Promiscuous Receptors and Neuroinflammation: The Formyl Peptide Class
Abstract
:1. Introduction
2. Inflammation
3. The Formyl Peptide Receptors
4. Cellular Expression of FPRs in the Central Nervous System
5. Roles of FPR1 in Neuroinflammation and Neurodegeneration
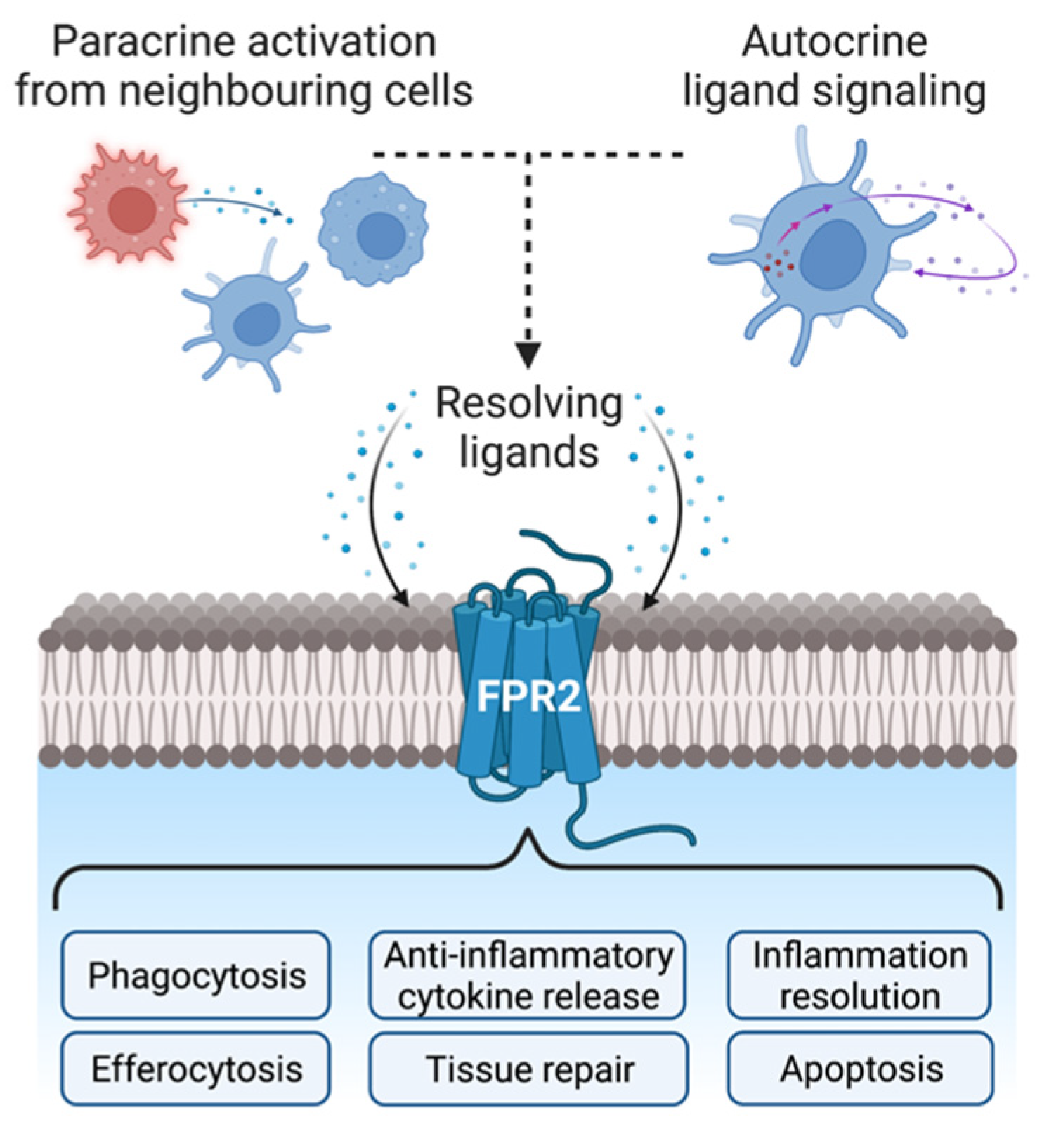
6. Roles of FPR2 in Neuroinflammation and Neurodegeneration
7. Expression Patterns of Endogenous FPR Ligand Annexin A1
8. Considerations for Therapeutic Development
9. Overall Conclusion
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wickstead, E.S.; Karim, H.A.; Manuel, R.E.; Biggs, C.S.; Getting, S.J.; McArthur, S. Reversal of β-Amyloid-Induced Microglial Toxicity In Vitro by Activation of Fpr2/3. Oxid. Med. Cell. Longev. 2020, 2020, 2139192. [Google Scholar] [CrossRef] [PubMed]
- Ye, R.D.; Boulay, F.; Wang, J.M.; Dahlgren, C.; Gerard, C.; Parmentier, M.; Serhan, C.N.; Murphy, P.M. International Union of Basic and Clinical Pharmacology. LXXIII. Nomenclature for the Formyl Peptide Receptor (FPR) Family. Pharmacol. Rev. 2009, 61, 119–161. [Google Scholar] [CrossRef] [PubMed]
- Hartt, J.K.; Barish, G.; Murphy, P.M.; Gao, J.L. N-formylpeptides induce two distinct concentration optima for mouse neutrophil chemotaxis by differential interaction with two N-formylpeptide receptor (FPR) subtypes. Molecular characterization of FPR2, A second mouse neutrophil FPR. J. Exp. Med. 1999, 190, 741–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wickstead, E.S.; Irving, M.A.; Getting, S.J.; McArthur, S. Exploiting formyl peptide receptor 2 to promote microglial resolution: A new approach to Alzheimer’s disease treatment. FEBS J. 2021, 289, 1801–1822. [Google Scholar] [CrossRef]
- McArthur, S.; Yazid, S.; Christian, H.; Sirha, R.; Flower, R.; Buckingham, J.; Solito, E. Annexin A1 regulates hormone exocytosis through a mechanism involving actin reorganization. FASEB J. 2009, 23, 4000–4010. [Google Scholar] [CrossRef]
- Sansbury, B.E.; Spite, M. Resolution of Acute Inflammation and the Role of Resolvins in Immunity, Thrombosis, and Vascular Biology. Circ. Res. 2016, 119, 113–130. [Google Scholar] [CrossRef]
- Iwasaki, A.; Medzhitov, R. Control of adaptive immunity by the innate immune system. Nat. Immunol. 2015, 16, 343–353. [Google Scholar] [CrossRef]
- Leng, F.; Edison, P. Neuroinflammation and microglial activation in Alzheimer disease: Where do we go from here? Nat. Rev. Neurol. 2021, 17, 157–172. [Google Scholar] [CrossRef]
- Lavelle, A.; Sokol, H. Gut microbiota-derived metabolites as key actors in inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 223–237. [Google Scholar] [CrossRef]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- McArthur, S.; Juban, G.; Gobbetti, T.; Desgeorges, T.; Theret, M.; Gondin, J.; Toller-Kawahisa, J.E.; Reutelingsperger, C.P.; Chazaud, B.; Perretti, M.; et al. Annexin A1 drives macrophage skewing to accelerate muscle regeneration through AMPK activation. J. Clin. Investig. 2020, 130, 1156–1167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, H.Q.; Ye, R.D. The Formyl Peptide Receptors: Diversity of Ligands and Mechanism for Recognition. Molecules 2017, 22, 455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhuang, Y.; Liu, H.; Edward Zhou, X.; Kumar Verma, R.; de Waal, P.W.; Jang, W.; Xu, T.-H.; Wang, L.; Meng, X.; Zhao, G.; et al. Structure of formylpeptide receptor 2-Gi complex reveals insights into ligand recognition and signaling. Nat. Commun. 2020, 11, 885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durstin, M.; Gao, J.L.; Tiffany, H.L.; McDermott, D.; Murphy, P.M. Differential expression of members of the N-formylpeptide receptor gene cluster in human phagocytes. Biochem. Biophys. Res. Commun. 1994, 201, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Kwon, W.Y.; Suh, G.J.; Jung, Y.S.; Park, S.M.; Oh, S.; Kim, S.H.; Lee, A.R.; Kim, J.Y.; Kim, H.; Kim, K.A.; et al. Circulating mitochondrial N-formyl peptides contribute to secondary nosocomial infection in patients with septic shock. Proc. Natl. Acad. Sci. USA 2021, 118, e2018538118. [Google Scholar] [CrossRef]
- Liang, W.; Chen, K.; Gong, W.; Yoshimura, T.; Le, Y.; Wang, Y.; Wang, J.M. The Contribution of Chemoattractant GPCRs, Formylpeptide Receptors, to Inflammation and Cancer. Front. Endocrinol. 2020, 11, 17. [Google Scholar] [CrossRef] [Green Version]
- Cattaneo, F.; Castaldo, M.; Parisi, M.; Faraonio, R.; Esposito, G.; Ammendola, R. Formyl Peptide Receptor 1 Modulates Endothelial Cell Functions by NADPH Oxidase-Dependent VEGFR2 Transactivation. Oxid. Med. Cell. Longev. 2018, 2018, 2609847. [Google Scholar] [CrossRef] [Green Version]
- Pessolano, E.; Belvedere, R.; Novizio, N.; Filippelli, A.; Perretti, M.; Whiteford, J.; Petrella, A. Mesoglycan connects Syndecan-4 and VEGFR2 through Annexin A1 and formyl peptide receptors to promote angiogenesis in vitro. FEBS J. 2021, 288, 6428–6446. [Google Scholar] [CrossRef]
- Lee, C.; Kim, J.; Han, J.; Oh, D.; Kim, M.; Jeong, H.; Kim, T.-J.; Kim, S.-W.; Kim, J.N.; Seo, Y.-S.; et al. Formyl peptide receptor 2 determines sex-specific differences in the progression of nonalcoholic fatty liver disease and steatohepatitis. Nat. Commun. 2022, 13, 578. [Google Scholar] [CrossRef]
- Panaro, M.A.; Acquafredda, A.; Sisto, M.; Lisi, S.; Maffione, A.B.; Mitolo, V. Biological Role of the N-Formyl Peptide Receptors. Immunopharmacol. Immunotoxicol. 2006, 28, 103–127. [Google Scholar] [CrossRef]
