Factors Associated with the Uptake of Genetic Testing for Cancer Risks: A Pathway Analysis Using the Health Information National Trends Survey Data
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
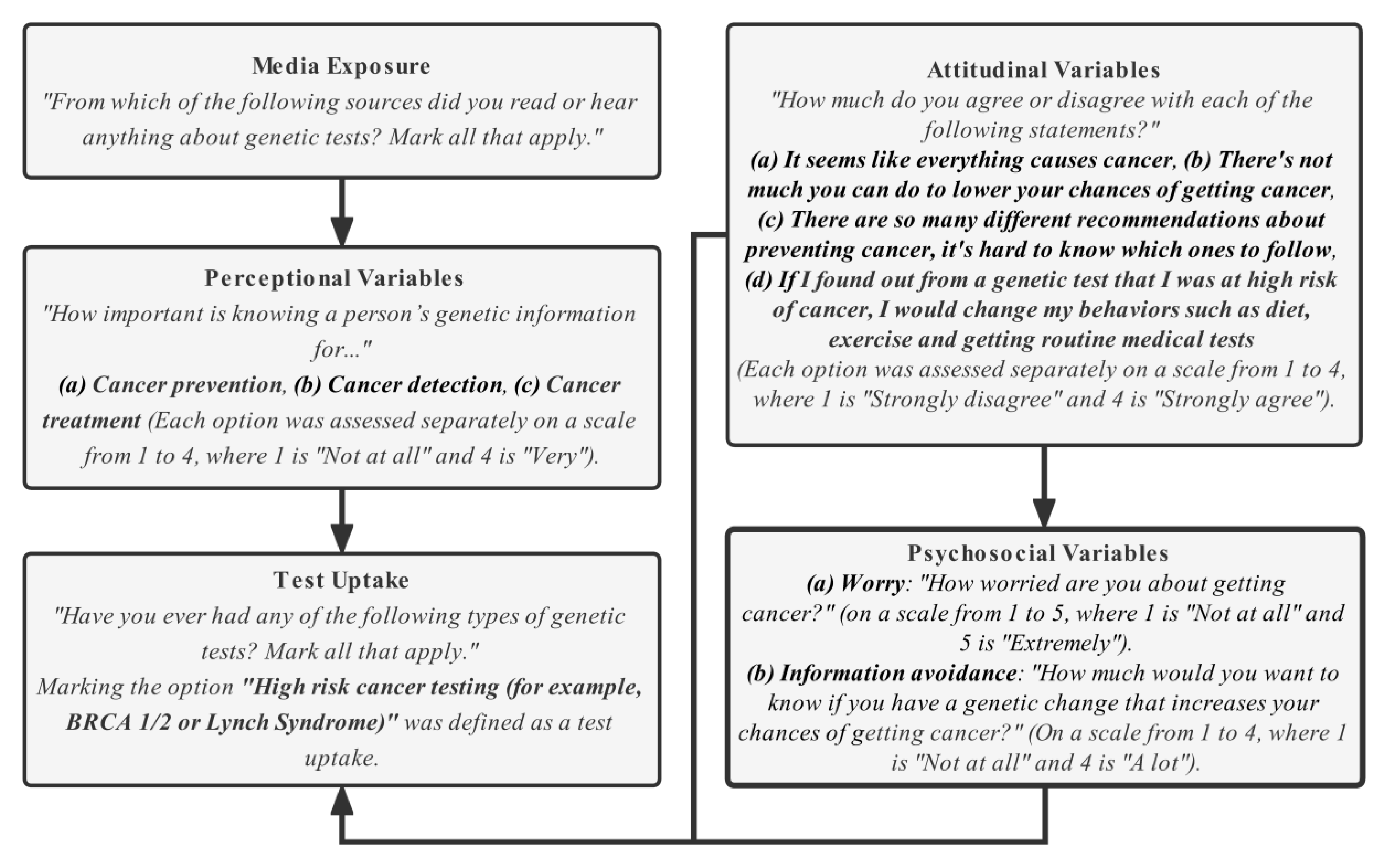
2.2. Study Variable
2.3. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Global Burden of Disease Cancer Collaboration; Fitzmaurice, C.; Abate, D.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdel-Rahman, O.; Abdelalim, A.; Abdoli, A.; Abdollahpour, I.; et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived with Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2019, 5, 1749–1768. [Google Scholar] [CrossRef]
- Young, K.; Bowers, A.; Bradford, N. Families’ Experiences of Child and Adolescent Brain Tumor: A Systematic Review and Synthesis of Qualitative Research. Psychooncology 2021, 30, 1643–1662. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, M.E.; Kurtz, J.C.; Given, C.W.; Given, B.A. Depression and Physical Health among Family Caregivers of Geriatric Patients with Cancer—A Longitudinal View. Med. Sci. Monit. 2004, 10, CR447–CR456. [Google Scholar] [PubMed]
- Kanter, C.; D’Agostino, N.M.; Daniels, M.; Stone, A.; Edelstein, K. Together and Apart: Providing Psychosocial Support for Patients and Families Living with Brain Tumors. Support Care Cancer 2014, 22, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Kristjanson, L.J.; Ashcroft, T. The Family’s Cancer Journey: A Literature Review. Cancer Nurs. 1994, 17, 1–17. [Google Scholar] [CrossRef]
- Duggan, C.; Dvaladze, A.L.; Tsu, V.; Jeronimo, J.; Constant, T.K.H.; Romanoff, A.; Scheel, J.R.; Patel, S.; Gralow, J.R.; Anderson, B.O. Resource-Stratified Implementation of a Community-Based Breast Cancer Management Programme in Peru. Lancet Oncol. 2017, 18, e607–e617. [Google Scholar] [CrossRef]
- Dare, A.J.; Knapp, G.C.; Romanoff, A.; Olasehinde, O.; Famurewa, O.C.; Komolafe, A.O.; Olatoke, S.; Katung, A.; Alatise, O.I.; Kingham, T.P. High-Burden Cancers in Middle-Income Countries: A Review of Prevention and Early Detection Strategies Targeting At-Risk Populations. Cancer Prev. Res. 2021, 14, 1061–1074. [Google Scholar] [CrossRef]
- Arbyn, M.; Ronco, G.; Anttila, A.; Meijer, C.J.L.M.; Poljak, M.; Ogilvie, G.; Koliopoulos, G.; Naucler, P.; Sankaranarayanan, R.; Peto, J. Evidence Regarding Human Papillomavirus Testing in Secondary Prevention of Cervical Cancer. Vaccine 2012, 30 (Suppl. S5), F88–F99. [Google Scholar] [CrossRef]
- Lamartina, L.; Grani, G.; Durante, C.; Filetti, S.; Cooper, D.S. Screening for Differentiated Thyroid Cancer in Selected Populations. Lancet Diabetes Endocrinol. 2020, 8, 81–88. [Google Scholar] [CrossRef]
