Subretinal Transplant of Human Amniotic Membrane in Advanced Age-Related Macular Degeneration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Study Exams
2.3. Statistical Analysis
2.4. Surgical Technique
3. Discussion
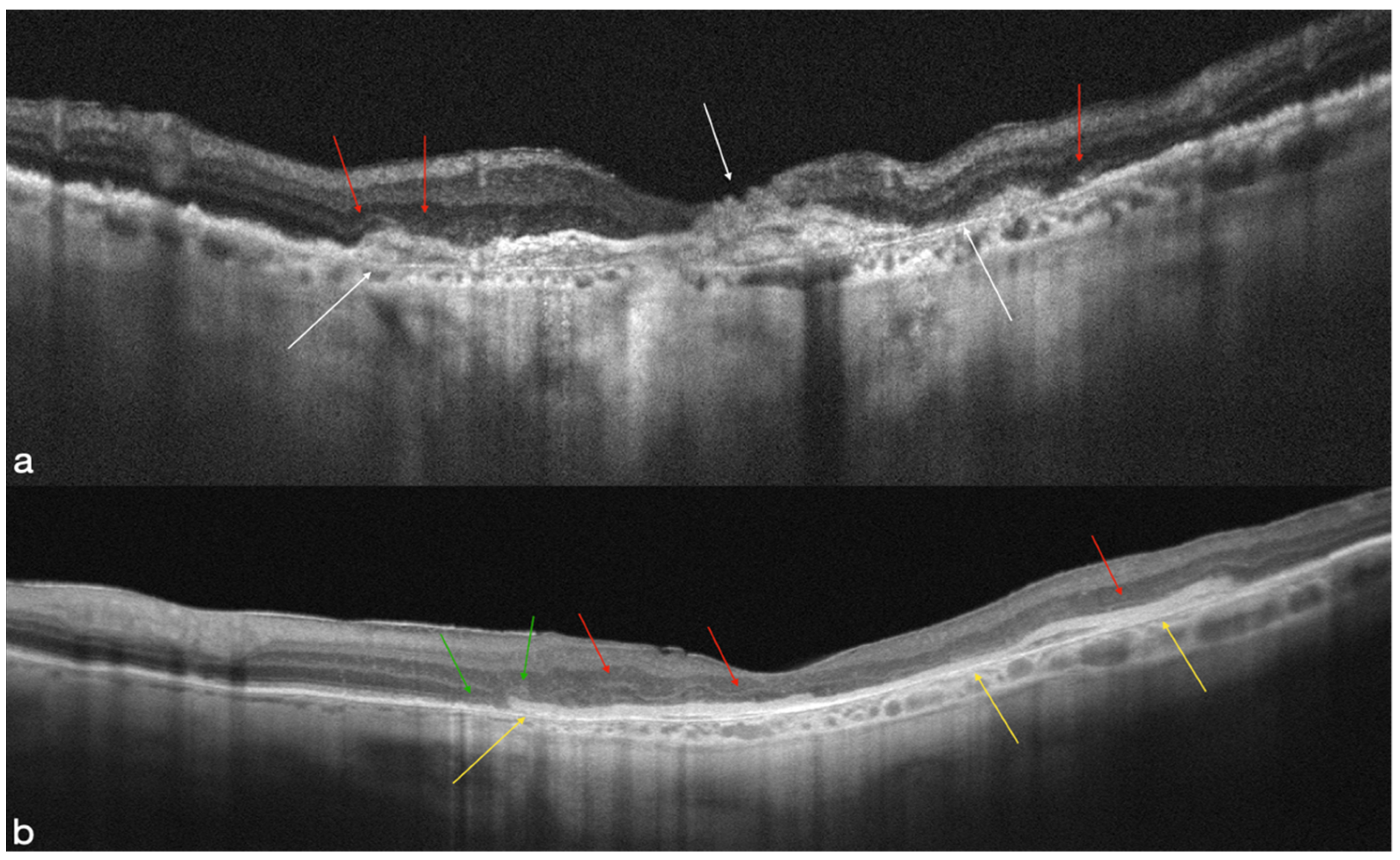
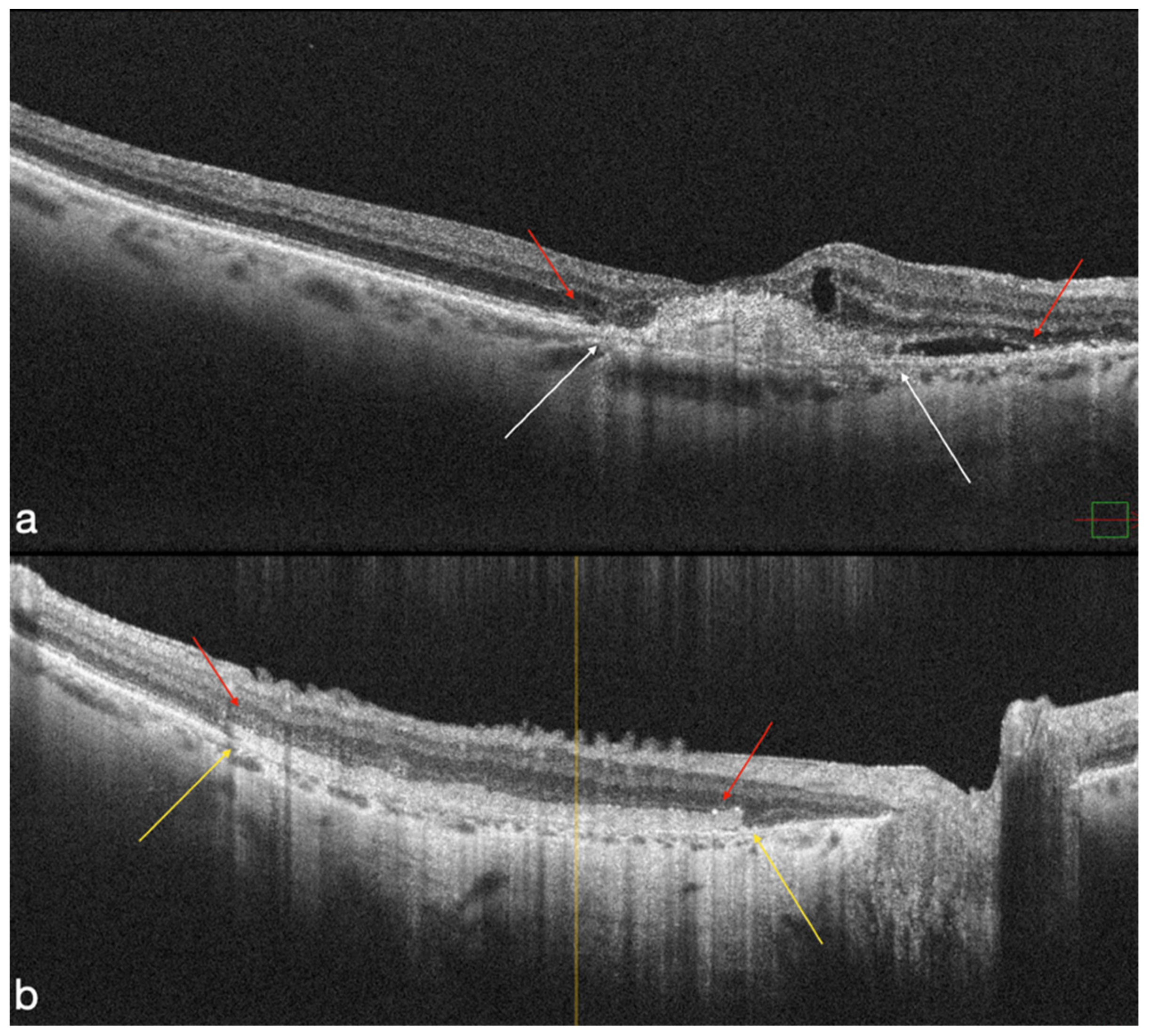
4. Results
4.1. MNV-Group
4.2. Atrophic Group
4.3. OCT-A Results
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wong, T.Y.; Chakravarthy, U.; Klein, R.; Mitchell, P.; Zlateva, G.; Buggage, R.; Fahrbach, K.; Probst, C.; Sledge, I. The natural history and prognosis of neovascular age-related macular degeneration: A systematic review of the literature and meta-analysis. Ophthalmology 2008, 115, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Au, A.; Parikh, V.S.; Singh, R.P.; Ehlers, J.P.; Yuan, A.; Rachitskaya, A.V.; E Sears, J.; Srivastava, S.K.; Kaiser, P.K.; Schachat, A.P.; et al. Comparison of anti-VEGF therapies on fibrovascular pigment epithelial detachments in age-related macular degeneration. Br. J. Ophthalmol. 2017, 101, 970–975. [Google Scholar] [CrossRef] [PubMed]
- Wylegala, A.; Wylegala, F.; Wylegala, E. Aflibercept Treatment Leads to Vascular Abnormalization of the Choroidal Neovascularization. J. Healthc. Eng. 2018, 2018, 8595278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizzo, S.; Caporossi, T.; Tartaro, R.; Finocchio, L.; Pacini, B.; Bacherini, D.; Virgili, G. Human Amniotic Membrane plug to restore Age related Macular Degeneration photoreceptors’ damage. Ophthalmol. Retina 2020, 4, 996–1007. [Google Scholar] [CrossRef] [PubMed]
- Tosi, G.M.; Traversi, C.; Schuerfeld, K.; Mittica, V.; Massaro-Giordano, M.; Tilanus, M.A.; Caporossi, A.; Toti, P. Amniotic membrane graft: Histopathological findings in five cases. J. Cell Physiol. 2005, 202, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Broughton, B.R.; Lim, R.; Arumugam, T.V.; Drummond, G.R.; Wallace, E.M.; Sobey, C.G. Post-stroke inflammation and the potential efficacy of novel stem cell therapies: Focus on amnion epithelial cells. Front. Cell Neurosci. 2012, 6, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otani, T.; Ochiai, D.; Masuda, H.; Abe, Y.; Fukutake, M.; Matsumoto, T.; Miyakoshi, K.; Tanaka, M. The neurorestorative effect of human amniotic fluid stem cells on the chronic phase of neonatal hypoxic-ischemic encephalopathy in mice. Pediatr. Res. 2018, 85, 97–104. [Google Scholar] [CrossRef]