- Lee, Y.B.; Nagai, A.; Kim, S.U. Cytokines, chemokines, and cytokine receptors in human microglia. J. Neurosci. Res. 2002, 69, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Showell, H.J.; Freer, R.J.; Zigmond, S.H.; Schiffmann, E.; Aswanikumar, S.; Corcoran, B.; Becker, E.L. The structure-activity relations of synthetic peptides as chemotactic factors and inducers of lysosomal secretion for neutrophils. J. Exp. Med. 1976, 143, 1154–1169. [Google Scholar] [CrossRef] [Green Version]
- Cuomo, P.; Papaianni, M.; Capparelli, R.; Medaglia, C. The Role of Formyl Peptide Receptors in Permanent and Low-Grade Inflammation: Helicobacter pylori Infection as a Model. Int. J. Mol. Sci. 2021, 22, 3706. [Google Scholar] [CrossRef] [PubMed]
- Weiß, E.; Kretschmer, D. Formyl-Peptide Receptors in Infection, Inflammation, and Cancer. Trends Immunol. 2018, 39, 815–829. [Google Scholar] [CrossRef]
- Quehenberger, O.; Prossnitz, E.R.; Cavanagh, S.L.; Cochrane, C.G.; Ye, R.D. Multiple domains of the N-formyl peptide receptor are required for high-affinity ligand binding. Construction and analysis of chimeric N-formyl peptide receptors. J. Biol. Chem. 1993, 268, 18167–18175. [Google Scholar] [CrossRef] [PubMed]
- Koo, C.; Lefkowitz, R.J.; Snyderman, R. The oligopeptide chemotactic factor receptor on human polymorphonuclear leukocyte membranes exists in two affinity states. Biochem. Biophys. Res. Commun. 1982, 106, 442–449. [Google Scholar] [CrossRef]
- Southgate, E.L.; He, R.L.; Gao, J.-L.; Murphy, P.M.; Nanamori, M.; Ye, R.D. Identification of formyl peptides from Listeria monocytogenes and Staphylococcus aureus as potent chemoattractants for mouse neutrophils. J. Immunol. 2008, 181, 1429–1437. [Google Scholar] [CrossRef] [Green Version]
- He, H.-Q.; Liao, D.; Wang, Z.-G.; Wang, Z.-L.; Zhou, H.-C.; Wang, M.-W.; Ye, R.D. Functional characterization of three mouse formyl peptide receptors. Mol. Pharmacol. 2013, 83, 389–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bufe, B.; Schumann, T.; Kappl, R.; Bogeski, I.; Kummerow, C.; Podgórska, M.; Smola, S.; Hoth, M.; Zufall, F. Recognition of bacterial signal peptides by mammalian formyl peptide receptors: A new mechanism for sensing pathogens. J. Biol. Chem. 2015, 290, 7369–7387. [Google Scholar] [CrossRef] [Green Version]
- Rabiet, M.-J.; Huet, E.; Boulay, F. Human mitochondria-derived N-formylated peptides are novel agonists equally active on FPR and FPRL1, while Listeria monocytogenes-derived peptides preferentially activate FPR. Eur. J. Immunol. 2005, 35, 2486–2495. [Google Scholar] [CrossRef]
- Seki, T.; Fukamizu, A.; Kiso, Y.; Mukai, H. Mitocryptide-2, a neutrophil-activating cryptide, is a specific endogenous agonist for formyl-peptide receptor-like 1. Biochem. Biophys. Res. Commun. 2011, 404, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Lind, S.; Gabl, M.; Holdfeldt, A.; Mårtensson, J.; Sundqvist, M.; Nishino, K.; Dahlgren, C.; Mukai, H.; Forsman, H. Identification of Residues Critical for FPR2 Activation by the Cryptic Peptide Mitocryptide-2 Originating from the Mitochondrial DNA-Encoded Cytochrome b. J. Immunol. 2019, 202, 2710–2719. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Fang, D.; Hou, X.; Le, Y.; Fang, J.; Wen, F.; Gong, W.; Chen, K.; Wang, J.M.; Su, S.B. HCV peptide (C5A), an amphipathic α-helical peptide of hepatitis virus C, is an activator of N-formyl peptide receptor in human phagocytes. J. Immunol. 2011, 186, 2087–2094. [Google Scholar] [CrossRef] [Green Version]
- Bellner, L.; Thorén, F.; Nygren, E.; Liljeqvist, J.-A.; Karlsson, A.; Eriksson, K. A proinflammatory peptide from herpes simplex virus type 2 glycoprotein G affects neutrophil, monocyte, and NK cell functions. J. Immunol. 2005, 174, 2235–2241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Betten, Å.; Bylund, J.; Cristophe, T.; Boulay, F.; Romero, A.; Hellstrand, K.; Dahlgren, C. A proinflammatory peptide from Helicobacter pylori activates monocytes to induce lymphocyte dysfunction and apoptosis. J. Clin. Investig. 2001, 108, 1221–1228. [Google Scholar] [CrossRef]
- Tiffany, H.L.; Lavigne, M.C.; Cui, Y.H.; Wang, J.M.; Leto, T.L.; Gao, J.L.; Murphy, P.M. Amyloid-beta induces chemotaxis and oxidant stress by acting at formylpeptide receptor 2, a G protein-coupled receptor expressed in phagocytes and brain. J. Biol. Chem. 2001, 276, 23645–23652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le, Y.; Gong, W.; Tiffany, H.L.; Tumanov, A.; Nedospasov, S.; Shen, W.; Dunlop, N.M.; Gao, J.L.; Murphy, P.M.; Oppenheim, J.J.; et al. Amyloid (beta)42 activates a G-protein-coupled chemoattractant receptor, FPR-like-1. J. Neurosci. 2001, 21, RC123. [Google Scholar] [CrossRef]
- Hayhoe, R.P.G.; Kamal, A.M.; Solito, E.; Flower, R.J.; Cooper, D.; Perretti, M. Annexin 1 and its bioactive peptide inhibit neutrophil-endothelium interactions under flow: Indication of distinct receptor involvement. Blood 2006, 107, 2123–2130. [Google Scholar] [CrossRef] [Green Version]
- Perretti, M.; Chiang, N.; La, M.; Fierro, I.M.; Marullo, S.; Getting, S.J.; Solito, E.; Serhan, C.N. Endogenous lipid- and peptide-derived anti-inflammatory pathways generated with glucocorticoid and aspirin treatment activate the lipoxin A4 receptor. Nat. Med. 2002, 8, 1296–1302. [Google Scholar] [CrossRef] [Green Version]
- Perretti, M.; Getting, S.J.; Solito, E.; Murphy, P.M.; Gao, J.L. Involvement of the receptor for formylated peptides in the in vivo anti-migratory actions of annexin 1 and its mimetics. Am. J. Pathol. 2001, 158, 1969–1973. [Google Scholar] [CrossRef]