- Khoury, M.J.; Gwinn, M.; Yoon, P.W.; Dowling, N.; Moore, C.A.; Bradley, L. The Continuum of Translation Research in Genomic Medicine: How Can We Accelerate the Appropriate Integration of Human Genome Discoveries into Health Care and Disease Prevention? Genet. Med. 2007, 9, 665–674. [Google Scholar] [CrossRef]
- Khoury, M.J.; Clauser, S.B.; Freedman, A.N.; Gillanders, E.M.; Glasgow, R.E.; Klein, W.M.P.; Schully, S.D. Population Sciences, Translational Research, and the Opportunities and Challenges for Genomics to Reduce the Burden of Cancer in the 21st Century. Cancer Epidemiol. Biomark. Prev. 2011, 20, 2105–2114. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.M.; Traverso, G.; Johnson, C.; Geng, L.; Favis, R.; Boynton, K.; Hibi, K.; Goodman, S.N.; D’Allessio, M.; Paty, P.; et al. Detecting Colorectal Cancer in Stool with the Use of Multiple Genetic Targets. J. Natl. Cancer Inst. 2001, 93, 858–865. [Google Scholar] [CrossRef] [PubMed]
- Miller-Wilson, L.-A.; Rutten, L.J.F.; Van Thomme, J.; Ozbay, A.B.; Limburg, P.J. Cross-Sectional Adherence with the Multi-Target Stool DNA Test for Colorectal Cancer Screening in a Large, Nationally Insured Cohort. Int. J. Colorectal Dis. 2021, 36, 2471–2480. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Wu, X.; Yin, J.; Li, M.; Shen, J.; Li, J.; Zhao, Y.; Zhao, Q.; Wu, J.; Wen, Q.; et al. Identification of Genetic Mutations in Cancer: Challenge and Opportunity in the New Era of Targeted Therapy. Front. Oncol. 2019, 9, 263. [Google Scholar] [CrossRef]
- Werner, H.M.J.; Mills, G.B.; Ram, P.T. Cancer Systems Biology: A Peek into the Future of Patient Care? Nat. Rev. Clin. Oncol. 2014, 11, 167–176. [Google Scholar] [CrossRef]
- Kastrinos, F.; Samadder, N.J.; Burt, R.W. Use of Family History and Genetic Testing to Determine Risk of Colorectal Cancer. Gastroenterology 2020, 158, 389–403. [Google Scholar] [CrossRef]
- Binefa, G.; Rodríguez-Moranta, F.; Teule, A.; Medina-Hayas, M. Colorectal Cancer: From Prevention to Personalized Medicine. World J. Gastroenterol. 2014, 20, 6786–6808. [Google Scholar] [CrossRef]
- Brittain, H.K.; Scott, R.; Thomas, E. The Rise of the Genome and Personalised Medicine. Clin. Med. 2017, 17, 545–551. [Google Scholar] [CrossRef]
- Marteau, T.M.; Lerman, C. Genetic Risk and Behavioural Change. BMJ 2001, 322, 1056–1059. [Google Scholar] [CrossRef]
- Meiser, B. Psychological Impact of Genetic Testing for Cancer Susceptibility: An Update of the Literature. Psychooncology 2005, 14, 1060–1074. [Google Scholar] [CrossRef] [PubMed]