- Murri, M.S.; Moshirfar, M.; Birdsong, O.C.; Ronquillo, Y.C.; Ding, Y.; Hoopes, P.C. Amniotic membrane extract and eye drops: A review of literature and clinical application. Clin. Ophthalmol. 2018, 12, 1105–1112. [Google Scholar] [CrossRef] [Green Version]
- Marcus, A.J.; Coyne, T.M.; Black, I.B.; Woodbury, D. Fate of amnion-derived stem cells transplanted to the fetal rat brain: Migration, survival and differentiation. J. Cell Mol. Med. 2008, 12, 1256–1264. [Google Scholar] [CrossRef] [Green Version]
- Letko, E.; Stechschulte, S.U.; Kenyon, K.R.; Sadeq, N.; Romero, T.R.; Samson, C.M.; Nguyen, Q.D.; Harper, S.L.; Primack, J.D.; Azar, D.T.; et al. Amniotic membrane inlay and overlay grafting for corneal epithelial defects and stromal ulcers. Arch. Ophthalmol. 2001, 119, 659–663. [Google Scholar] [CrossRef]
- de Roth, A. Plastic Repair of Conjunctival Defects with Fetal Membranes. Arch. Ophthalmol. 1940, 23, 522–525. [Google Scholar] [CrossRef]
- Rizzo, S.; Caporossi, T.; Tartaro, R.; Finocchio, L.; Franco, F.; Barca, F.; Giansanti, F. A Human Amniotic Membrane Plug to Promote Retinal Breaks Repair and Recurrent Macular Hole Closure. Retina 2019, 39 (Suppl. S1), S95–S103. [Google Scholar] [CrossRef] [PubMed]
- Caporossi, T.; Tartaro, R.; De Angelis, L.; Pacini, B.; Rizzo, S. A human amniotic membrane plug to repair retinal detachment associated with large macular tear. Acta Ophthalmol. 2019, 97, 821–823. [Google Scholar] [CrossRef] [PubMed]
- Caporossi, T.; Pacini, B.; Bacherini, D.; Barca, F.; Faraldi, F.; Rizzo, S. Human amniotic membrane plug to promote failed macular hole closure. Sci. Rep. 2020, 10, 18264. [Google Scholar] [CrossRef]
- Caporossi, T.; Tartaro, R.; Finocchio, L.; Pacini, B.; De Angelis, L.; Bacherini, D.; Rizzo, S. Human Amniotic Membrane to Treat Macular Holes That Failed to Close, Sulfur Hexafluoride Endotamponade Versus Air Endotamponade: A Prospective Comparative Study. Retina 2021, 41, 735–743. [Google Scholar] [CrossRef]
- Caporossi, T.; De Angelis, L.; Pacini, B.; Tartaro, R.; Finocchio, L.; Barca, F.; Rizzo, S. A human Amniotic Membrane plug to manage high myopic macular hole associated with retinal detachment. Acta Ophthalmol. 2019, 98, e252–e256. [Google Scholar] [CrossRef]