- Guo, Z.; Hu, Q.; Xu, L.; Guo, Z.-N.; Ou, Y.; He, Y.; Yin, C.; Sun, X.; Tang, J.; Zhang, J.H. Lipoxin A4 Reduces Inflammation Through Formyl Peptide Receptor 2/p38 MAPK Signaling Pathway in Subarachnoid Hemorrhage Rats. Stroke 2016, 47, 490–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiore, S.; Maddox, J.F.; Perez, H.D.; Serhan, C.N. Identification of a human cDNA encoding a functional high affinity lipoxin A4 receptor. J. Exp. Med. 1994, 180, 253–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiore, S.; Ryeom, S.W.; Weller, P.F.; Serhan, C.N. Lipoxin recognition sites. Specific binding of labeled lipoxin A4 with human neutrophils. J. Biol. Chem. 1992, 267, 16168–16176. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, S.; Recchiuti, A.; Chiang, N.; Yacoubian, S.; Lee, C.-H.; Yang, R.; Petasis, N.A.; Serhan, C.N. Resolvin D1 binds human phagocytes with evidence for proresolving receptors. Proc. Natl. Acad. Sci. USA 2010, 107, 1660–1665. [Google Scholar] [CrossRef] [Green Version]
- Smole, U.; Gour, N.; Phelan, J.; Hofer, G.; Köhler, C.; Kratzer, B.; Tauber, P.A.; Xiao, X.; Yao, N.; Dvorak, J.; et al. Serum amyloid A is a soluble pattern recognition receptor that drives type 2 immunity. Nat. Immunol. 2020, 21, 756–765. [Google Scholar] [CrossRef]
- Liang, T.S.; Wang, J.M.; Murphy, P.M.; Gao, J.L. Serum amyloid A is a chemotactic agonist at FPR2, a low-affinity N-formylpeptide receptor on mouse neutrophils. Biochem. Biophys. Res. Commun. 2000, 270, 331–335. [Google Scholar] [CrossRef] [Green Version]
- Su, S.B.; Gong, W.; Gao, J.L.; Shen, W.; Murphy, P.M.; Oppenheim, J.J.; Wang, J.M. A seven-transmembrane, G protein-coupled receptor, FPRL1, mediates the chemotactic activity of serum amyloid A for human phagocytic cells. J. Exp. Med. 1999, 189, 395–402. [Google Scholar] [CrossRef]
- Kim, S.-H.; Kim, Y.N.; Jang, Y.-S. Cutting Edge: LL-37-Mediated Formyl Peptide Receptor-2 Signaling in Follicular Dendritic Cells Contributes to B Cell Activation in Peyer’s Patch Germinal Centers. J. Immunol. 2017, 198, 629–633. [Google Scholar] [CrossRef] [Green Version]
- De Yang, B.; Chen, Q.; Schmidt, A.P.; Anderson, G.M.; Wang, J.M.; Wooters, J.; Oppenheim, J.J.; Chertov, O.; Yang, D.; Chen, Q.; et al. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J. Exp. Med. 2000, 192, 1069–1074. [Google Scholar] [CrossRef] [Green Version]
- Wenzel-Seifert, K.; Seifert, R. Cyclosporin H is a potent and selective formyl peptide receptor antagonist. Comparison with N-t-butoxycarbonyl-L-phenylalanyl-L-leucyl-L-phenylalanyl-L-leucyl-L-phenylalanine and cyclosporins A, B, C, D, and E. J. Immunol. 1993, 150, 4591–4599. [Google Scholar]
- Karlsson, J.; Fu, H.; Boulay, F.; Dahlgren, C.; Hellstrand, K.; Movitz, C. Neutrophil NADPH-oxidase activation by an annexin AI peptide is transduced by the formyl peptide receptor (FPR), whereas an inhibitory signal is generated independently of the FPR family receptors. J. Leukoc. Biol. 2005, 78, 762–771. [Google Scholar] [CrossRef] [PubMed]
- Gavins, F.N.E.; Yona, S.; Kamal, A.M.; Flower, R.J.; Perretti, M. Leukocyte antiadhesive actions of annexin 1: ALXR- and FPR-related anti-inflammatory mechanisms. Blood 2003, 101, 4140–4147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christophe, T.; Karlsson, A.; Dugave, C.; Rabiet, M.J.; Boulay, F.; Dahlgren, C. The synthetic peptide Trp-Lys-Tyr-Met-Val-Met-NH2 specifically activates neutrophils through FPRL1/lipoxin A4 receptors and is an agonist for the orphan monocyte-expressed chemoattractant receptor FPRL2. J. Biol. Chem. 2001, 276, 21585–21593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bae, Y.-S.; Lee, H.Y.; Jo, E.J.; Kim, J.I.; Kang, H.-K.; Ye, R.D.; Kwak, J.-Y.; Ryu, S.H. Identification of peptides that antagonize formyl peptide receptor-like 1-mediated signaling. J. Immunol. 2004, 173, 607–614. [Google Scholar] [CrossRef] [Green Version]
- Bürli, R.W.; Xu, H.; Zou, X.; Muller, K.; Golden, J.; Frohn, M.; Adlam, M.; Plant, M.H.; Wong, M.; McElvain, M.; et al. Potent hFPRL1 (ALXR) agonists as potential anti-inflammatory agents. Bioorg. Med. Chem. Lett. 2006, 16, 3713–3718. [Google Scholar] [CrossRef]
- Qin, C.X.; May, L.T.; Li, R.; Cao, N.; Rosli, S.; Deo, M.; Alexander, A.E.; Horlock, D.; Bourke, J.E.; Yang, Y.H.; et al. Small-molecule-biased formyl peptide receptor agonist compound 17b protects against myocardial ischaemia-reperfusion injury in mice. Nat. Commun. 2017, 8, 14232. [Google Scholar] [CrossRef] [Green Version]
- Nanamori, M.; Cheng, X.; Mei, J.; Sang, H.; Xuan, Y.; Zhou, C.; Wang, M.-W.; Ye, R.D. A novel nonpeptide ligand for formyl peptide receptor-like 1. Mol. Pharmacol. 2004, 66, 1213–1222. [Google Scholar] [CrossRef] [Green Version]
- Zhou, C.; Zhang, S.; Nanamori, M.; Zhang, Y.; Liu, Q.; Li, N.; Sun, M.; Tian, J.; Ye, P.P.; Cheng, N.; et al. Pharmacological characterization of a novel nonpeptide antagonist for formyl peptide receptor-like 1. Mol. Pharmacol. 2007, 72, 976–983. [Google Scholar] [CrossRef]
- Osei-Owusu, P.; Charlton, T.M.; Kim, H.K.; Missiakas, D.; Schneewind, O. FPR1 is the plague receptor on host immune cells. Nature 2019, 574, 57–62. [Google Scholar] [CrossRef]
- Wang, J.; Ye, R.D. Agonist concentration-dependent changes in FPR1 conformation lead to biased signaling for selective activation of phagocyte functions. Proc. Natl. Acad. Sci. USA 2022, 119, e2201249119. [Google Scholar] [CrossRef]
- Lammers, K.M.; Chieppa, M.; Liu, L.; Liu, S.; Omatsu, T.; Janka-Junttila, M.; Casolaro, V.; Reinecker, H.-C.; Parent, C.A.; Fasano, A. Gliadin Induces Neutrophil Migration via Engagement of the Formyl Peptide Receptor, FPR1. PLoS ONE 2015, 10, e0138338. [Google Scholar] [CrossRef] [PubMed]
