Quercetin Derivatives in Combating Spinal Cord Injury: A Mechanistic and Systematic Review
Abstract
:1. Introduction
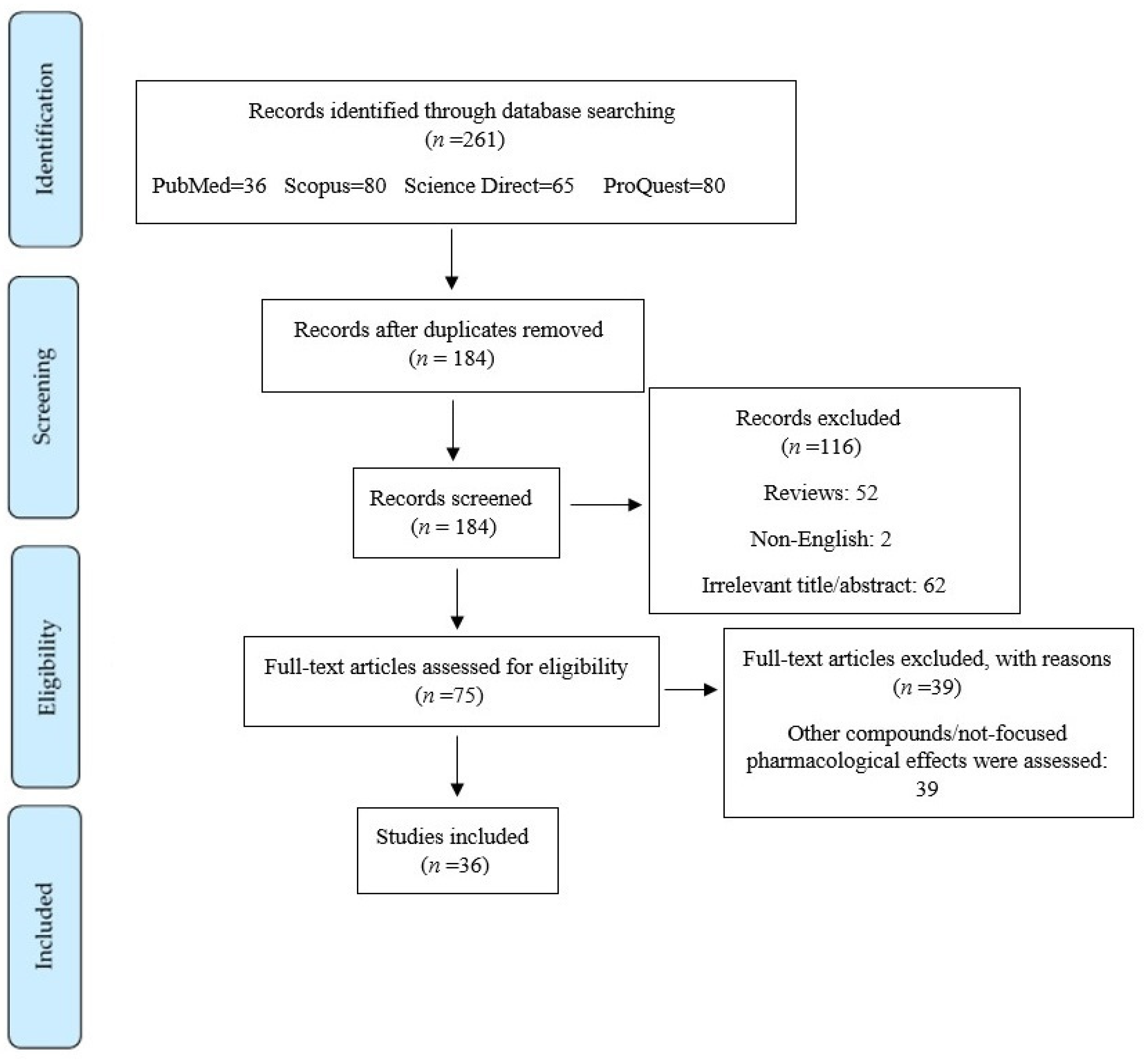
2. Materials and Methods
3. Results and Discussion
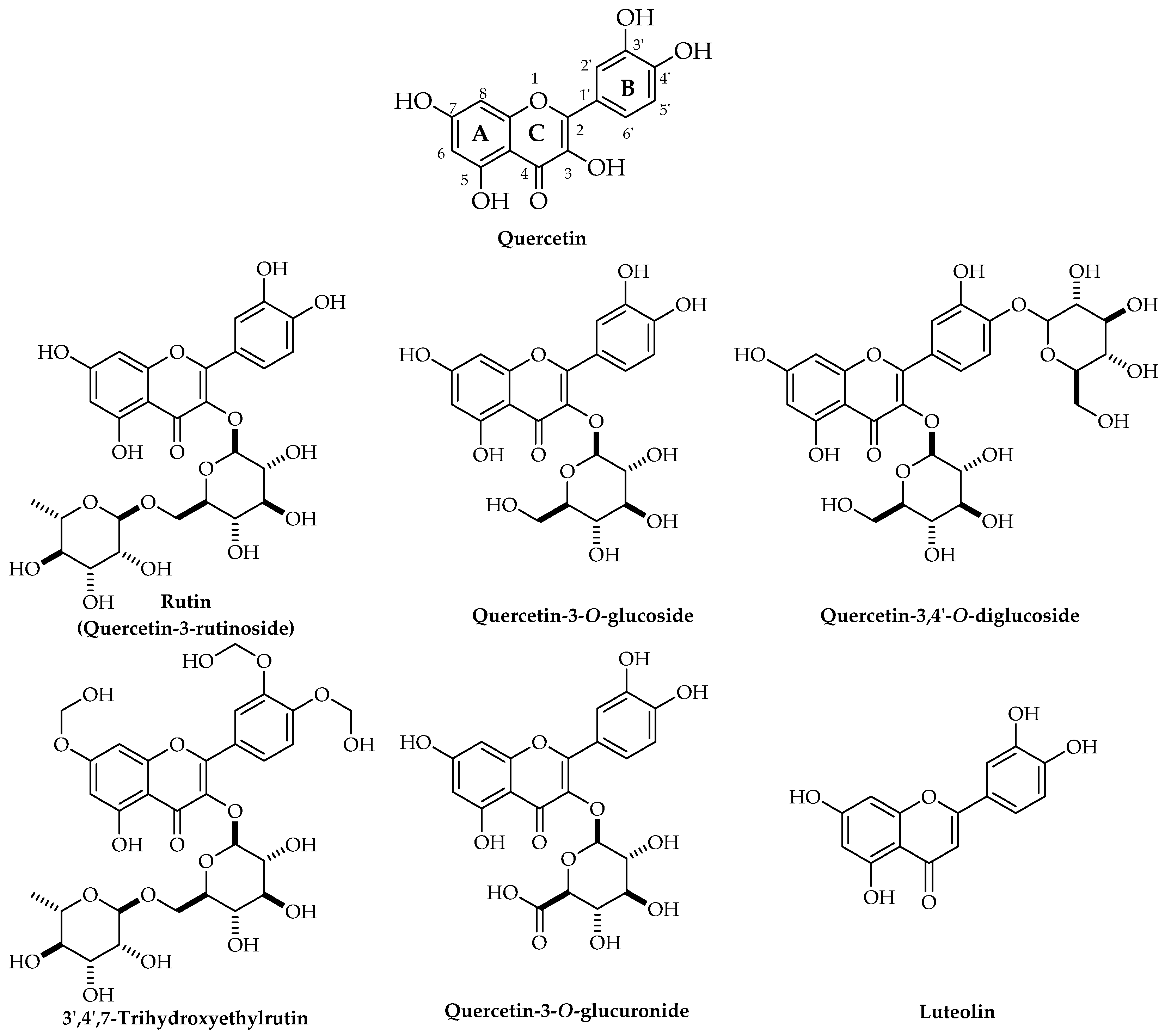
3.1. Biological Sources, Chemical Structure, and Pharmacokinetics of Quercetin
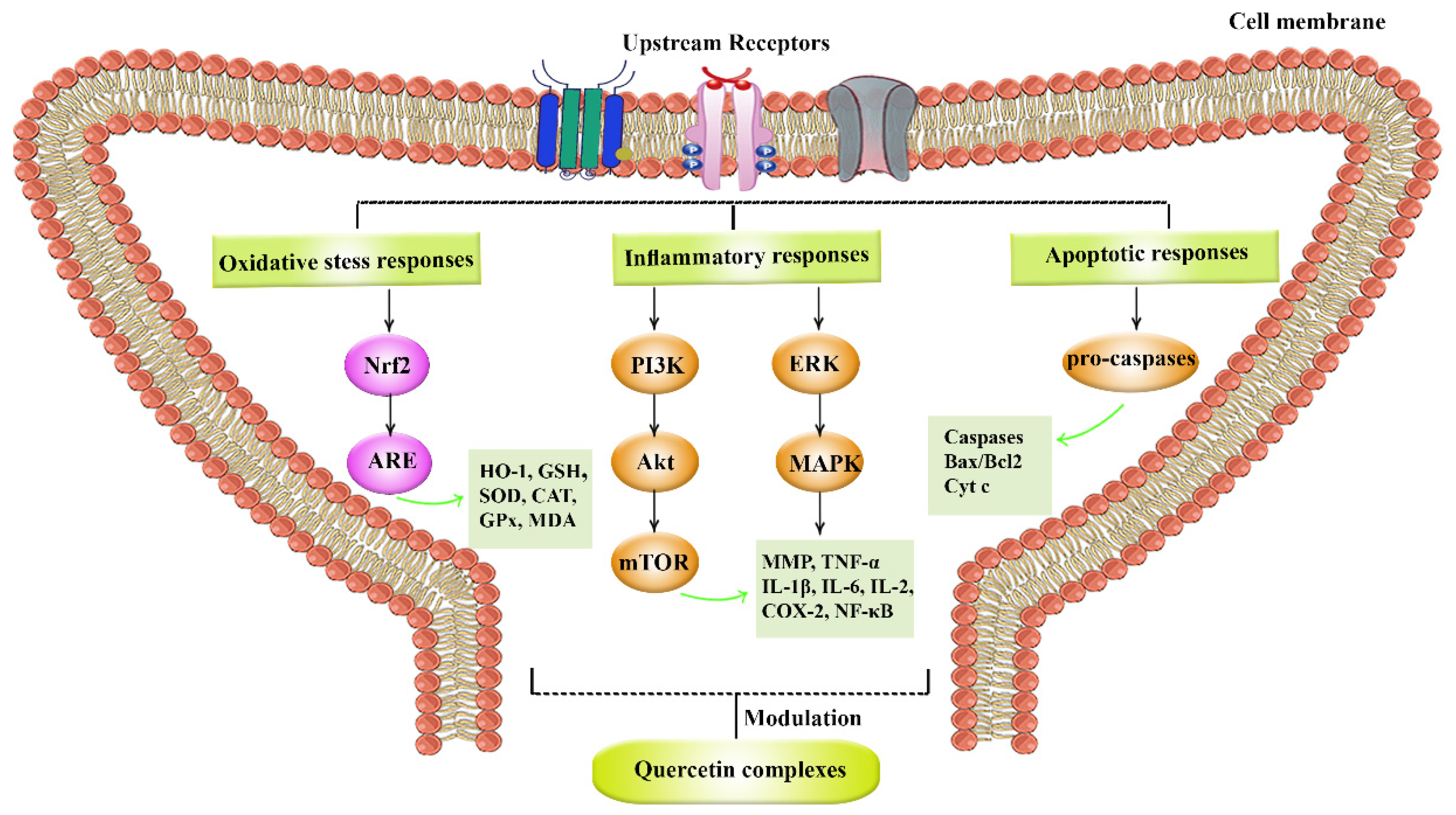
3.2. Neuroprotective Potentials of Quercetin Derivatives
3.3. Pathophysiological Mechanisms of Spinal Cord Injury
3.3.1. Inflammatory Responses
3.3.2. ROS and Free Radicals
3.3.3. NMDA, Excitatory Amino Acids, and Opiate Receptors
3.3.4. Apoptosis
3.3.5. Local Vascular Effects
3.4. Quercetin Derivatives against Spinal Cord Injury
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Alizadeh, A.; Dyck, S.M.; Karimi-Abdolrezaee, S. Traumatic Spinal Cord Injury: An Overview of Pathophysiology, Models and Acute Injury Mechanisms. Front. Neurol. 2019, 10, 282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, S.; Shi, Z.; Cao, F.; Li, J.; Feng, S. Epidemiological features of spinal cord injury in China: A systematic review. Front. Neurol. 2018, 9, 683. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, S.; Sabouri, S.; Kiani, A.; Farzaei, M.H.; Rashidi, K.; Mohammadi-Farani, A.; Mohammadi-Noori, E.; Abbaszadeh, F. Intrathecal administration of naringenin improves motor dysfunction and neuropathic pain following compression spinal cord injury in rats: Relevance to its antioxidant and anti-inflammatory activities. Korean J. Pain 2022, 35, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, S.; Dargahi, L.; Abbaszadeh, F.; Jorjani, M. Effects of astaxanthin on sensory-motor function in a compression model of spinal cord injury: Involvement of ERK and AKT signalling pathway. Eur. J. Pain 2019, 23, 750–764. [Google Scholar] [CrossRef] [PubMed]
- Abbaszadeh, F.; Fakhri, S.; Khan, H. Targeting apoptosis and autophagy following spinal cord injury: Therapeutic approaches to polyphenols and candidate phytochemicals. Pharmacol. Res. 2020, 160, 105069. [Google Scholar] [CrossRef]
- Fakhri, S.; Abbaszadeh, F.; Jorjani, M. On the therapeutic targets and pharmacological treatments for pain relief following spinal cord injury: A mechanistic review. Biomed. Pharmacother. 2021, 139, 111563. [Google Scholar] [CrossRef]
- Jawad, M.; Khan, H.; Pervaiz, S.; Bawazeer, S.S.; Abu-Izneid, T.; Saeed, M.; Kamal, M.A. Pharmacological validation of the anxiolytic, muscle relaxant and sedative like activities of Capsicum annuum in animal model. Bangladesh J. Pharmacol. 2017, 12, 439–447. [Google Scholar] [CrossRef] [Green Version]