- Caporossi, T.; De Angelis, L.; Pacini, B.; Rizzo, S. Amniotic membrane for retinal detachment due to paravascular retinal breaks over patchy chorioretinal atrophy in pathologic myopia. Eur. J. Ophthalmol. 2020, 30, 392–395. [Google Scholar] [CrossRef]
- Niknejad, H.; Yazdanpanah, G.; Ahmadiani, A. Induction of apoptosis, stimulation of cell-cycle arrest and inhibition of angiogenesis make human amnion-derived cells promising sources for cell therapy of cancer. Cell Tissue Res. 2016, 363, 599–608. [Google Scholar] [CrossRef]
- Marangon, F.B.; Alfonso, E.C.; Miller, D.; Remonda, N.M.; Muallem, M.S.; Tseng, S.C. Incidence of microbial infection after amniotic membrane transplantation. Cornea 2004, 23, 264–269. [Google Scholar] [CrossRef]
- Holladay, J.T. Visual acuity measurements. J. Cataract. Refract. Surg. 2004, 30, 287–290. [Google Scholar] [CrossRef]
- da Cruz, L.; Fynes, K.; Georgiadis, O.; Kerby, J.; Luo, Y.H.; Ahmado, A.; Vernon, A.; Daniels, J.T.; Nommiste, B.; Hasan, S.M.; et al. Phase 1 clinical study of an embryonic stem cell-derived retinal pigment epithelium patch in age-related macular degeneration. Nat. Biotechnol. 2018, 36, 328–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomson, J.A.; Itskovitz-Eldor, J.; Shapiro, S.S.; Waknitz, M.A.; Swiergiel, J.J.; Marshall, V.S.; Jones, J.M. Embryonic stem cell lines derived from human blastocysts. Science 1998, 282, 1145–1147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGill, T.J.; Wilson, D.J.; Stoddard, J.; Renner, L.M.; Neuringer, M. Cell Transplantation for Retinal Degeneration: Transition from Rodent to Nonhuman Primate Models. Adv. Exp. Med. Biol. 2018, 1074, 641–647. [Google Scholar] [CrossRef]
- Capeans, C.; Piñeiro, A.; Pardo, M.; Sueiro-López, C.; Blanco, M.J.; Domínguez, F.; Sánchez-Salorio, M. Amniotic membrane as support for human retinal pigment epithelium (RPE) cell growth. Acta Ophthalmol. Scand. 2003, 81, 271–277. [Google Scholar] [CrossRef]
- Ohno-Matsui, K.; Ichinose, S.; Nakahama, K.I.; Yoshida, T.; Kojima, A.; Mochizuki, M.; Morita, I. The effects of amniotic membrane on retinal pigment epithelial cell differentiation. Mol. Vis. 2005, 11, 1–10. [Google Scholar]
- Kiilgaard, J.F.; Scherfig, E.; Prause, J.U.; la Cour, M. Transplantation of amniotic membrane to the subretinal space in pigs. Stem Cells Int. 2012, 2012, 716968. [Google Scholar] [CrossRef] [Green Version]