- Stempel, H.; Jung, M.; Pérez-Gómez, A.; Leinders-Zufall, T.; Zufall, F.; Bufe, B. Strain-specific Loss of Formyl Peptide Receptor 3 in the Murine Vomeronasal and Immune Systems. J. Biol. Chem. 2016, 291, 9762–9775. [Google Scholar] [CrossRef] [Green Version]
- Dufton, N.; Hannon, R.; Brancaleone, V.; Dalli, J.; Patel, H.B.; Gray, M.; D’Acquisto, F.; Buckingham, J.C.; Perretti, M.; Flower, R.J. Anti-inflammatory role of the murine formyl-peptide receptor 2: Ligand-specific effects on leukocyte responses and experimental inflammation. J. Immunol. 2010, 184, 2611–2619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schepetkin, I.A.; Khlebnikov, A.I.; Giovannoni, M.P.; Kirpotina, L.N.; Cilibrizzi, A.; Quinn, M.T. Development of small molecule non-peptide formyl peptide receptor (FPR) ligands and molecular modeling of their recognition. Curr. Med. Chem. 2014, 21, 1478–1504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, H.-Q.; Troksa, E.L.; Caltabiano, G.; Pardo, L.; Ye, R.D. Structural Determinants for the Interaction of Formyl Peptide Receptor 2 with Peptide Ligands. J. Biol. Chem. 2014, 289, 2295–2306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stepniewski, T.M.; Filipek, S. Non-peptide ligand binding to the formyl peptide receptor FPR2—A comparison to peptide ligand binding modes. Bioorg. Med. Chem. 2015, 23, 4072–4081. [Google Scholar] [CrossRef]
- Ferrari, C.; Macchiarulo, A.; Costantino, G.; Pellicciari, R. Pharmacophore model for bile acids recognition by the FPR receptor. J. Comput. Aided Mol. Des. 2006, 20, 295–303. [Google Scholar] [CrossRef]
- Bena, S.; Brancaleone, V.; Wang, J.M.; Perretti, M.; Flower, R.J. Annexin A1 interaction with the FPR2/ALX receptor: Identification of distinct domains and downstream associated signaling. J. Biol. Chem. 2012, 287, 24690–24697. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, Y.; Wang, L.; Guo, J.; Sun, D.; Wang, Y.; Liu, W.; Xu, H.E.; Zhang, C. Molecular recognition of formylpeptides and diverse agonists by the formylpeptide receptors FPR1 and FPR2. Nat. Commun. 2022, 13, 1054. [Google Scholar] [CrossRef]
- Chen, T.; Xiong, M.; Zong, X.; Ge, Y.; Zhang, H.; Wang, M.; Won Han, G.; Yi, C.; Ma, L.; Ye, R.D.; et al. Structural basis of ligand binding modes at the human formyl peptide receptor 2. Nat. Commun. 2020, 11, 1208. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Lin, X.; Zong, X.; Han, S.; Wang, M.; Su, Y.; Ma, L.; Chu, X.; Yi, C.; Zhao, Q.; et al. Structural basis of FPR2 in recognition of Aβ42 and neuroprotection by humanin. Nat. Commun. 2022, 13, 1775. [Google Scholar] [CrossRef] [PubMed]
- Ries, M.; Loiola, R.; Shah, U.N.; Gentleman, S.M.; Solito, E.; Sastre, M. The anti-inflammatory Annexin A1 induces the clearance and degradation of the amyloid-β peptide. J. Neuroinflamm. 2016, 13, 234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ries, M.; Watts, H.; Mota, B.C.; Lopez, M.Y.; Donat, C.K.; Baxan, N.; Pickering, J.A.; Chau, T.W.; Semmler, A.; Gurung, B.; et al. Annexin A1 restores cerebrovascular integrity concomitant with reduced amyloid-β and tau pathology. Brain 2021, 144, 1526–1541. [Google Scholar] [CrossRef] [PubMed]
- Solito, E.; Sastre, M. Microglia function in Alzheimer’s disease. Front. Pharmacol. 2012, 3, 14. [Google Scholar] [CrossRef] [Green Version]
- Luo, Z.Z.; Gao, Y.; Sun, N.; Zhao, Y.; Wang, J.; Tian, B.; Shi, J. Enhancing the interaction between annexin-1 and formyl peptide receptors regulates microglial activation to protect neurons from ischemia-like injury. J. Neuroimmunol. 2014, 276, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Iribarren, P.; Huang, J.; Zhang, L.; Gong, W.; Cho, E.H.; Lockett, S.; Dunlop, N.M.; Wang, J.M. Induction of the Formyl Peptide Receptor 2 in Microglia by IFN- and Synergy with CD40 Ligand. J. Immunol. 2007, 178, 1759–1766. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Li, Y.; Han, J.; Zhu, Z.; Li, M.; Liu, Q.; Wang, Y.; Shi, F.-D. Formyl peptide receptor 1 signaling potentiates inflammatory brain injury. Sci. Transl. Med. 2021, 13, eabe9890. [Google Scholar] [CrossRef]
- Calvello, R.; Cianciulli, A.; Porro, C.; Moda, P.; De Nuccio, F.; Nicolardi, G.; Giannotti, L.; Panaro, M.A.; Lofrumento, D.D. Formyl Peptide Receptor (FPR)1 Modulation by Resveratrol in an LPS-Induced Neuroinflammatory Animal Model. Nutrients 2021, 13, 1418. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, G.; Chen, X.; Xue, X.; Guo, Q.; Liu, M.; Zhao, J. Formyl peptide receptors promotes neural differentiation in mouse neural stem cells by ROS generation and regulation of PI3K-AKT signaling. Sci. Rep. 2017, 7, 206. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Zhang, L.; Chen, X.; Xue, X.; Guo, Q.; Liu, M.; Zhao, J. Formylpeptide Receptors Promote the Migration and Differentiation of Rat Neural Stem Cells. Sci. Rep. 2016, 6, 25946. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Wang, Z.-J.; Lok, K.-H.; Yin, M. β-amyloid42 induces desensitization of CXC chemokine receptor-4 via formyl peptide receptor in neural stem/progenitor cells. Biol. Pharm. Bull. 2012, 35, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-C.; Baik, S.H.; Han, S.-H.; Cho, H.J.; Choi, H.; Kim, H.J.; Choi, H.; Lee, W.; Kim, D.K.; Mook-Jung, I. Annexin A1 restores Aβ1-42-induced blood-brain barrier disruption through the inhibition of RhoA-ROCK signaling pathway. Aging Cell 2017, 16, 149–161. [Google Scholar] [CrossRef]