- Karim, N.; Khan, I.; Khan, H.; Ayub, B.; Abdel-Halim, H.; Gavande, N. Anxiolytic potential of natural flavonoids. SM J. Endocrinol. Metab. 2018, 4, 1018s. [Google Scholar]
- Fakhri, S.; Moradi, S.Z.; Nouri, Z.; Cao, H.; Wang, H.; Khan, H.; Xiao, J. Modulation of integrin receptor by polyphenols: Downstream Nrf2-Keap1/ARE and associated cross-talk mediators in cardiovascular diseases. Crit. Rev. Food Sci. Nutr. 2022, 8, 1–25. [Google Scholar] [CrossRef]
- Fakhri, S.; Tomas, M.; Capanoglu, E.; Hussain, Y.; Abbaszadeh, F.; Lu, B.; Hu, X.; Wu, J.; Zou, L.; Smeriglio, A. Antioxidant and anticancer potentials of edible flowers: Where do we stand? Crit. Rev. Food Sci. Nutr. 2022, 62, 8589–8645. [Google Scholar] [CrossRef]
- Naseri, R.; Farzaei, F.; Fakhri, S.; El-Senduny, F.F.; Altouhamy, M.; Bahramsoltani, R.; Ebrahimi, F.; Rahimi, R.; Farzaei, M.H. Polyphenols for diabetes associated neuropathy: Pharmacological targets and clinical perspective. Daru 2019, 27, 781–798. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, S.; Piri, S.; Majnooni, M.B.; Farzaei, M.H.; Echeverria, J. Targeting neurological manifestations of coronaviruses by candidate phytochemicals: A mechanistic approach. Front. Pharmacol. 2021, 11, 621099. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi Pour, P.; Fakhri, S.; Asgary, S.; Farzaei, M.H.; Echeverria, J. The signaling pathways, and therapeutic targets of antiviral agents: Focusing on the antiviral approaches and clinical perspectives of anthocyanins in the management of viral diseases. Front. Pharmacol. 2019, 10, 1207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Septembre-Malaterre, A.; Boumendjel, A.; Seteyen, A.-L.S.; Boina, C.; Gasque, P.; Guiraud, P.; Sélambarom, J. Focus on the high therapeutic potentials of quercetin and its derivatives. Phytomed. Plus 2022, 2, 100220. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Ullah, H.; Martorell, M.; Valdes, S.E.; Belwal, T.; Tejada, S.; Sureda, A.; Kamal, M.A. Flavonoids nanoparticles in cancer: Treatment, prevention and clinical prospects. Semin. Cancer Biol. 2021, 69, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Solanki, I.; Parihar, P.; Mansuri, M.L.; Parihar, M.S. Flavonoid-based therapies in the early management of neurodegenerative diseases. Adv. Nutr. 2015, 6, 64–72. [Google Scholar] [CrossRef] [Green Version]