- Zhu, D.; Jin, X.; Zhou, J. Transplantation of amniotic membrane for choroidal hole to treat suprachoroidal silicone oil migration. Acta Ophthalmol. 2017, 95, e522–e523. [Google Scholar] [CrossRef]
- Kashani, A.H.; Lebkowski, J.S.; Rahhal, F.M.; Avery, R.L.; Salehi-Had, H.; Dang, W.; Lin, C.-M.; Mitra, D.; Zhu, D.; Thomas, B.B.; et al. A bioengineered retinal pigment epithelial monolayer for advanced, dry age-related macular degeneration. Sci. Transl. Med. 2018, 10, eaao4097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Zeeburg, E.J.; Maaijwee, K.J.; Missotten, T.O.; Heimann, H.; van Meurs, J.C. A free retinal pigment epithelium-choroid graft in patients with exudative age-related macular degeneration: Results up to 7 years. Am. J. Ophthalmol. 2012, 153, 120–127.e2. [Google Scholar] [CrossRef]
- Parolini, B.; Di Salvatore, A.; Pinackatt, S.J.; Baldi, A.; Besozzi, G.; Finzi, A.; Cardillo, D.; Sallam, A.B.; Frisina, R. Long-Term Results of Autologous Retinal Pigment Epithelium and Choroid Transplantation for the Treatment of Exudative and Atrophic Maculopathies. Retina 2018, 40, 507–520. [Google Scholar] [CrossRef]
- van Romunde, S.H.M.; Polito, A.; Peroglio Deiro, A.; Guerriero, M.; Pertile, G. Retinal Pigment Epithelium-Choroid Graft with A Peripheral Retinotomy for Exudative Age-Related Macular Degeneration: Long-Term Outcome. Retina 2017, 39, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, T.; Sato, T.; Hara-Ueno, C.; Fukushima, Y.; Sayanagi, K.; Shiraki, N.; Sawa, M.; Ikuno, Y.; Sakaguchi, H.; Nishida, K. Retinal Microvasculature and Visual Acuity in Eyes with Branch Retinal Vein Occlusion: Imaging Analysis by Optical Coherence Tomography Angiography. Invest Ophthalmol Vis. Sci. 2017, 58, 2087–2094. [Google Scholar] [CrossRef] [PubMed]
- Avery, R.L.; Fekrat, S.; Hawkins, B.S.; Bressler, N.M. Natural history of subfoveal subretinal hemorrhage in age-related macular degeneration. Retina 1996, 16, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Bressler, N.M.; Bressler, S.B.; Childs, A.L.; A Haller, J.; Hawkins, B.S.; Lewis, H.; MacCumber, M.W.; Marsh, M.J.; Redford, M.; Sternberg, P.; et al. Surgery for hemorrhagic choroidal neovascular lesions of age-related macular degeneration: Ophthalmic findings: SST report no. 13. Ophthalmology 2004, 111, 1993–2006. [Google Scholar] [CrossRef] [PubMed]