- Ho, C.F.-Y.; Ismail, N.B.; Koh, J.K.-Z.; Gunaseelan, S.; Low, Y.-H.; Ng, Y.-K.; Chua, J.J.-E.; Ong, W.-Y. Localisation of Formyl-Peptide Receptor 2 in the Rat Central Nervous System and Its Role in Axonal and Dendritic Outgrowth. Neurochem. Res. 2018, 43, 1587–1598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Ma, S.; Ke, X.; Yi, Y.; Yu, H.; Yu, D.; Li, Q.; Shang, Y.; Lu, Y.; Pei, L. The mechanism of Annexin A1 to modulate TRPV1 and nociception in dorsal root ganglion neurons. Cell Biosci. 2021, 11, 167. [Google Scholar] [CrossRef] [PubMed]
- Brandenburg, L.-O.; Konrad, M.; Wruck, C.; Koch, T.; Pufe, T.; Lucius, R. Involvement of formyl-peptide-receptor-like-1 and phospholipase D in the internalization and signal transduction of amyloid beta 1-42 in glial cells. Neuroscience 2008, 156, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.J.; Tao, T.; Wang, H.; Zhou, Y.; Gao, X.; Gao, Y.Y.; Hang, C.H.; Li, W. Functions of resolvin D1-ALX/FPR2 receptor interaction in the hemoglobin-induced microglial inflammatory response and neuronal injury. J. Neuroinflamm. 2020, 17, 239. [Google Scholar] [CrossRef]
- Slowik, A.; Merres, J.; Elfgen, A.; Jansen, S.; Mohr, F.; Wruck, C.J.; Pufe, T.; Brandenburg, L.O. Involvement of formyl peptide receptors in receptor for advanced glycation end products (RAGE)—And amyloid beta 1-42-induced signal transduction in glial cells. Mol. Neurodegener. 2012, 7, 1. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Wu, M. Pattern recognition receptors in health and diseases. Signal Transduct. Target. Ther. 2021, 6, 291. [Google Scholar] [CrossRef]
- Medzhitov, R. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 2001, 1, 135–145. [Google Scholar] [CrossRef]
- Fitzgerald, K.A.; Kagan, J.C. Toll-like Receptors and the Control of Immunity. Cell 2020, 180, 1044–1066. [Google Scholar] [CrossRef]
- Jeong, Y.S.; Bae, Y.-S. Formyl peptide receptors in the mucosal immune system. Exp. Mol. Med. 2020, 52, 1694–1704. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Raoof, M.; Chen, Y.; Sumi, Y.; Sursal, T.; Junger, W.; Brohi, K.; Itagaki, K.; Hauser, C.J. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 2010, 464, 104–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorward, D.A.; Lucas, C.D.; Chapman, G.B.; Haslett, C.; Dhaliwal, K.; Rossi, A.G. The Role of Formylated Peptides and Formyl Peptide Receptor 1 in Governing Neutrophil Function during Acute Inflammation. Am. J. Pathol. 2015, 185, 1172–1184. [Google Scholar] [CrossRef] [Green Version]
- Prevete, N.; Liotti, F.; Marone, G.; Melillo, R.M.; de Paulis, A. Formyl peptide receptors at the interface of inflammation, angiogenesis and tumor growth. Pharmacol. Res. 2015, 102, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.M.; Roy, S.; Galla, S.L.; Tomcho, J.C.; Bearss, N.R.; Waigi, E.W.; Mell, B.; Cheng, X.; Saha, P.; Vijay-Kumar, M.; et al. FPR-1 (Formyl Peptide Receptor-1) Activation Promotes Spontaneous, Premature Hypertension in Dahl Salt-Sensitive Rats. Hypertens 2021, 77, 1191–1202. [Google Scholar] [CrossRef] [PubMed]
- Le, Y.; Yang, Y.; Cui, Y.; Yazawa, H.; Gong, W.; Qiu, C.; Wang, J.M. Receptors for chemotactic formyl peptides as pharmacological targets. Int. Immunopharmacol. 2002, 2, 1–13. [Google Scholar] [CrossRef]
- Prossnitz, E.R.; Quehenberger, O.; Cochrane, C.G.; Ye, R.D. Signal transducing properties of the N-formyl peptide receptor expressed in undifferentiated HL60 cells. J. Immunol. 1993, 151, 5704–5715. [Google Scholar]
- Zhang, M.; Gao, J.-L.; Chen, K.; Yoshimura, T.; Liang, W.; Gong, W.; Li, X.; Huang, J.; McDermott, D.H.; Murphy, P.M.; et al. A Critical Role of Formyl Peptide Receptors in Host Defense against Escherichia coli. J. Immunol. 2020, 204, 2464–2473. [Google Scholar] [CrossRef]
- Oldekamp, S.; Pscheidl, S.; Kress, E.; Soehnlein, O.; Jansen, S.; Pufe, T.; Wang, J.M.; Tauber, S.C.; Brandenburg, L.-O. Lack of formyl peptide receptor 1 and 2 leads to more severe inflammation and higher mortality in mice with of pneumococcal meningitis. Immunology 2014, 143, 447–461. [Google Scholar] [CrossRef]
- Fusco, R.; Gugliandolo, E.; Siracusa, R.; Scuto, M.; Cordaro, M.; D’Amico, R.; Evangelista, M.; Peli, A.; Peritore, A.F.; Impellizzeri, D.; et al. Formyl Peptide Receptor 1 Signaling in Acute Inflammation and Neural Differentiation Induced by Traumatic Brain Injury. Biology 2020, 9, 238. [Google Scholar] [CrossRef]
- Al-Madol, M.A.; Shaqura, M.; John, T.; Likar, R.; Ebied, R.S.; Schäfer, M.; Mousa, S.A. Comparative Expression Analyses of Pro-versus Anti-Inflammatory Mediators within Synovium of Patients with Joint Trauma, Osteoarthritis, and Rheumatoid Arthritis. Mediat. Inflamm. 2017, 2017, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, M.; Cheng, N.; Gao, W.; Zhang, M.; Zhang, Y.; Ye, R.D.; Wang, M. Characterization of Quin-C1 for its anti-inflammatory property in a mouse model of bleomycin-induced lung injury. Acta Pharmacol. Sin. 2011, 32, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, C.M.V.; Fock, R.A.; Hastreiter, A.A.; Reutelingsperger, C.; Perretti, M.; Paredes-Gamero, E.J.; Farsky, S.H.P. Extracellular annexin-A1 promotes myeloid/granulocytic differentiation of hematopoietic stem/progenitor cells via the Ca2+/MAPK signalling transduction pathway. Cell Death Discov. 2019, 5, 135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, Y.; Flores, J.; Klebe, D.; Li, P.; McBride, D.W.; Tang, J.; Zhang, J.H. Annexin A1 attenuates neuroinflammation through FPR2/p38/COX-2 pathway after intracerebral hemorrhage in male mice. J. Neurosci. Res. 2019, 98, 168–178. [Google Scholar] [CrossRef] [Green Version]