- Yeung, A.W.K.; Choudhary, N.; Tewari, D.; El-Demerdash, A.; Horbanczuk, O.K.; Das, N.; Pirgozliev, V.; Lucarini, M.; Durazzo, A.; Souto, E.B.; et al. Quercetin: Total-scale literature landscape analysis of a valuable nutraceutical with numerous potential applications in the promotion of human and animal health—A review. Anim. Sci. Pap. Rep. 2021, 39, 13. [Google Scholar]
- Kawabata, K.; Mukai, R.; Ishisaka, A. Quercetin and related polyphenols: New insights and implications for their bioactivity and bioavailability. Food Funct. 2015, 6, 1399–1417. [Google Scholar] [CrossRef] [PubMed]
- Harwood, M.; Danielewska-Nikiel, B.; Borzelleca, J.; Flamm, G.; Williams, G.; Lines, T. A critical review of the data related to the safety of quercetin and lack of evidence of in vivo toxicity, including lack of genotoxic/carcinogenic properties. Food Chem. Toxicol. 2007, 45, 2179–2205. [Google Scholar] [CrossRef]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, inflammation and immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef] [Green Version]
- Rishitha, N.; Muthuraman, A. Therapeutic evaluation of solid lipid nanoparticle of quercetin in pentylenetetrazole induced cognitive impairment of zebrafish. Life Sci. 2018, 199, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, H.; Gao, Y.; Li, L.; Tang, C.; Wen, G.; Zhou, Y.; Zhou, M.; Mao, L.; Fan, Y. Protective effects of quercetin on mitochondrial biogenesis in experimental traumatic brain injury via the Nrf2 signaling pathway. PLoS ONE 2016, 11, e0164237. [Google Scholar] [CrossRef] [PubMed]
- El-Horany, H.E.; El-latif, R.N.A.; ElBatsh, M.M.; Emam, M.N. Ameliorative effect of quercetin on neurochemical and behavioral deficits in rotenone rat model of Parkinson’s disease: Modulating autophagy (quercetin on experimental Parkinson’s disease). J. Biochem. Mol. Toxicol. 2016, 30, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Sandhir, R.; Mehrotra, A. Quercetin supplementation is effective in improving mitochondrial dysfunctions induced by 3-nitropropionic acid: Implications in Huntington’s disease. Biochim. Biophys. Acta 2013, 1832, 421–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moradi, S.Z.; Momtaz, S.; Bayrami, Z.; Farzaei, M.H.; Abdollahi, M. Nanoformulations of herbal extracts in treatment of neurodegenerative disorders. Front. Bioeng. Biotechnol. 2020, 8, 238. [Google Scholar] [CrossRef] [PubMed]
