Age-Dependent Decline in Common Femoral Artery Flow-Mediated Dilation and Wall Shear Stress in Healthy Subjects
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Study Design and Protocol
2.3. FMD and Blood Flow Analysis
2.4. Blood Pressure, Pulse Wave Velocity and ABPI Measurements
2.5. Statistical Analysis and Sample Size Calculation
3. Results
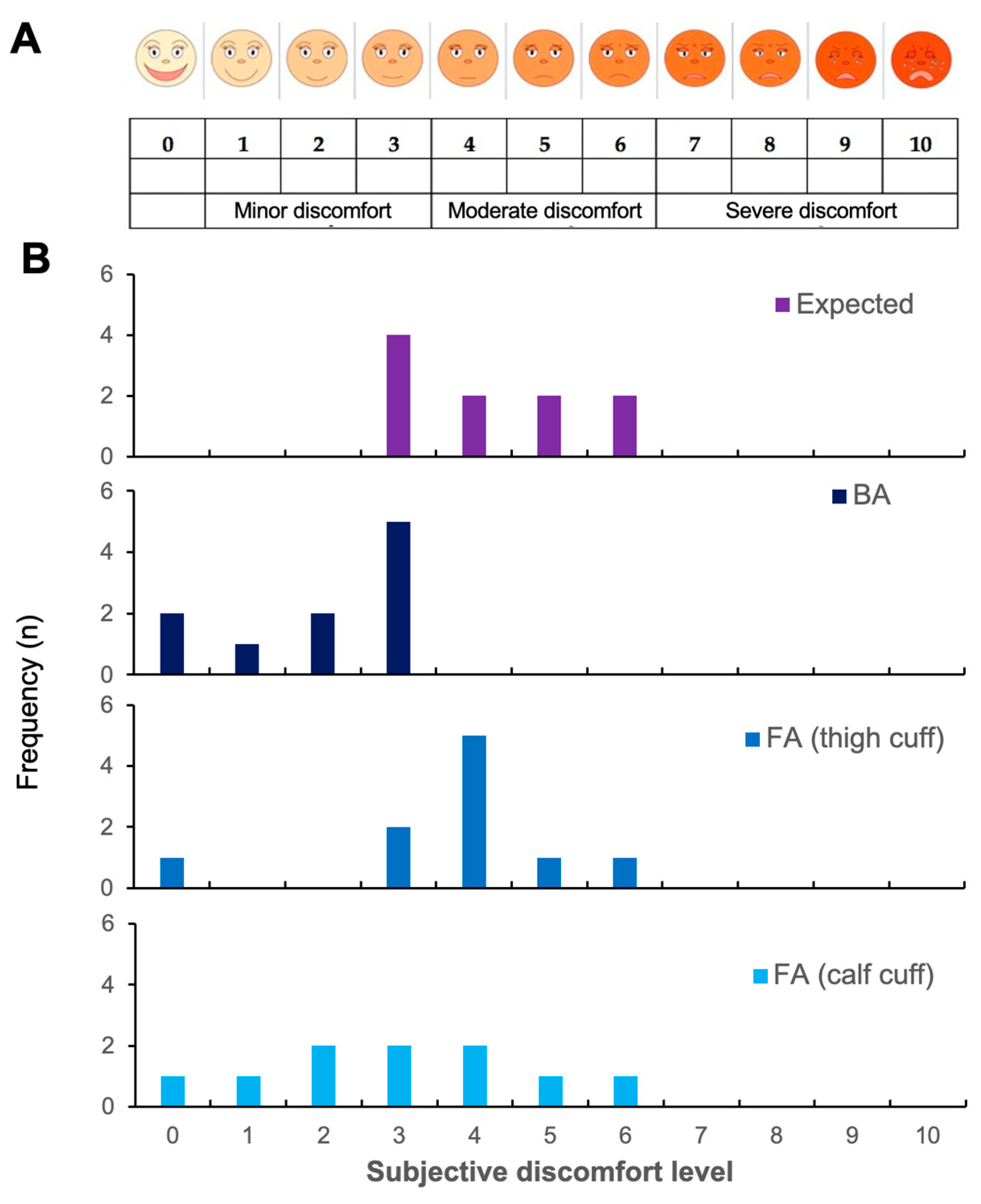
3.1. Reproducibility and Acceptability
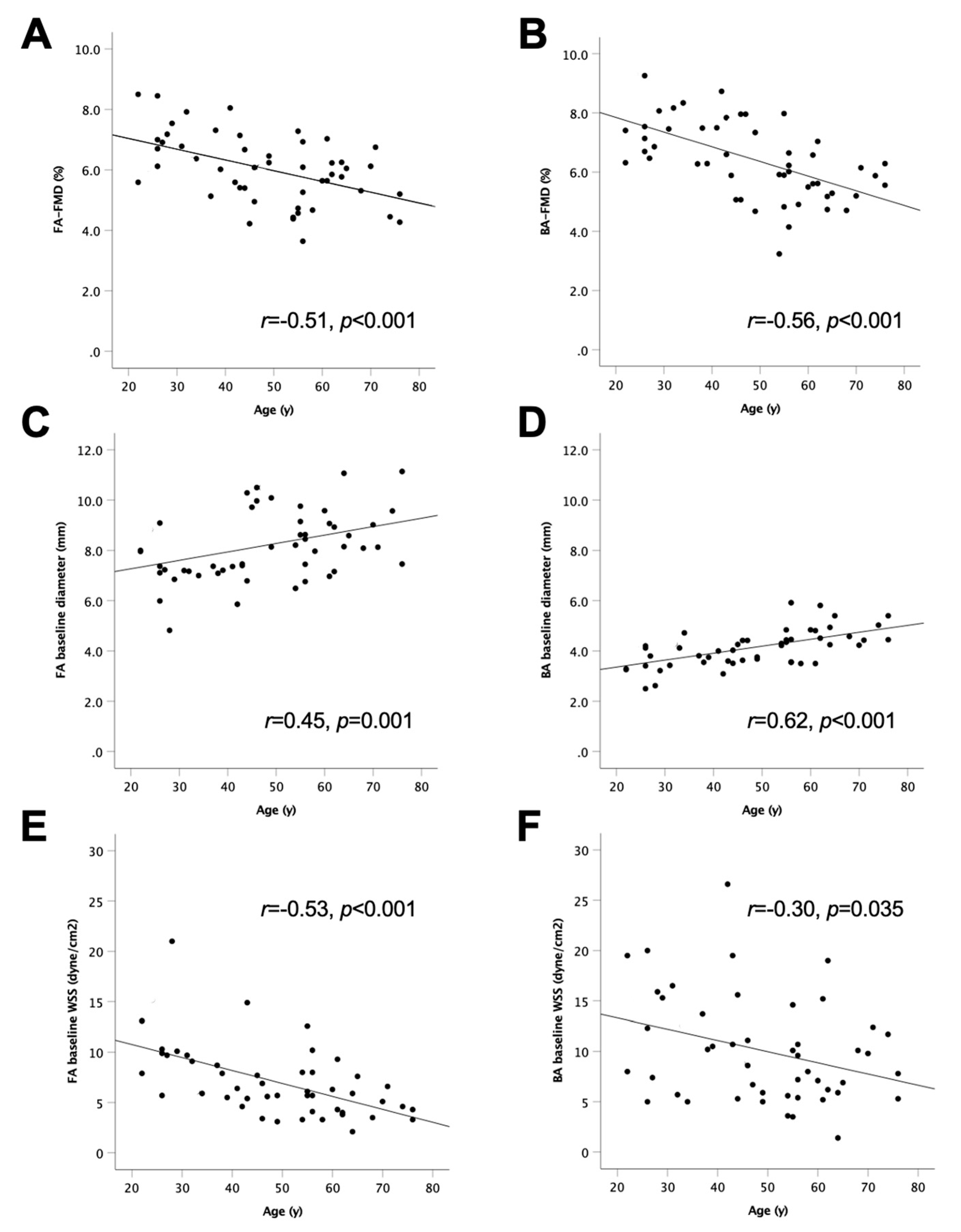
3.2. Age-Dependent Decline in FA-FMD and BA-FMD
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Song, P.; Rudan, D.; Zhu, Y.; Fowkes, F.J.I.; Rahimi, K.; Fowkes, F.G.R.; Rudan, I. Global, regional, and national prevalence and risk factors for peripheral artery disease in 2015: An updated systematic review and analysis. Lancet Glob. Health 2019, 7, e1020–e1030. [Google Scholar] [CrossRef] [PubMed]
- Stoberock, K.; Kaschwich, M.; Nicolay, S.S.; Mahmoud, N.; Heidemann, F.; Rieß, H.C.; Debus, E.S.; Behrendt, C.-A. The interrelationship between diabetes mellitus and peripheral arterial disease—A systematic review. Vasa 2021, 50, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration—About Biomarkers and Qualification. Available online: https://www.fda.gov/drugs/biomarker-qualification-program/about-biomarkers-and-qualification (accessed on 1 November 2022).