- McArthur, S.; Gobbetti, T.; Kusters, D.H.M.; Reutelingsperger, C.P.; Flower, R.J.; Perretti, M. Definition of a Novel Pathway Centered on Lysophosphatidic Acid To Recruit Monocytes during the Resolution Phase of Tissue Inflammation. J. Immunol. 2015, 195, 1500733. [Google Scholar] [CrossRef] [Green Version]
- Cooray, S.N.; Gobbetti, T.; Montero-Melendez, T.; McArthur, S.; Thompson, D.; Clark, A.J.L.; Flower, R.J.; Perretti, M. Ligand-specific conformational change of the G-protein-coupled receptor ALX/FPR2 determines proresolving functional responses. Proc. Natl. Acad. Sci. USA 2013, 110, 18232–18237. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Liu, C.; Chang, D.-Y.; Zhan, R.; Zhao, M.; Man Lam, S.; Shui, G.; Zhao, M.-H.; Zheng, L.; Chen, M. The Attenuation of Diabetic Nephropathy by Annexin A1 via Regulation of Lipid Metabolism through the AMPK/PPARα/CPT1b Pathway. Diabetes 2021, 70, 2192–2203. [Google Scholar] [CrossRef]
- Vital, S.A.; Senchenkova, E.Y.; Ansari, J.; Gavins, F.N.E. Targeting AnxA1/Formyl Peptide Receptor 2 Pathway Affords Protection against Pathological Thrombo-Inflammation. Cells 2020, 9, 2473. [Google Scholar] [CrossRef]
- Birkl, D.; O’Leary, M.N.; Quiros, M.; Azcutia, V.; Schaller, M.; Reed, M.; Nishio, H.; Keeney, J.; Neish, A.S.; Lukacs, N.W.; et al. Formyl peptide receptor 2 regulates monocyte recruitment to promote intestinal mucosal wound repair. FASEB J. 2019, 33, 13632–13643. [Google Scholar] [CrossRef] [Green Version]
- Kao, W.; Gu, R.; Jia, Y.; Wei, X.; Fan, H.; Harris, J.; Zhang, Z.; Quinn, J.; Morand, E.F.; Yang, Y.H. A formyl peptide receptor agonist suppresses inflammation and bone damage in arthritis. Br. J. Pharmacol. 2014, 171, 4087–4096. [Google Scholar] [CrossRef] [Green Version]
- Senchenkova, E.Y.; Ansari, J.; Becker, F.; Vital, S.A.; Al-Yafeai, Z.; Sparkenbaugh, E.M.; Pawlinski, R.; Stokes, K.Y.; Carroll, J.L.; Dragoi, A.-M.; et al. Novel Role for the AnxA1-Fpr2/ALX Signaling Axis as a Key Regulator of Platelet Function to Promote Resolution of Inflammation. Circulation 2019, 140, 319–335. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Gao, W.; Li, L.; Hao, J.; Yang, B.; Wang, T.; Li, L.; Bai, X.; Li, F.; Ren, H.; et al. Annexin A1 protects against cerebral ischemia-reperfusion injury by modulating microglia/macrophage polarization via FPR2/ALX-dependent AMPK-mTOR pathway. J. Neuroinflamm. 2021, 18, 119. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.D.; Sagare, A.P.; Zlokovic, B.V. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 2018, 14, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Zhang, Z.; Shim, J.-K.; Venkatraman, T.N.; Lascola, C.D.; Quinones, Q.J.; Mathew, J.P.; Terrando, N.; Podgoreanu, M.V. Annexin A1 Bioactive Peptide Promotes Resolution of Neuroinflammation in a Rat Model of Exsanguinating Cardiac Arrest Treated by Emergency Preservation and Resuscitation. Front. Neurosci. 2019, 13, 608. [Google Scholar] [CrossRef] [Green Version]
- Fang, Y.; An, N.; Zhu, L.; Gu, Y.; Qian, J.; Jiang, G.; Zhao, R.; Wei, W.; Xu, L.; Zhang, G.; et al. Autophagy-Sirt3 axis decelerates hematopoietic aging. Aging Cell 2020, 19, e13232. [Google Scholar] [CrossRef]
- Diao, Z.; Ji, Q.; Wu, Z.; Zhang, W.; Cai, Y.; Wang, Z.; Hu, J.; Liu, Z.; Wang, Q.; Bi, S.; et al. SIRT3 consolidates heterochromatin and counteracts senescence. Nucleic Acids Res. 2021, 49, 4203–4219. [Google Scholar] [CrossRef]
- Chang, T.-C.; Hsu, M.-F.; Shih, C.-Y.; Wu, K.K. 5-methoxytryptophan protects MSCs from stress induced premature senescence by upregulating FoxO3a and mTOR. Sci. Rep. 2017, 7, 11133. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Lin, Y.; Wang, L.; Zhan, H.; Luo, X.; Zeng, Y.; Wu, W.; Zhang, X.; Wang, F. TREM2 ameliorates neuroinflammatory response and cognitive impairment via PI3K/AKT/FoxO3a signaling pathway in Alzheimer’s disease mice. Aging 2020, 12, 20862–20879. [Google Scholar] [CrossRef]
- Du, S.; Jin, F.; Maneix, L.; Gedam, M.; Xu, Y.; Catic, A.; Wang, M.C.; Zheng, H. FoxO3 deficiency in cortical astrocytes leads to impaired lipid metabolism and aggravated amyloid pathology. Aging Cell 2021, 20, e13432. [Google Scholar] [CrossRef]
- Lee, J.; Kim, Y.; Liu, T.; Hwang, Y.J.; Hyeon, S.J.; Im, H.; Lee, K.; Alvarez, V.E.; McKee, A.C.; Um, S.-J.; et al. SIRT3 deregulation is linked to mitochondrial dysfunction in Alzheimer’s disease. Aging Cell 2018, 17, e12679. [Google Scholar] [CrossRef] [Green Version]
- Sanphui, P.; Biswas, S.C. FoxO3a is activated and executes neuron death via Bim in response to β-amyloid. Cell Death Dis. 2013, 4, e625. [Google Scholar] [CrossRef] [Green Version]
- Wong, H.-K.A.; Veremeyko, T.; Patel, N.; Lemere, C.A.; Walsh, D.M.; Esau, C.; Vanderburg, C.; Krichevsky, A.M. De-repression of FOXO3a death axis by microRNA-132 and -212 causes neuronal apoptosis in Alzheimer’s disease. Hum. Mol. Genet. 2013, 22, 3077–3092. [Google Scholar] [CrossRef] [PubMed]
- Trojan, E.; Tylek, K.; Leśkiewicz, M.; Lasoń, W.; Brandenburg, L.-O.; Leopoldo, M.; Lacivita, E.; Basta-Kaim, A. The N-Formyl Peptide Receptor 2 (FPR2) Agonist MR-39 Exhibits Anti-Inflammatory Activity in LPS-Stimulated Organotypic Hippocampal Cultures. Cells 2021, 10, 1524. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Ye, R.D. Microglial Aβ Receptors in Alzheimer’s Disease. Cell Mol. Neurobiol. 2014, 35, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Schröder, N.; Schaffrath, A.; Welter, J.A.; Putzka, T.; Griep, A.; Ziegler, P.; Brandt, E.; Samer, S.; Heneka, M.T.; Kaddatz, H.; et al. Inhibition of formyl peptide receptors improves the outcome in a mouse model of Alzheimer disease. J. Neuroinflamm. 2020, 17, 131. [Google Scholar] [CrossRef] [Green Version]