- Vadaparampil, S.T.; Miree, C.A.; Wilson, C.; Jacobsen, P.B. Psychosocial and Behavioral Impact of Genetic Counseling and Testing. Breast Dis. 2006, 27, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.S. Assessing the Psychological Impact of Genetic Susceptibility Testing. Hastings Cent. Rep. 2019, 49 (Suppl. S1), S38–S43. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, A.; Werrett, J.; Burgess, L.; Chapman, C.; Clifford, C. Cancer Genetic Predisposition: Information Needs of Patients Irrespective of Risk Level. Fam. Cancer 2009, 8, 403–412. [Google Scholar] [CrossRef]
- Reitz, F.; Barth, J.; Bengel, J. Predictive Value of Breast Cancer Cognitions and Attitudes toward Genetic Testing on Women’s Interest in Genetic Testing for Breast Cancer Risk. Psychosoc. Med. 2004, 1, Doc03. [Google Scholar]
- Agurs-Collins, T.; Ferrer, R.; Ottenbacher, A.; Waters, E.A.; O’Connell, M.E.; Hamilton, J.G. Public Awareness of Direct-to-Consumer Genetic Tests: Findings from the 2013 U.S. Health Information National Trends Survey. J. Cancer Educ. 2015, 30, 799–807. [Google Scholar] [CrossRef]
- Klein, W.M.P.; O’Connell, M.E.; Bloch, M.H.; Czajkowski, S.M.; Green, P.A.; Han, P.K.J.; Moser, R.P.; Nebeling, L.C.; Vanderpool, R.C. Behavioral Research in Cancer Prevention and Control: Emerging Challenges and Opportunities. J. Natl. Cancer Inst. 2022, 114, 179–186. [Google Scholar] [CrossRef]
- Quinlivan, J.A.; Battikhi, Z.; Petersen, R.W. What Factors Impact upon a Woman’s Decision to Undertake Genetic Cancer Testing? Front. Oncol. 2014, 3, 325. [Google Scholar] [CrossRef][Green Version]
- Wade, C.H.; Shiloh, S.; Woolford, S.W.; Roberts, J.S.; Alford, S.H.; Marteau, T.M.; Biesecker, B.B. Modelling Decisions to Undergo Genetic Testing for Susceptibility to Common Health Conditions: An Ancillary Study of the Multiplex Initiative. Psychol. Health 2012, 27, 430–444. [Google Scholar] [CrossRef]
- Wolff, K.; Nordin, K.; Brun, W.; Berglund, G.; Kvale, G. Affective and Cognitive Attitudes, Uncertainty Avoidance and Intention to Obtain Genetic Testing: An Extension of the Theory of Planned Behaviour. Psychol. Health 2011, 26, 1143–1155. [Google Scholar] [CrossRef]
- Helmes, A.W. Application of the Protection Motivation Theory to Genetic Testing for Breast Cancer Risk. Prev. Med. 2002, 35, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Bright, D.; Worley, M.; Porter, B.L. Patient Perceptions of Pharmacogenomic Testing in the Community Pharmacy Setting. Res. Social. Adm. Pharm. 2021, 17, 744–749. [Google Scholar] [CrossRef] [PubMed]