- Augustin, H.G.; Koh, G.Y. Organotypic vasculature: From descriptive heterogeneity to functional pathophysiology. Science 2017, 357, eaal2379. [Google Scholar] [CrossRef] [PubMed]
- Heinen, Y.; Stegemann, E.; Sansone, R.; Benedens, K.; Wagstaff, R.; Balzer, J.; Rassaf, T.; Lauer, T.; Kelm, M.; Heiss, C. Local association between endothelial dysfunction and intimal hyperplasia: Relevance in peripheral artery disease. J. Am. Heart Assoc. 2015, 4, e001472. [Google Scholar] [CrossRef] [PubMed]
- Bapir, M.; Untracht, G.R.; Cooke, D.; McVey, J.H.; Skene, S.S.; Campagnolo, P.; Whyte, M.B.; Dikaios, N.; Rodriguez-Mateos, A.; Sampson, D.D.; et al. Cocoa flavanol consumption improves lower extremity endothelial function in healthy individuals and people with type 2 diabetes. Food Funct. 2022, 13, 10439–10448. [Google Scholar] [CrossRef]
- Heiss, C.; Rodriguez-Mateos, A.; Bapir, M.; Skene, S.S.; Sies, H.; Kelm, M. Flow-mediated dilation reference values for evaluation of endothelial function and cardiovascular health. Cardiovasc. Res. 2022, cvac095. [Google Scholar] [CrossRef]
- Heiss, C.; Keymel, S.; Niesler, U.; Ziemann, J.; Kelm, M.; Kalka, C. Impaired progenitor cell activity in age-related endothelial dysfunction. J. Am. Coll.Cardiol. 2005, 45, 1441–1448. [Google Scholar] [CrossRef]
- Frank, U.; Nikol, S.; Belch, J.; Boc, V.; Brodmann, M.; Carpentier, P.H.; Chraim, A.; Canning, C.; Dimakakos, E.; Gottsater, A.; et al. ESVM Guideline on peripheral arterial disease. Vasa 2019, 48, 1–79. [Google Scholar] [CrossRef]
- Thijssen, D.H.J.; Bruno, R.M.; van Mil, A.; Holder, S.M.; Faita, F.; Greyling, A.; Zock, P.L.; Taddei, S.; Deanfield, J.E.; Luscher, T.; et al. Expert consensus and evidence-based recommendations for the assessment of flow-mediated dilation in humans. Eur. Heart J. 2019, 40, 2534–2547. [Google Scholar] [CrossRef]
- Sansone, R.; Stanske, B.; Keymel, S.; Schuler, D.; Horn, P.; Saeed, D.; Boeken, U.; Westenfeld, R.; Lichtenberg, A.; Kelm, M.; et al. Macrovascular and microvascular function after implantation of left ventricular assist devices in end-stage heart failure: Role of microparticles. J. Heart Lung Transplant. 2015, 34, 921–932. [Google Scholar] [CrossRef]
- Salazar, M.R.; Espeche, W.G.; Aizpurua, M.; Sisnieguez, C.E.; Sisnieguez, B.C.; Dulbecco, C.A.; March, C.E.; Stavile, R.N.; Ferrari, E.H.; Correa, M.; et al. Should the first blood pressure reading be discarded? J. Hum. Hypertens. 2015, 29, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, K.S.; Gotlieb, A.I. The role of shear stress in the pathogenesis of atherosclerosis. Lab. Investig. 2005, 85, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Peretz, A.; Leotta, D.F.; Sullivan, J.H.; Trenga, C.A.; Sands, F.N.; Aulet, M.R.; Paun, M.; Gill, E.A.; Kaufman, J.D. Flow mediated dilation of the brachial artery: An investigation of methods requiring further standardization. BMC Cardiovasc. Disord. 2007, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.N.; Slysz, J.T.; King, T.J.; Coates, A.M.; King, R.T.; Burr, J.F. Blood flow restriction in the presence or absence of muscle contractions does not preserve vasculature structure and function following 14-days of limb immobilization. Eur. J. Appl. Physiol. 2021, 121, 2437–2447. [Google Scholar] [CrossRef] [PubMed]