- Purvis, G.S.D.; Collino, M.; Loiola, R.A.; Baragetti, A.; Chiazza, F.; Brovelli, M.; Sheikh, M.H.; Collotta, D.; Cento, A.; Mastrocola, R.; et al. Identification of Annexina1 as an endogenous regulator of RhoA, and its role in the pathophysiology and experimental therapy of type-2 diabetes. Front. Immunol. 2019, 10, 571. [Google Scholar] [CrossRef] [Green Version]
- Gobbetti, T.; Cooray, S.N. Annexin A1 and resolution of inflammation: Tissue repairing properties and signalling signature. Biol. Chem. 2016, 397, 981–993. [Google Scholar] [CrossRef]
- Loiola, R.A.; Wickstead, E.S.; Solito, E.; McArthur, S. Estrogen Promotes Pro-resolving Microglial Behavior and Phagocytic Cell Clearance Through the Actions of Annexin A1. Front. Endocrinol. 2019, 10, 420. [Google Scholar] [CrossRef]
- Moss, S.E.; Morgan, R.O. The annexins. Genome Biol. 2004, 5, 219. [Google Scholar] [CrossRef] [Green Version]
- Cristante, E.; McArthur, S.; Mauro, C.; Maggioli, E.; Romero, I.A.; Wylezinska-Arridge, M.; Couraud, P.O.; Lopez-Tremoleda, J.; Christian, H.C.; Weksler, B.B.; et al. Identification of an essential endogenous regulator of blood-brain barrier integrity, and its pathological and therapeutic implications. Proc. Natl. Acad. Sci. USA 2013, 110, 832–841. [Google Scholar] [CrossRef] [Green Version]
- Gil, C.D.; La, M.; Perretti, M.; Oliani, S.M. Interaction of human neutrophils with endothelial cells regulates the expression of endogenous proteins annexin 1, galectin-1 and galectin-3. Cell Biol. Int. 2006, 30, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Bergström, I.; Lundberg, A.K.; Jönsson, S.; Särndahl, E.; Ernerudh, J.; Jonasson, L. Annexin A1 in blood mononuclear cells from patients with coronary artery disease: Its association with inflammatory status and glucocorticoid sensitivity. PLoS ONE 2017, 12, e0174177. [Google Scholar] [CrossRef]
- Purvis, G.S.D.; Solito, E.; Thiemermann, C. Annexin-A1: Therapeutic Potential in Microvascular Disease. Front. Immunol. 2019, 10, 938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gussenhoven, R.; Klein, L.; Ophelders, D.R.M.G.; Habets, D.H.J.; Giebel, B.; Kramer, B.W.; Schurgers, L.J.; Reutelingsperger, C.P.M.; Wolfs, T.G.A.M. Annexin A1 as Neuroprotective Determinant for Blood-Brain Barrier Integrity in Neonatal Hypoxic-Ischemic Encephalopathy. J. Clin. Med. 2019, 8, 137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoyles, L.; Pontifex, M.G.; Rodriguez-Ramiro, I.; Anis-Alavi, M.A.; Jelane, K.S.; Snelling, T.; Solito, E.; Fonseca, S.; Carvalho, A.L.; Carding, S.R.; et al. Regulation of blood-brain barrier integrity by microbiome-associated methylamines and cognition by trimethylamine N-oxide. Microbiome 2021, 9, 235. [Google Scholar] [CrossRef]
- Liu, J.-H.; Feng, D.; Zhang, Y.-F.; Shang, Y.; Wu, Y.; Li, X.-F.; Pei, L. Chloral Hydrate Preconditioning Protects Against Ischemic Stroke via Upregulating Annexin A1. CNS Neurosci. Ther. 2015, 21, 718–726. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Gao, Y.; Yu, X.; Zhao, B.; Liu, L.; Zhao, Y.; Luo, Z.; Shi, J. Annexin-1 Mediates Microglial Activation and Migration via the CK2 Pathway during Oxygen-Glucose Deprivation/Reperfusion. Int. J. Mol. Sci. 2016, 17, 1770. [Google Scholar] [CrossRef] [Green Version]
- Shijo, M.; Hamasaki, H.; Honda, H.; Suzuki, S.O.; Tachibana, M.; Ago, T.; Kitazono, T.; Iihara, K.; Iwaki, T. Upregulation of Annexin A1 in Reactive Astrocytes and Its Subtle Induction in Microglia at the Boundaries of Human Brain Infarcts. J. Neuropathol. Exp. Neurol. 2019, 78, 961–970. [Google Scholar] [CrossRef]
- Eberhard, D.A.; Brown, M.D.; VandenBerg, S.R. Alterations of annexin expression in pathological neuronal and glial reactions. Immunohistochemical localization of annexins I, II (p36 and p11 subunits), IV, and VI in the human hippocampus. Am. J. Pathol. 1994, 145, 640–649. [Google Scholar]
- Dreier, R.; Schmid, K.W.; Gerke, V.; Riehemann, K. Differential expression of annexins I, II and IV in human tissues: An immunohistochemical study. Histochem. Cell Biol. 1998, 110, 137–148. [Google Scholar] [CrossRef]
- Xia, Q.; Mao, M.; Zeng, Z.; Luo, Z.; Zhao, Y.; Shi, J.; Li, X. Inhibition of SENP6 restrains cerebral ischemia-reperfusion injury by regulating Annexin-A1 nuclear translocation-associated neuronal apoptosis. Theranostics 2021, 11, 7450–7470. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zheng, L.; Xia, Q.; Liu, L.; Mao, M.; Zhou, H.; Zhao, Y.; Shi, J. A novel cell-penetrating peptide protects against neuron apoptosis after cerebral ischemia by inhibiting the nuclear translocation of annexin A1. Cell Death Differ. 2019, 26, 260–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makani, V.; Sultana, R.; Sie, K.S.; Orjiako, D.; Tatangelo, M.; Dowling, A.; Cai, J.; Pierce, W.; Butterfield, D.A.; Hill, J.; et al. Annexin A1 complex mediates oxytocin vesicle transport. J. Neuroendocrinol. 2013, 25, 1241–1254. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, X.; Gong, J.; Li, L.; Chen, L.; Zheng, L.; Chen, Z.; Shi, J.; Zhang, H. Annexin A1 nuclear translocation induces retinal ganglion cell apoptosis after ischemia-reperfusion injury through the p65/IL-1β pathway. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1350–1358. [Google Scholar] [CrossRef]