- Emanuel, A.S.; Kiviniemi, M.T.; Howell, J.L.; Hay, J.L.; Waters, E.A.; Orom, H.; Shepperd, J.A. Avoiding Cancer Risk Information. Soc. Sci. Med. 2015, 147, 113–120. [Google Scholar] [CrossRef]
- Godard, B.; Pratte, A.; Dumont, M.; Simard-Lebrun, A.; Simard, J. Factors Associated with an Individual’s Decision to Withdraw from Genetic Testing for Breast and Ovarian Cancer Susceptibility: Implications for Counseling. Genet. Test 2007, 11, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Meiser, B.; Collins, V.; Warren, R.; Gaff, C.; St John, D.J.B.; Young, M.-A.; Harrop, K.; Brown, J.; Halliday, J. Psychological Impact of Genetic Testing for Hereditary Non-Polyposis Colorectal Cancer. Clin. Genet. 2004, 66, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Gritz, E.R.; Peterson, S.K.; Vernon, S.W.; Marani, S.K.; Baile, W.F.; Watts, B.G.; Amos, C.I.; Frazier, M.L.; Lynch, P.M. Psychological Impact of Genetic Testing for Hereditary Nonpolyposis Colorectal Cancer. J. Clin. Oncol. 2005, 23, 1902–1910. [Google Scholar] [CrossRef]
- Michie, S.; French, D.P.; Marteau, T.M. Predictive Genetic Testing: Mediators and Moderators of Anxiety. Int. J. Behav. Med. 2002, 9, 309–321. [Google Scholar] [CrossRef]
- Lynch, J.; Parrott, A.; Hopkin, R.J.; Myers, M. Media Coverage of Direct-to-Consumer Genetic Testing. J. Genet. Couns. 2011, 20, 486–494. [Google Scholar] [CrossRef]
- Lowery, J.T.; Byers, T.; Axell, L.; Ku, L.; Jacobellis, J. The Impact of Direct-to-Consumer Marketing of Cancer Genetic Testing on Women According to Their Genetic Risk. Genet. Med. 2008, 10, 888–894. [Google Scholar] [CrossRef]
- Myers, M.F.; Chang, M.-H.; Jorgensen, C.; Whitworth, W.; Kassim, S.; Litch, J.A.; Armstrong, L.; Bernhardt, B.; Faucett, W.A.; Irwin, D.; et al. Genetic Testing for Susceptibility to Breast and Ovarian Cancer: Evaluating the Impact of a Direct-to-Consumer Marketing Campaign on Physicians’ Knowledge and Practices. Genet. Med. 2006, 8, 361–370. [Google Scholar] [CrossRef][Green Version]
- Goddard, K.A.B.; Moore, C.; Ottman, D.; Szegda, K.L.; Bradley, L.; Khoury, M.J. Awareness and Use of Direct-to-Consumer Nutrigenomic Tests, United States, 2006. Genet. Med. 2007, 9, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Mouchawar, J.; Laurion, S.; Ritzwoller, D.P.; Ellis, J.; Kulchak-Rahm, A.; Hensley-Alford, S. Assessing Controversial Direct-to-Consumer Advertising for Hereditary Breast Cancer Testing: Reactions from Women and Their Physicians in a Managed Care Organization. Am. J. Manag. Care 2005, 11, 601–608. [Google Scholar] [PubMed]