- Moradi, S.Z.; Jalili, F.; Farhadian, N.; Joshi, T.; Wang, M.; Zou, L.; Cao, H.; Farzaei, M.H.; Xiao, J. Polyphenols and neurodegenerative diseases: Focus on neuronal regeneration. Crit. Rev. Food Sci. Nutr. 2022, 62, 3421–3436. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, S.; Piri, S.; Moradi, S.Z.; Khan, H. Phytochemicals Targeting Oxidative Stress, Interconnected Neuroinflammatory, and Neuroapoptotic Pathways Following Radiation. Curr. Neuropharmacol. 2022, 20, 836–856. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, S.; Pesce, M.; Patruno, A.; Moradi, S.Z.; Iranpanah, A.; Farzaei, M.H.; Sobarzo-Sánchez, E. Attenuation of Nrf2/Keap1/ARE in Alzheimer’s disease by plant secondary metabolites: A mechanistic review. Molecules 2020, 25, 4926. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, S.; Moradi, S.Z.; Yarmohammadi, A.; Narimani, F.; Wallace, C.E.; Bishayee, A. Modulation of TLR/NF-κB/NLRP signaling by bioactive phytocompounds: A promising strategy to augment cancer chemotherapy and immunotherapy. Front. Oncol. 2022, 12, 834072. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, S.; Iranpanah, A.; Gravandi, M.M.; Moradi, S.Z.; Ranjbari, M.; Majnooni, M.B.; Echeverría, J.; Qi, Y.; Wang, M.; Liao, P. Natural products attenuate PI3K/Akt/mTOR signaling pathway: A promising strategy in regulating neurodegeneration. Phytomedicine 2021, 91, 153664. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, S.; Abbaszadeh, F.; Moradi, S.Z.; Cao, H.; Khan, H.; Xiao, J. Effects of polyphenols on oxidative stress, inflammation, and interconnected pathways during spinal cord injury. Oxid. Med. Cell. Longev. 2022, 2022, 8100195. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Fu, Y.; Botchway, B.O.; Zhang, Y.; Zhang, Y.; Jin, T.; Liu, X. Quercetin Can Improve Spinal Cord Injury by Regulating the mTOR Signaling Pathway. Front. Neurol. 2022, 13, 905640. [Google Scholar] [CrossRef] [PubMed]
- Biesaga, M.; Pyrzynska, K. Analytical procedures for determination of quercetin and its glycosides in plant material. Crit. Rev. Anal. Chem. 2009, 39, 95–107. [Google Scholar] [CrossRef]
- Shi, Z.-H.; Li, N.-G.; Tang, Y.-P.; Shi, Q.-P.; Zhang, W.; Zhang, P.-X.; Dong, Z.-X.; Li, W.; Zhang, X.; Fu, H.-A. Synthesis, biological evaluation and SAR analysis of O-alkylated analogs of quercetin for anticancer. Bioorg. Med. Chem. Lett. 2014, 24, 4424–4427. [Google Scholar] [CrossRef] [PubMed]
- Abraham, M.H.; Acree, W.E., Jr. On the solubility of quercetin. J. Mol. Liq. 2014, 197, 157–159. [Google Scholar] [CrossRef]