- Ernst, S.; Lange, C.; Wilbers, A.; Goebeler, V.; Gerke, V.; Rescher, U. An annexin 1 N-terminal peptide activates leukocytes by triggering different members of the formyl peptide receptor family. J. Immunol. 2004, 172, 7669–7676. [Google Scholar] [CrossRef] [Green Version]
- Braun, B.J.; Slowik, A.; Leib, S.L.; Lucius, R.; Varoga, D.; Wruck, C.J.; Jansen, S.; Podschun, R.; Pufe, T.; Brandenburg, L.-O. The formyl peptide receptor like-1 and scavenger receptor MARCO are involved in glial cell activation in bacterial meningitis. J. Neuroinflamm. 2011, 8, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandenburg, L.-O.; Konrad, M.; Wruck, C.J.; Koch, T.; Lucius, R.; Pufe, T. Functional and physical interactions between formyl-peptide-receptors and scavenger receptor MARCO and their involvement in amyloid beta 1-42-induced signal transduction in glial cells. J. Neurochem. 2010, 113, 749–760. [Google Scholar] [CrossRef]
- Deane, R.; Singh, I.; Sagare, A.P.; Bell, R.D.; Ross, N.T.; LaRue, B.; Love, R.; Perry, S.; Paquette, N.; Deane, R.J.; et al. A multimodal RAGE-specific inhibitor reduces amyloid β-mediated brain disorder in a mouse model of Alzheimer disease. J. Clin. Investig. 2012, 122, 1377–1392. [Google Scholar] [CrossRef] [Green Version]
- Rabiet, M.-J.; Macari, L.; Dahlgren, C.; Boulay, F. N-formyl peptide receptor 3 (FPR3) departs from the homologous FPR2/ALX receptor with regard to the major processes governing chemoattractant receptor regulation, expression at the cell surface, and phosphorylation. J. Biol. Chem. 2011, 286, 26718–26731. [Google Scholar] [CrossRef] [Green Version]
- Wagener, B.M.; Marjon, N.A.; Revankar, C.M.; Prossnitz, E.R. Adaptor protein-2 interaction with arrestin regulates GPCR recycling and apoptosis. Traffic 2009, 10, 1286–1300. [Google Scholar] [CrossRef] [Green Version]
- Gilbert, T.L.; Bennett, T.A.; Maestas, D.C.; Cimino, D.F.; Prossnitz, E.R. Internalization of the human N-formyl peptide and C5a chemoattractant receptors occurs via clathrin-independent mechanisms. Biochemistry 2001, 40, 3467–3475. [Google Scholar] [CrossRef] [PubMed]
- Huet, E.; Boulay, F.; Barral, S.; Rabiet, M.-J. The role of beta-arrestins in the formyl peptide receptor-like 1 internalization and signaling. Cell. Signal. 2007, 19, 1939–1948. [Google Scholar] [CrossRef] [PubMed]

| Ligand | Origin | Selectivity | pKD | pEC50 | Model | Refs |
|---|---|---|---|---|---|---|
| Formylated bacterial ligands | ||||||
| fMLF | E. coli | FPR1 | 6.4–9.3 | 4.6 | Human neutrophils, L cells, RBL-2H3 | [2,25,26] |
| fMIFL | S. aureus | FPR1, FPR2 | n.d. | n.d. | Mouse neutrophils, RBL-2H3 | [27,28] |
| fMIVTLF | Listeria | FPR1, FPR2 | n.d. | n.d. | RBL-2H3 | [28] |
| fMVMKFK | Haemophilus | FPR1, FPR2 | n.d. | 6.1, 8.1 | HEK293 | [29] |
| Formylated mitochondrial ligands | ||||||
| fMLKLIV | Mitochondria | FPR1, FPR2 | n.d. | 7.4, 7.3 | HL-60 | [30] |
| fMMYALF | Mitochondria | FPR1, FPR2 | n.d. | 8.0, 7.8 | HL-60, RBL-2H3 | [28,30] |
| Mitocryptide-2 | Mitochondria | FPR2 | n.d. | 6.2–6.4 | Human neutrophils, HEK293T | [31,32] |
| Non-formylated pathogen-derived ligands | ||||||
| C5a peptide | Hepatitis C virus | FPR2 | n.d. | n.d. | Human monocytes and neutrophils, HEK293, RBL-2H3 | [33] |
| gG-2p20 | Herpes simplex virus | FPR1 | n.d. | 6.2–6.3 | Human monocytes and neutrophils | [34] |
| Hp(2-20) | Helicobacter pylori | FPR2 | n.d. | 6.5 | Human monocytes | [35] |
| Non-mitochondrial host-derived ligands | ||||||
| Aβ | Host | FPR2 | n.d. | 7.0 | Human monocytes, mouse neutrophils, HEK293, RBL-2H3 | [36,37] |
| Annexin A1 | Host | FPR2 | 6.5 | n.d. | Human neutrophils, HEK293 | [11,38,39,40] |
| Lipoxin A4 | Host | FPR2 | 8.8–9.3 | ~12.0 | Human neutrophils | [41,42,43,44] |
| Resolvin D1 | Host | FPR2 | ~11.9 | n.d. | Human neutrophils | [44] |
| Serum Amyloid A | Host | FPR2 | n.d. | 6.6–7.3 | Human monocytes and neutrophils, HEK293 | [45,46,47] |
| LL-37 | Host | FPR2 | n.d. | 6.0 | Human monocytes, neutrophils, and T cells, HEK293, RBL-2H3 | [48,49] |
| Natural peptide ligands | ||||||
| Cyclosporin H | T. inflatum & T. polysporum | FPR1 | 7.0 (pIC50) | n.d. | Human neutrophils | [50] |
| Synthetic peptide ligands | ||||||
| Ac9-25 | Synthetic | FPR1 | n.d. | 4.7 | Human neutrophils, HL-60 | [51] |
| Ac2-26 | Synthetic | FPR1, FPR2 | 5.9 | 5.8–6.1 | Human neutrophils, HEK293 | [38,39,52] |
| WKYMVm | Synthetic | FPR2 | 10.1 | n.d. | human neutrophils, HL-60 | [53] |
| WRW4 | Synthetic | FPR2 | 6.6 (pIC50) | n.d. | Human neutrophils, RBL-2H3 | [54] |
| Small molecule ligands | ||||||
| Compound 43 | Synthetic | FPR1, FPR2 | n.d. | n.d. | CHO, RBL-2H3 | [28,55,56] |
| Compound 17b | Synthetic | FPR1, FPR2 | n.d. | n.d. | CHO | [28,56] |
| Quin-C1 | Synthetic | FPR2 | n.d. | 5.7–6.2 | Human neutrophils, RBL-2H3 | [28,57] |
| Quin-C7 | Synthetic | FPR2 | 5.2 (pIC50) | n.d. | HeLa, RBL-2H3 | [58] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wickstead, E.S.; Solito, E.; McArthur, S. Promiscuous Receptors and Neuroinflammation: The Formyl Peptide Class. Life 2022, 12, 2009. https://doi.org/10.3390/life12122009
Wickstead ES, Solito E, McArthur S. Promiscuous Receptors and Neuroinflammation: The Formyl Peptide Class. Life. 2022; 12(12):2009. https://doi.org/10.3390/life12122009
Chicago/Turabian StyleWickstead, Edward S., Egle Solito, and Simon McArthur. 2022. "Promiscuous Receptors and Neuroinflammation: The Formyl Peptide Class" Life 12, no. 12: 2009. https://doi.org/10.3390/life12122009
APA StyleWickstead, E. S., Solito, E., & McArthur, S. (2022). Promiscuous Receptors and Neuroinflammation: The Formyl Peptide Class. Life, 12(12), 2009. https://doi.org/10.3390/life12122009