- Roberts, K.A.; Draijer, R.; Hopkins, N.D.; de Graaf, Y.; Holder, S.M.; Carter, S.E.; Thijssen, D.H.J.; Low, D.A. Impact of green tea on the deleterious cardiometabolic effects of 7-days unhealthy lifestyle in young healthy males. Physiol. Rep. 2021, 9, e14720. [Google Scholar] [CrossRef]
- Naylor, L.H.; Spence, A.L.; Donker, S.C.M.; Thijssen, D.H.J.; Green, D.J. Is there an athlete’s artery? A comparison of brachial and femoral artery structure and function in male strength, power and endurance athletes. J. Sci. Med. Sport 2021, 24, 635–640. [Google Scholar] [CrossRef]
- Taylor, F.C.; Dunstan, D.W.; Homer, A.R.; Dempsey, P.C.; Kingwell, B.A.; Climie, R.E.; Owen, N.; Cohen, N.D.; Larsen, R.N.; Grace, M.; et al. Acute effects of interrupting prolonged sitting on vascular function in type 2 diabetes. Am. J. Physiol. Heart Circ. Physiol 2021, 320, H393–H403. [Google Scholar] [CrossRef]
- Braith, R.W.; Conti, C.R.; Nichols, W.W.; Choi, C.Y.; Khuddus, M.A.; Beck, D.T.; Casey, D.P. Enhanced external counterpulsation improves peripheral artery flow-mediated dilation in patients with chronic angina: A randomized sham-controlled study. Circulation 2010, 122, 1612–1620. [Google Scholar] [CrossRef]
- Holder, S.M.; Bruno, R.M.; Shkredova, D.A.; Dawson, E.A.; Jones, H.; Hopkins, N.D.; Hopman, M.T.E.; Bailey, T.G.; Coombes, J.S.; Askew, C.D.; et al. Reference intervals for brachial artery flow-mediated dilation and the relation with cardiovascular risk factors. Hypertension 2021, 77, 1469–1480. [Google Scholar] [CrossRef]
- Caro, C.G. Discovery of the role of wall shear in atherosclerosis. Arter. Thromb. Vasc. Biol. 2009, 29, 158–161. [Google Scholar] [CrossRef]
- Mahler, G.J.; Frendl, C.M.; Cao, Q.; Butcher, J.T. Effects of shear stress pattern and magnitude on mesenchymal transformation and invasion of aortic valve endothelial cells. Biotechnol. Bioeng. 2014, 111, 2326–2337. [Google Scholar] [CrossRef] [PubMed]
- Avis, S.R.; Vernon, S.T.; Hagstrom, E.; Figtree, G.A. Coronary artery disease in the absence of traditional risk factors: A call for action. Eur. Heart J. 2021, 42, 3822–3824. [Google Scholar] [CrossRef] [PubMed]
- Bapir, M.; Campagnolo, P.; Rodriguez-Mateos, A.; Skene, S.S.; Heiss, C. Assessing variability in vascular response to cocoa with personal devices: A series of double-blind randomized crossover n-of-1 trials. Front. Nutr. 2022, 9, 886597. [Google Scholar] [CrossRef]
- Domagala, T.B.; Szeffler, A.; Dobrucki, L.W.; Dropinski, J.; Polanski, S.; Leszczynska-Wiloch, M.; Kotula-Horowitz, K.; Wojciechowski, J.; Wojnowski, L.; Szczeklik, A.; et al. Nitric oxide production and endothelium-dependent vasorelaxation ameliorated by N1-methylnicotinamide in human blood vessels. Hypertension 2012, 59, 825–832. [Google Scholar] [CrossRef]