- Hennen, L.; Sauter, A.; Van Den Cruyce, E. Direct to Consumer Genetic Testing: Insights from an Internet Scan. New Genet. Soc. 2010, 29, 167–186. [Google Scholar] [CrossRef]
- Keller, M.; Jost, R.; Kadmon, M.; Wüllenweber, H.-P.; Haunstetter, C.M.; Willeke, F.; Jung, C.; Gebert, J.; Sutter, C.; Herfarth, C.; et al. Acceptance of and Attitude toward Genetic Testing for Hereditary Nonpolyposis Colorectal Cancer: A Comparison of Participants and Nonparticipants in Genetic Counseling. Dis. Colon Rectum 2004, 47, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Bellardita, L.; Colciago, R.R.; Frasca, S.; De Santis, M.C.; Gay, S.; Palorini, F.; La Rocca, E.; Valdagni, R.; Rancati, T.; Lozza, L. Breast cancer patient perspective on opportunities and challenges of a genetic test aimed to predict radio-induced side effects before treatment: Analysis of the Italian branch of the REQUITE project. Radiol. Med. 2021, 126, 1366–1373. [Google Scholar] [CrossRef]
- Spencer, S.A.; Rodgers, C.; Coffey, V. Factors Influencing Breast Cancer Genetic Testing Among High Risk African American Women: A Systematic Review. Internet J. Allied Health Sci. Pract. 2019, 17, 1. [Google Scholar] [CrossRef]
- Harris, A.; Kelly, S.E.; Wyatt, S. Counseling Customers: Emerging Roles for Genetic Counselors in the Direct-to-Consumer Genetic Testing Market. J. Genet. Couns. 2013, 22, 277–288. [Google Scholar] [CrossRef]
- Koeller, D.R.; Uhlmann, W.R.; Carere, D.A.; Green, R.C.; Roberts, J.S. PGen Study Group Utilization of Genetic Counseling after Direct-to-Consumer Genetic Testing: Findings from the Impact of Personal Genomics (PGen) Study. J. Genet. Couns. 2017, 26, 1270–1279. [Google Scholar] [CrossRef]
- Ruhl, G.L.; Hazel, J.W.; Clayton, E.W.; Malin, B.A. Public Attitudes Toward Direct to Consumer Genetic Testing. AMIA Annu. Symp. Proc. 2019, 2019, 774–783. [Google Scholar]
- Darst, B.F.; Madlensky, L.; Schork, N.J.; Topol, E.J.; Bloss, C.S. Perceptions of Genetic Counseling Services in Direct-to-Consumer Personal Genomic Testing. Clin. Genet. 2013, 84, 335–339. [Google Scholar] [CrossRef]
- The Role of the Genetic Counsellor: A Systematic Review of Research Evidence|European Journal of Human Genetics. Available online: https://www.nature.com/articles/ejhg2014116 (accessed on 14 October 2022).