| Patient ID/Age (YO)/Sex | Eye | Lens Status | Preoperative BCVA—Snellen (LogMAR) | 1-Month BCVA—Snellen (LogMAR) | 3-Month BCVA—Snellen (LogMAR) | 6-Month BCVA—Snellen (LogMAR) | 12-Month BCVA—Snellen (LogMAR) |
|---|---|---|---|---|---|---|---|
| 1/70/F | Right | phakic | 20/2000 (2) | 20/400 (1.3) | 20/100 (0.7) | 20/2000 (2) | 20/400 (1.3) |
| 2/68/M | Right | pseudophakic | 20/2000 (2) | 20/2000 (2) | 20/400 (1.3) | 20/400 (1.3) | 20/200 (1) |
| 3/80/F | Right | pseudophakic | 20/2000 (2) | 20/2000 (2) | 20/400 (1.3) | 20/100 (0.7) | 20/100 (0.7) |
| 4/80/M | Right | phakic | 20/2000 (2) | 20/2000 (2) | 20/2000 (2) | 20/2000 (2) | 20/2000 (2) |
| 5/72/F | Left | pseudophakic | 20/2000 (2) | 20/2000 (2) | 20/2000 (2) | 20/400 (1.3) | 20/400 (1.3) |
| 6/68/M | Right | phakic | 20/2000 (2) | 20/400 (1.3) | 20/400 (1.3) | 20/400 (1.3) | 20/400 (1.3) |
| 7/85/F | Left | pseudophakic | 20/2000 (2) | 20/2000 (2) | 20/800 (1.6) | 20/800 (1.6) | 20/400 (1.3) |
| 8/86/F | Left | pseudophakic | 20/2000 (2) | 20/2000 (2) | 20/400 (1.3) | 20/400 (1.3) | 20/400 (1.3) |
| 9/79/M | Right | pseudophakic | 20/800 (1.6) | 20/200 (1) | 20/200 (1) | 20/125 (0.8) | 20/100 (0.7) |
| 10/85/M | Right | pseudophakic | 20/800 (1.6) | 20/200 (1) | 20/200 (1) | 20/200 (1) | 20/200 (1) |
| 11/87/M | Right | pseudophakic | 20/2000 (2) | 20/2000 (2) | 20/2000 (2) | 20/400 (1.3) | 20/200 (1) |
| 12/83/F | Left | phakic | 20/2000 (2) | 20/2000 (2) | 20/2000 (2) | 20/2000 (2) | 20/2000 (2) |
| 13/75/F | Left | phakic | 20/200 (1) | 20/200 (1) | 20/160 (0.9) | 20/100 (0.7) | 20/80 (0.6) |
| 14/84/M | Right | pseudophakic | 20/2000 (2) | 20/400 (1.3) | 20/400 (1.3) | 20/200 (1) | 20/200 (1) |
| 15/73/M | Left | phakic | 20/2000 (2) | 20/160 (0.9) | 20/160 (0.9) | 20/200 (1) | 20/125 (0.8) |
| 15/73/M | Right | phakic | 20/20,000 (3) | 20/400 (1.3) | 20/400 (1.3) | 20/400 (1.3) | 20/400 (1.3) |
| 16/80/F | Right | pseudophakic | 20/2000 (2) | 20/2000 (2) | 20/2000 (2) | 20/2000 (2) | 20/2000 (2) |
| 17/88/F | Left | pseudophakic | 20/2000 (2) | 20/2000 (2) | 20/400 (1.3) | 20/400 (1.3) | 20/400 (1.3) |
| 18/80/M | Right | pseudophakic | 20/800 (1.6) | 20/2000 (2) | 20/400 (1.3) | 20/400 (1.3) | 20/200 (1) |
| 19/78/M | Right | pseudophakic | 20/800 (1.6) | 20/200 (1) | 20/200 (1) | 20/200 (1) | 20/200 (1) |
| 20/78/F | Right | phakic | 20/20,000 (3) | 20/20,000 (3) | 20/20,000 (3) | 20/20,000 (3) | 20/20,000 (3) |