- Modena, M.G.; Bonetti, L.; Coppi, F.; Bursi, F.; Rossi, R. Prognostic role of reversible endothelial dysfunction in hypertensive postmenopausal women. J. Am. Coll. Cardiol. 2002, 40, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Vlachopoulos, C.; Xaplanteris, P.; Aboyans, V.; Brodmann, M.; Cifkova, R.; Cosentino, F.; De Carlo, M.; Gallino, A.; Landmesser, U.; Laurent, S.; et al. The role of vascular biomarkers for primary and secondary prevention. A position paper from the European Society of Cardiology Working Group on peripheral circulation: Endorsed by the Association for Research into Arterial Structure and Physiology (ARTERY) Society. Atherosclerosis 2015, 241, 507–532. [Google Scholar] [CrossRef] [PubMed]
- Yamakoshi, T.; Rolfe, P.; Yamakoshi, K.I. Peripheral arterial elasticity changes derived by volume-oscillometry in reaction to hyperemia as a possible assessment of flow-mediated vasodilatation. Sci. Rep. 2022, 12, 19479. [Google Scholar] [CrossRef] [PubMed]

| A | n (m/f) | 2/8 |
| Age | 28 ± 7 | |
| Height (m) | 1.68 ± 0.10 | |
| Weight (kg) | 72.0 ± 15.0 | |
| BMI (kg/m2) | 25.5 ± 5.6 | |
| Smoker (n) | 5 | |
| Systolic blood pressure (mmHg) | 118.0 ± 2.3 | |
| Diastolic blood pressure (mmHg) | 75.0 ± 1.9 | |
| B | n (m/f) | 4/6 |
| Age | 53 ± 10 | |
| Height (m) | 1.73 ± 0.11 | |
| Weight (kg) | 70.5 ± 12.7 | |
| BMI (kg/m2) | 23.6 ± 3.9 | |
| Smoker (n) | 0 | |
| Systolic blood pressure (mmHg) | 114.7 ± 11.3 | |
| Diastolic blood pressure (mmHg) | 73.3 ± 9.8 |
| A | n (m/f) | 23/27 | ||
| Age | 48 ± 15 | |||
| Height (m) | 1.73 ± 0.10 | |||
| Weight (kg) | 70.0 ±12.2 | |||
| BMI (kg/m2) | 24.0 ± 3.4 | |||
| Smoker (n) | 0 | |||
| Systolic blood pressure (mmHg) | 118.5 ± 14.7 | |||
| Diastolic blood pressure (mmHg) | 71.0 ± 10.5 | |||
| Ankle brachial pressure index | 0.99 ± 0.09 | |||
| B | FA | BA | p | |
| FMD (%) | 6.0 ± 1.1 | 6.4 ± 1.3 | 0.030 | |
| Baseline diameter (mm) | 8.11 ± 1.36 | 4.12 ± 0.74 | <0.001 | |
| Baseline mean flow velocity (cm/s) | 19.4 ± 7.9 | 14.4 ± 6.4 | <0.001 | |
| Baseline flow rate (mL/min) | 607 ± 296 | 119 ± 61 | <0.001 | |
| Baseline WSS (dyne/cm2) | 7.0 ± 3.5 | 10.2 ± 5.3 | <0.001 | |
| Baseline SR (/s) | 20.0 ± 10.1 | 29.0 ± 15.1 | <0.001 | |
| PORH mean flow velocity (cm/s) | 49.7 ± 17.0 | 71.2 ± 25.4 | <0.001 | |
| PORH flow rate (mL/min) | 1703 ± 658 | 689 ± 365 | <0.001 | |
| PORH WSS (dyne/cm2) | 18.1 ± 8.4 | 48.9 ± 18.7 | <0.001 | |
| PORH SR (/s) | 51.7 ± 24.1 | 139.8 ± 53.4 | <0.001 | |
| PORH flow reserve | 3.1 ± 1.1 | 7.2 ± 7.0 | <0.001 |
| FA-FMD | BA-FMD | Age | Height | Weight | BMI | SBP | DBP | FA Baseline Diameter | FA Baseline Flow Rate | FA Baseline WSS | BA Baseline Diameter | BA Baseline Flow Rate | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BA-FMD | r = 0.61, p < 0.001 | ||||||||||||
| Age | r = −0.51, p < 0.001 | r = −0.56,p < 0.001 | |||||||||||
| Height | r = −0.04, p = 0.815 | r = 0.17, p = 0.273 | r = 0.06, p = 0.719 | ||||||||||
| Weight | r = −0.05, p = 0.745 | r = −0.06, p = 0.700 | r = 0.13, p = 0.394 | r = 0.58, p < 0.001 | |||||||||
| BMI | r = −0.01, p = 0.932 | r = −0.18, p = 0.217 | r = 0.20, p = 0.154 | r = −0.01, p = 0.931 | r = 0.79, p < 0.001 | ||||||||
| SBP | r = −0.13, p = 0.379 | r = −0.02, p = 0.894 | r = 0.29, p = 0.042 | r = 0.34, p = 0.024 | r = 0.51, p < 0.001 | r = 0.39, p = 0.005 | |||||||