- Allen, C.G.; McBride, C.M.; Haardörfer, R.; Roberts, M.C. Associations Between Objective Television Exposure and Cancer Perceptions in a National Sample of Adults. Cancer Control 2019, 26, 1073274819846603. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.C.; Turbitt, E.; Klein, W.M.P. Psychosocial, Attitudinal, and Demographic Correlates of Cancer-Related Germline Genetic Testing in the 2017 Health Information National Trends Survey. J. Community Genet. 2019, 10, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Persoskie, A.; Ferrer, R.A.; Klein, W.M.P. Association of Cancer Worry and Perceived Risk with Doctor Avoidance: An Analysis of Information Avoidance in a Nationally Representative US Sample. J. Behav. Med. 2014, 37, 977–987. [Google Scholar] [CrossRef] [PubMed]
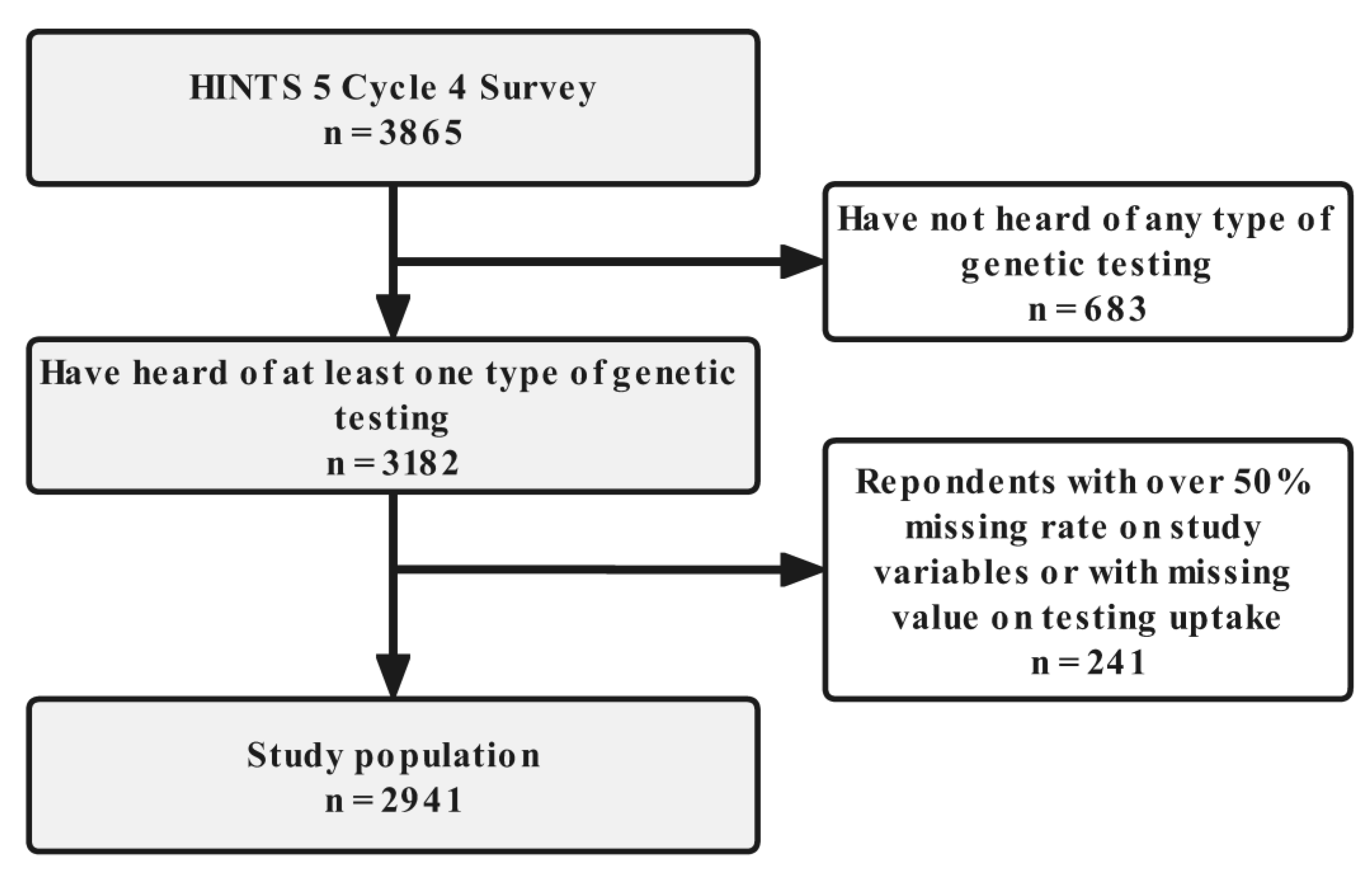

| Characteristics | Did Genetic Testing (n = 142) | Did Not (n = 2799) | Total (n = 2941) | p Value |
|---|---|---|---|---|
| Gender, n (%) | ||||
| Male | 30 (21.1) | 1154 (41.2) | 1184 (40.3) | <0.01 |
| Female | 112 (78.9) | 1645 (58.8) | 1757 (59.7) | |
| Age group, n (%) | ||||
| 18–34 | 10 (7.0) | 408 (14.6) | 418 (14.2) | |
| 35–49 | 32 (22.5) | 548 (19.6) | 580 (19.7) | 0.085 |
| 50–64 | 56 (39.4) | 912 (32.6) | 968 (32.9) | |
| 65–74 | 30 (21.1) | 619 (22.1) | 649 (22.1) | |
| 75 and above | 14 (9.9) | 312 (11.1) | 326 (11.1) | |
| BMI category, n (%) | ||||
| Underweight | 3 (2.1) | 37 (1.3) | 40 (1.4) | |
| Normal | 51 (35.9) | 852 (30.4) | 903 (30.7) | 0.340 |
| Overweight | 44 (31.0) | 914 (32.7) | 958 (32.6) | |
| Obesity | 44 (31.0) | 996 (35.6) | 1040 (35.4) | |
| Marital status, n (%) | ||||
| Married/Living together | 74 (52.1) | 1545 (55.2) | 1619 (55.0) | 0.071 |
| Divorced/Separated | 50 (35.2) | 761 (27.2) | 811 (27.6) | |
| Single/Never married | 18 (12.7) | 493 (17.6) | 511 (17.4) | |
| Location, n (%) | ||||
| Metropolitan | 127 (89.4) | 2459 (87.9) | 2586 (87.9) | |
| Micropolitan | 10 (7.0) | 201 (7.2) | 211 (7.2) | 0.961 |
| Small town | 4 (2.8) | 93 (3.3) | 97 (3.3) | |
| Rural | 1 (0.7) | 46 (1.6) | 47 (1.6) | |
| Race, n (%) | ||||
| Non-Hispanic White | 100 (70.4) | 1914 (68.4) | 2014 (68.5) | |
| Non-Hispanic Black | 20 (14.1) | 397 (14.2) | 417 (14.2) | 0.920 |
| Hispanic | 17 (12.0) | 360 (12.9) | 377 (12.8) | |
| Non-Hispanic other | 5 (3.5) | 128 (4.6) | 133 (4.5) | |
| Education, n (%) | ||||
| Less than High School | 12 (8.5) | 122 (4.4) | 134 (4.6) | |
| High School | 20 (14.1) | 442 (15.8) | 462 (15.7) | 0.053 |
| Some College | 45 (31.7) | 849 (30.2) | 891 (30.3) | |
| Bachelor | 30 (21.1) | 813 (29.0) | 843 (28.7) | |
| Post-Baccalaureate | 35 (24.6) | 576 (20.6) | 611 (20.8) | |
| Occupation, n (%) | ||||
| Employed | 76 (53.5) | 1516 (54.2) | 1592 (54.1) | |
| Unemployed | 4 (2.8) | 159 (5.7) | 163 (5.5) | 0.020 |
| Retired | 38 (26.8) | 855 (30.5) | 893 (30.4) | |