- Nathiya, S.; Durga, M.; Thiyagarajan, D. Quercetin, encapsulated quercetin and its application-a review. Int. J. Pharm. Pharm. 2014, 6, 20–26. [Google Scholar]
- Cai, X.; Fang, Z.; Dou, J.; Yu, A.; Zhai, G. Bioavailability of quercetin: Problems and promises. Curr. Med. Chem. 2013, 20, 2572–2582. [Google Scholar] [CrossRef]
- Hirpara, K.V.; Aggarwal, P.; Mukherjee, A.J.; Joshi, N.; Burman, A.C. Quercetin and its derivatives: Synthesis, pharmacological uses with special emphasis on anti-tumor properties and prodrug with enhanced bio-availability. Anticancer Agents Med. Chem. 2009, 9, 138–161. [Google Scholar] [CrossRef]
- Fakhri, S.; Abbaszadeh, F.; Dargahi, L.; Jorjani, M. Astaxanthin: A mechanistic review on its biological activities and health benefits. Pharmacol. Res. 2018, 136, 1–20. [Google Scholar] [CrossRef]
- Alizadeh, S.R.; Ebrahimzadeh, M.A. O-Glycoside quercetin derivatives: Biological activities, mechanisms of action, and structure–activity relationship for drug design, a review. Phytother. Res. 2022, 36, 778–807. [Google Scholar] [CrossRef]
- Islam, M.S.; Quispe, C.; Hossain, R.; Islam, M.T.; Al-Harrasi, A.; Al-Rawahi, A.; Martorell, M.; Mamurova, A.; Seilkhan, A.; Altybaeva, N. Neuropharmacological effects of quercetin: A literature-based review. Front. Pharmacol. 2021, 12, 665031. [Google Scholar] [CrossRef]
- Caruana, M.; Cauchi, R.; Vassallo, N. Putative role of red wine polyphenols against brain pathology in Alzheimer’s and Parkinson’s disease. Front. Nutr. 2016, 3, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jantan, I.; Ahmad, W.; Bukhari, S.N.A. Plant-derived immunomodulators: An insight on their preclinical evaluation and clinical trials. Front. Plant Sci. 2015, 6, 655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barreca, D.; Bellocco, E.; DOnofrio, G.; Fazel Nabavi, S.; Daglia, M.; Rastrelli, L.; Mohammad Nabavi, S. Neuroprotective effects of quercetin: From chemistry to medicine. CNS Neurol. Disord. Drug Targets 2016, 15, 964–975. [Google Scholar] [CrossRef] [PubMed]
- Ay, M.; Luo, J.; Langley, M.; Jin, H.; Anantharam, V.; Kanthasamy, A.; Kanthasamy, A.G. Molecular mechanisms underlying protective effects of quercetin against mitochondrial dysfunction and progressive dopaminergic neurodegeneration in cell culture and MitoPark transgenic mouse models of Parkinson’s Disease. J. Neurochem. 2017, 141, 766–782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- David, A.V.A.; Arulmoli, R.; Parasuraman, S. Overviews of biological importance of quercetin: A bioactive flavonoid. Pharmacogn. Rev. 2016, 10, 84. [Google Scholar]