| 21/75/F | Left | pseudophakic | 20/400 (1.3) | 20/200 (1) | 20/200 (1) | 20/100 (0.7) | 20/80 (0.6) |
| 21/75/F | Right | pseudophakic | 20/200 (1) | 20/200 (1) | 20/125 (0.8) | 20/100 (0.7) | 20/63 (0.5) |
| Patient ID/Age (YO)/Sex | Eye | Lens Status | Preoperative BCVA—Snellen (LogMAR) | 1-Month BCVA—Snellen (LogMAR) | 3-Month BCVA—Snellen (LogMAR) | 6-Month BCVA—Snellen (LogMAR) | 12-Month BCVA—Snellen (LogMAR) |
|---|---|---|---|---|---|---|---|
| 1/80/M | Right | phakic | 20/2000 (2) | 20/2000 (2) | 20/2000 (2) | 20/2000 (2) | 20/2000 (2) |
| 2/85/F | Left | pseudophakic | 20/2000 (2) | 20/2000 (2) | 20/800 (1.6) | 20/400 (1.3) | 20/400 (1.3) |
| 3/86/F | Left | pseudophakic | 20/2000 (2) | 20/2000 (2) | 20/400 (1.3) | 20/400 (1.3) | 20/400 (1.3) |
| 4/79/M | Right | pseudophakic | 20/800 (1.6) | 20/200 (1) | 20/200 (1) | 20/125 (0.8) | 20/100 (0.7) |
| 5/85/M | Right | pseudophakic | 20/800 (1.6) | 20/200 (1) | 20/200 (1) | 20/200 (1) | 20/200 (1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caporossi, T.; Scampoli, A.; Baldascino, A.; Gambini, G.; Pacini, B.; Governatori, L.; Bacherini, D.; Carlà, M.M.; Crincoli, E.; Rizzo, C.; et al. Subretinal Transplant of Human Amniotic Membrane in Advanced Age-Related Macular Degeneration. Life 2022, 12, 1998. https://doi.org/10.3390/life12121998
Caporossi T, Scampoli A, Baldascino A, Gambini G, Pacini B, Governatori L, Bacherini D, Carlà MM, Crincoli E, Rizzo C, et al. Subretinal Transplant of Human Amniotic Membrane in Advanced Age-Related Macular Degeneration. Life. 2022; 12(12):1998. https://doi.org/10.3390/life12121998
Chicago/Turabian StyleCaporossi, Tomaso, Alessandra Scampoli, Antonio Baldascino, Gloria Gambini, Bianca Pacini, Lorenzo Governatori, Daniela Bacherini, Matteo Mario Carlà, Emanuele Crincoli, Clara Rizzo, and et al. 2022. "Subretinal Transplant of Human Amniotic Membrane in Advanced Age-Related Macular Degeneration" Life 12, no. 12: 1998. https://doi.org/10.3390/life12121998
APA StyleCaporossi, T., Scampoli, A., Baldascino, A., Gambini, G., Pacini, B., Governatori, L., Bacherini, D., Carlà, M. M., Crincoli, E., Rizzo, C., Kilian, R., & Rizzo, S. (2022). Subretinal Transplant of Human Amniotic Membrane in Advanced Age-Related Macular Degeneration. Life, 12(12), 1998. https://doi.org/10.3390/life12121998









