X.-X. Functional Characterization of the Ryanodine Receptor Gene in Diaphorina citri. Life 2022, 12, 2005. https://doi.org/10.3390/life12122005
Liu T-S, Sun X-L, Bin M-L, Yi G-J, Zhang X-X. Functional Characterization of the Ryanodine Receptor Gene in Diaphorina citri. Life. 2022; 12(12):2005. https://doi.org/10.3390/life12122005
Chicago/Turabian StyleLiu, Tian-Sheng, Xue-Li Sun, Min-Liang Bin, Gan-Jun Yi, and Xin-Xin Zhang. 2022. "Functional Characterization of the Ryanodine Receptor Gene in Diaphorina citri" Life 12, no. 12: 2005. https://doi.org/10.3390/life12122005