- Ozgen, S.; Kilinc, O.K.; Selamoğlu, Z. Antioxidant activity of quercetin: A mechanistic review. TURJAF 2016, 4, 1134–1138. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.-M.; Li, S.-Q.; Wu, W.-L.; Zhu, X.-Y.; Wang, Y.; Yuan, H.-Y. Effects of long-term treatment with quercetin on cognition and mitochondrial function in a mouse model of Alzheimer’s disease. Neurochem. Res. 2014, 39, 1533–1543. [Google Scholar] [CrossRef]
- Richetti, S.; Blank, M.; Capiotti, K.; Piato, A.; Bogo, M.; Vianna, M.; Bonan, C. Quercetin and rutin prevent scopolamine-induced memory impairment in zebrafish. Behav. Brain. Res. 2011, 217, 10–15. [Google Scholar] [CrossRef]
- Khan, M.T.H.; Orhan, I.; Şenol, F.; Kartal, M.; Şener, B.; Dvorská, M.; Šmejkal, K.; Šlapetová, T. Cholinesterase inhibitory activities of some flavonoid derivatives and chosen xanthone and their molecular docking studies. Chem. Biol. Interact. 2009, 181, 383–389. [Google Scholar] [CrossRef]
- Shimmyo, Y.; Kihara, T.; Akaike, A.; Niidome, T.; Sugimoto, H. Flavonols and flavones as BACE-1 inhibitors: Structure–activity relationship in cell-free, cell-based and in silico studies reveal novel pharmacophore features. Biochim. Biophys. Acta 2008, 1780, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Ullah, H.; Aschner, M.; Cheang, W.S.; Akkol, E.K. Neuroprotective effects of quercetin in Alzheimer’s disease. Biomolecules 2019, 10, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rifaai, R.A.; Mokhemer, S.A.; Saber, E.A.; Abd El-Aleem, S.A.; El-Tahawy, N.F.G. Neuroprotective effect of quercetin nanoparticles: A possible prophylactic and therapeutic role in alzheimer’s disease. J. Chem. Neuroanat. 2020, 107, 101795. [Google Scholar] [CrossRef] [PubMed]
- Akyuz, E.; Paudel, Y.N.; Polat, A.K.; Dundar, H.E.; Angelopoulou, E. Enlightening the neuroprotective effect of quercetin in epilepsy: From mechanism to therapeutic opportunities. Epilepsy Behav. 2021, 115, 107701. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Raj, K.; Singh, S. Neuroprotective effect of quercetin in combination with piperine against rotenone-and iron supplement–induced Parkinson’s disease in experimental rats. Neurotox. Res. 2020, 37, 198–209. [Google Scholar] [CrossRef]
- Ghaffari, F.; Moghaddam, A.H.; Zare, M. Neuroprotective effect of quercetin nanocrystal in a 6-hydroxydopamine model of Parkinson disease: Biochemical and behavioral evidence. Basic. Clin. Neurosci. 2018, 9, 317–324. [Google Scholar] [CrossRef]