| DBP | r = −0.27, p = 0.055 | r = −0.06, p = 0.691 | r = 0.30, p = 0.032 | r = 0.37, p = 0.012 | r = 0.38, p = 0.010 | r = 0.22, p = 0.132 | r = 0.80, p < 0.001 | ||||||
| FA baseline diameter | r = −0.40, p = 0.004 | r = −0.30,p = 0.032 | r = 0.45, p = 0.001 | r = 0.42, p = 0.004 | r = 0.27, p = 0.153 | r = −0.05, p = 0.709 | r = 0.27, p = 0.055 | r = 0.24, p = 0.099 | |||||
| FA baseline flow rate | r = −0.06, p = 0.666 | r = 0.08, p = 0.568 | r = −0.02, p = 0.871 | r = 0.37, p = 0.015 | r = −0.08, p = 0.629 | r = −0.22, p = 0.129 | r = 0.14, p = 0.330 | r = 0.17, p = 0.234 | r = 0.51, p < 0.001 | ||||
| FA baseline WSS | r = 0.38, p = 0.008 | r = 0.32, p = 0.026 | r = −0.53,p < 0.001 | r = −0.13, p = 0.418 | r = −0.26, p = 0.098 | r = −0.13, p = 0.373 | r = −0.10, p = 0.484 | r = −0.06, p = 0.695 | r = −0.54, p < 0.001 | r = 0.32, p = 0.024 | |||
| BA baseline diameter | r = −0.43, p = 0.002 | r = −0.42,p = 0.003 | r = 0.62, p < 0.001 | r = 0.40, p = 0.006 | r = 0.39, p = 0.008 | r = 0.29, p = 0.042 | r = 0.34, p = 0.014 | r = 0.35, p = 0.012 | r = 0.43, p = 0.002 | r = 0.18, p = 0.217 | r = −0.37,p = 0.010 | ||
| BA baseline flow rate | r = −0.27, p = 0.062 | r = 0.05, p = 0.727 | r = 0.37, p = 0.010 | r = 0.47, p = 0.001 | r = 0.41, p = 0.006 | r = 0.21, p = 0.150 | r = 0.28, p = 0.050 | r = 0.34, p = 0.016 | r = 0.26, p = 0.078 | r = 0.26, p = 0.081 | r = −0.14, p = 0.339 | r = 0.59, p < 0.001 | |
| BA baseline WSS | r = 0.09, p = 0.539 | r = 0.51, p < 0.001 | r = −0.30,p = 0.035 | r = −0.01, p = 0.953 | r = 0.06, p = 0.681 | r = −0.11, p = 0.472 | r = −0.09, p = 0.550 | r = 0.07, p = 0.611 | r = −0.26, p = 0.073 | r = 0.00, p = 0.987 | r = 0.24, p = 0.098 | r = −0.41,p = 0.004 | r = 0.40, p = 0.005 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bapir, M.; Untracht, G.R.; Hunt, J.E.A.; McVey, J.H.; Harris, J.; Skene, S.S.; Campagnolo, P.; Dikaios, N.; Rodriguez-Mateos, A.; Sampson, D.D.; et al. Age-Dependent Decline in Common Femoral Artery Flow-Mediated Dilation and Wall Shear Stress in Healthy Subjects. Life 2022, 12, 2023. https://doi.org/10.3390/life12122023
Bapir M, Untracht GR, Hunt JEA, McVey JH, Harris J, Skene SS, Campagnolo P, Dikaios N, Rodriguez-Mateos A, Sampson DD, et al. Age-Dependent Decline in Common Femoral Artery Flow-Mediated Dilation and Wall Shear Stress in Healthy Subjects. Life. 2022; 12(12):2023. https://doi.org/10.3390/life12122023
Chicago/Turabian StyleBapir, Mariam, Gavrielle R. Untracht, Julie E. A. Hunt, John H. McVey, Jenny Harris, Simon S. Skene, Paola Campagnolo, Nikolaos Dikaios, Ana Rodriguez-Mateos, David D. Sampson, and et al. 2022. "Age-Dependent Decline in Common Femoral Artery Flow-Mediated Dilation and Wall Shear Stress in Healthy Subjects" Life 12, no. 12: 2023. https://doi.org/10.3390/life12122023
APA StyleBapir, M., Untracht, G. R., Hunt, J. E. A., McVey, J. H., Harris, J., Skene, S. S., Campagnolo, P., Dikaios, N., Rodriguez-Mateos, A., Sampson, D. D., Sampson, D. M., & Heiss, C. (2022). Age-Dependent Decline in Common Femoral Artery Flow-Mediated Dilation and Wall Shear Stress in Healthy Subjects. Life, 12(12), 2023. https://doi.org/10.3390/life12122023