| Other | 24 (16.9) | 269 (9.6) | 293 (10.0) | |
| Income, n (%) | ||||
| Less than USD 20,000 | 24 (16.9) | 443 (15.8) | 467 (15.9) | |
| USD 20,000 to <USD 35,000 | 11 (7.7) | 301 (10.8) | 312 (10.6) | 0.843 |
| USD 35,000 to <USD 50,000 | 16 (11.3) | 328 (11.7) | 344 (11.7) | |
| USD 50,000 to <USD 75,000 | 24 (16.9) | 464 (16.6) | 488 (16.6) | |
| USD 75,000 or More | 67 (47.2) | 1263 (45.1) | 1330 (45.2) | |
| Insurance, n (%) | ||||
| Yes | 141 (99.3) | 2665 (95.2) | 2806 (95.4) | 0.021 |
| No | 1 (0.7) | 134 (4.8) | 135 (4.6) | |
| Smoke, n (%) | ||||
| Current | 15 (10.6) | 310 (11.1) | 325 (11.1) | 0.925 |
| Former | 37 (26.1) | 690 (24.7) | 727 (24.7) | |
| Never | 90 (63.4) | 1799 (64.3) | 1889 (64.2) | |
| Moderate drink, n (%) | ||||
| Yes | 106 (74.6) | 2215 (79.1) | 2321 (78.9) | 0.241 |
| No | 36 (25.4) | 584 (20.9) | 620 (21.1) | |
| Sufficient exercise per week, n (%) | ||||
| Yes | 55 (38.7) | 1074 (38.4) | 1129 (38.4) | 0.999 |
| No | 87 (61.3) | 1725 (61.6) | 1812 (61.6) | |
| General health score, n (%) | ||||
| Excellent | 18 (12.7) | 343 (12.3) | 361 (12.3) | |
| Very good | 44 (31.0) | 1062 (37.9) | 1106 (37.6) | 0.250 |
| Good | 57 (40.1) | 1008 (36.0) | 1065 (36.2) | |
| Fair | 17 (12.0) | 327 (11.7) | 344 (11.7) | |
| Poor | 6 (4.2) | 59 (2.1) | 65 (2.2) | |
| Family cancer history, n (%) | ||||
| Yes | 126 (88.7) | 2099 (75.0) | 2225 (75.7) | <0.01 |
| No | 9 (6.3) | 504 (18.0) | 513 (17.4) | |
| Not sure | 7 (4.9) | 196 (7.0) | 203 (6.9) | |
| Ever had cancer, n (%) | ||||
| Yes | 50 (35.2) | 423 (15.1) | 473 (16.1) | <0.01 |
| No | 92 (64.8) | 2376 (84.9) | 2468 (83.9) |
| Characteristics | Did Genetic Testing (n = 142) | Did Not (n = 2799) | Total (n = 2941) | p Value |
|---|---|---|---|---|
| Information source | ||||
| Newspaper, n (%) | ||||
| Yes | 29 (20.4) | 417 (14.9) | 446 (15.2) | 0.095 |
| No | 113 (79.6) | 2382 (85.1) | 2495 (84.8) | |
| Magazine, n (%) | ||||
| Yes | 31 (21.8) | 493 (17.6) | 524 (17.8) | 0.242 |
| No | 111 (78.2) | 2306 (82.4) | 2417 (82.2) | |
| Radio, n (%) | ||||
| Yes | 17 (12.0) | 464 (16.6) | 481 (16.4) | 0.183 |
| No | 125 (88.0) | 2335 (83.4) | 2460 (83.6) | |
| Healthcare provider, n (%) | ||||
| Yes | 85 (59.9) | 485 (17.3) | 570 (19.4) | <0.01 |
| No | 57 (40.1) | 2314 (82.7) | 2371 (80.6) | |
| Genetic counsellor, n (%) | ||||
| Yes | 39 (27.5) | 66 (2.4) | 105 (3.6) | <0.01 |
| No | 103 (72.5) | 2733 (97.6) | 2836 (96.4) | |
| Family member, n (%) | ||||
| Yes | 55 (38.7) | 1032 (36.9) | 1087 (37.0) | 0.712 |
| No | 87 (61.3) | 1767 (63.1) | 1854 (63.0) | |