- Anjum, A.; Yazid, M.D.; Fauzi Daud, M.; Idris, J.; Ng, A.M.H.; Selvi Naicker, A.; Ismail, O.H.R.; Athi Kumar, R.K.; Lokanathan, Y. Spinal Cord Injury: Pathophysiology, Multimolecular Interactions, and Underlying Recovery Mechanisms. Int. J. Mol. Sci. 2020, 21, 7533. [Google Scholar] [CrossRef]
- Zhang, Y.; Al Mamun, A.; Yuan, Y.; Lu, Q.; Xiong, J.; Yang, S.; Wu, C.; Wu, Y.; Wang, J. Acute spinal cord injury: Pathophysiology and pharmacological intervention (Review). Mol. Med. Rep. 2021, 23, 417. [Google Scholar] [CrossRef]
- Hachem, L.D.; Fehlings, M.G. Pathophysiology of spinal cord injury. Clin. Neurosurg. 2021, 32, 305–313. [Google Scholar] [CrossRef]
- Sharma, H.S. Early microvascular reactions and blood–spinal cord barrier disruption are instrumental in pathophysiology of spinal cord injury and repair: Novel therapeutic strategies including nanowired drug delivery to enhance neuroprotection. J. Neural. Transm. 2011, 118, 155–176. [Google Scholar] [CrossRef]
- Eldahan, K.C.; Rabchevsky, A.G. Autonomic dysreflexia after spinal cord injury: Systemic pathophysiology and methods of management. Auton. Neurosci. 2018, 209, 59–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fakhri, S.; Moradi, S.Z.; Ash-Rafzadeh, A.; Bishayee, A. Targeting cellular senescence in cancer by plant secondary metabolites: A systematic review. Pharmacol. Res. 2021, 177, 105961. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, S.; Moradi, S.Z.; Farzaei, M.H.; Bishayee, A. Modulation of dysregulated cancer metabolism by plant secondary metabolites: A mechanistic review. Semin. Cancer. Biol. 2020, 80, 276–305. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, S.; Nouri, Z.; Moradi, S.Z.; Akkol, E.K.; Piri, S.; Sobarzo-Sanchez, E.; Farzaei, M.H.; Echeverría, J. Targeting multiple signal transduction pathways of SARS-CoV-2: Approaches to COVID-19 therapeutic candidates. Molecules 2021, 26, 2917. [Google Scholar] [CrossRef]
- Moghaddam, R.H.; Samimi, Z.; Moradi, S.Z.; Little, P.J.; Xu, S.; Farzaei, M.H. Naringenin and naringin in cardiovascular disease prevention: A preclinical review. Eur. J. Pharmacol. 2020, 887, 173535. [Google Scholar] [CrossRef]
- Aarabi, B.; Olexa, J.; Chryssikos, T.; Galvagno, S.M.; Hersh, D.S.; Wessell, A.; Sansur, C.; Schwartzbauer, G.; Crandall, K.; Shanmuganathan, K. Extent of spinal cord decompression in motor complete (American Spinal Injury Association Impairment Scale Grades A and B) traumatic spinal cord injury patients: Post-operative magnetic resonance imaging analysis of standard operative approaches. J. Neurotrauma 2019, 36, 862–876. [Google Scholar] [CrossRef] [Green Version]