| Friend, n (%) | ||||
| Yes | 38 (26.8) | 797 (28.5) | 835 (28.4) | 0.729 |
| No | 104 (73.2) | 2002 (71.5) | 2106 (71.6) | |
| Social media, n (%) | ||||
| Yes | 42 (29.6) | 921 (32.9) | 963 (32.7) | 0.464 |
| No | 100 (70.4) | 1878 (67.1) | 1978 (67.3) | |
| Television, n (%) | ||||
| Yes | 73 (51.4) | 1769 (63.2) | 1842 (62.6) | <0.01 |
| No | 69 (48.6) | 1030 (36.8) | 1099 (37.4) | |
| Internet, n (%) | ||||
| Yes | 74 (52.1) | 1369 (48.9) | 1443 (49.1) | 0.510 |
| No | 68 (47.9) | 1430 (51.1) | 1498 (50.9) |
| Characteristics | Did Genetic Testing (n = 142) | Did Not (n = 2799) | Total (n = 2941) | p Value |
|---|---|---|---|---|
| Perception | ||||
| Preventing cancer, n (%) | <0.001 | |||
| A lot | 91 (64.1) | 1285 (45.9) | 1376 (46.8) | |
| Somewhat | 38 (26.8) | 949 (33.9) | 987 (33.6) | |
| A little | 9 (6.3) | 395 (14.1) | 404 (13.7) | |
| Not at all | 4 (2.8) | 170 (6.1) | 174 (5.9) | |
| Detecting cancer, n (%) | <0.001 | |||
| A lot | 105 (73.9) | 1515 (54.1) | 1620 (55.1) | |
| Somewhat | 32 (22.5) | 868 (31.0) | 900 (30.6) | |
| A little | 3 (2.1) | 297 (10.6) | 300 (10.2) | |
| Not at all | 2 (1.4) | 119 (4.3) | 121 (4.1) | |
| Treating cancer, n (%) | <0.001 | |||
| A lot | 88 (62.0) | 1334 (47.7) | 1422 (48.4) | |
| Somewhat | 32 (22.5) | 884 (31.6) | 916 (31.1) | |
| A little | 9 (6.3) | 394 (14.1) | 403 (13.7) | |
| Not at all | 13 (9.2) | 187 (6.7) | 200 (6.8) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, X.; Huang, J.; Yi, Y.; Zhang, L.; Li, T.; Chen, Y. Factors Associated with the Uptake of Genetic Testing for Cancer Risks: A Pathway Analysis Using the Health Information National Trends Survey Data. Life 2022, 12, 2024. https://doi.org/10.3390/life12122024
Dong X, Huang J, Yi Y, Zhang L, Li T, Chen Y. Factors Associated with the Uptake of Genetic Testing for Cancer Risks: A Pathway Analysis Using the Health Information National Trends Survey Data. Life. 2022; 12(12):2024. https://doi.org/10.3390/life12122024
Chicago/Turabian StyleDong, Xiangning, Jingxian Huang, Yanze Yi, Lanwei Zhang, Tenglong Li, and Ying Chen. 2022. "Factors Associated with the Uptake of Genetic Testing for Cancer Risks: A Pathway Analysis Using the Health Information National Trends Survey Data" Life 12, no. 12: 2024. https://doi.org/10.3390/life12122024
APA StyleDong, X., Huang, J., Yi, Y., Zhang, L., Li, T., & Chen, Y. (2022). Factors Associated with the Uptake of Genetic Testing for Cancer Risks: A Pathway Analysis Using the Health Information National Trends Survey Data. Life, 12(12), 2024. https://doi.org/10.3390/life12122024