- Baklaushev, V.; Durov, O.; Kim, S.; Gulaev, E.; Gubskiy, I.; Konoplyannikov, M.; Zabozlaev, F.; Zhang, C.; Agrba, V.; Orlov, S. Development of a motor and somatosensory evoked potentials-guided spinal cord Injury model in non-human primates. J. Neurosci. Methods 2019, 311, 200–214. [Google Scholar] [CrossRef]
- De Menezes, M.F.; Nicola, F.; da Silva, I.R.V.; Vizuete, A.; Elsner, V.R.; Xavier, L.L.; Gonçalves, C.A.S.; Netto, C.A.; Mestriner, R.G. Glial fibrillary acidic protein levels are associated with global histone H4 acetylation after spinal cord injury in rats. Neural. Regen. Res. 2018, 13, 1945–1952. [Google Scholar]
- Winkler, T.; Sharma, H.S.; Stålberg, E.; Badgaiyan, R.D.; Gordh, T.; Westman, J. An L-type calcium channel blocker, nimodipine influences trauma induced spinal cord conduction and axonal injury in the rat. Acta Neurochir. Suppl. 2003, 86, 425–432. [Google Scholar]
- Burnside, E.R.; De Winter, F.; Didangelos, A.; James, N.D.; Andreica, E.-C.; Layard-Horsfall, H.; Muir, E.M.; Verhaagen, J.; Bradbury, E.J. Immune-evasive gene switch enables regulated delivery of chondroitinase after spinal cord injury. Brain 2018, 141, 2362–2381. [Google Scholar] [CrossRef] [Green Version]
- Casper, D.S.; Zmistowski, B.; Schroeder, G.D.; McKenzie, J.C.; Mangan, J.; Vatson, J.; Hilibrand, A.S.; Vaccaro, A.R.; Kepler, C.K. Preinjury patient characteristics and postinjury neurological status are associated with mortality following spinal cord injury. Spine 2018, 43, 895–899. [Google Scholar] [CrossRef] [PubMed]
- David, S.; Kroner, A.; Greenhalgh, A.D.; Zarruk, J.G.; López-Vales, R. Myeloid cell responses after spinal cord injury. J. Neuroimmunol. 2018, 321, 97–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huber, E.; David, G.; Thompson, A.J.; Weiskopf, N.; Mohammadi, S.; Freund, P. Dorsal and ventral horn atrophy is associated with clinical outcome after spinal cord injury. Neurology 2018, 90, e1510–e1522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, P.; Ma, X. Effect of rutin on spinal cord injury through inhibition of the expression of MIP-2 and activation of MMP-9, and downregulation of Akt phosphorylation. Mol. Med. Rep. 2015, 12, 7554–7560. [Google Scholar] [CrossRef] [Green Version]
- Song, H.-l.; Zhang, X.; Wang, W.-z.; Liu, R.-h.; Zhao, K.; Liu, M.-y.; Gong, W.-m.; Ning, B. Neuroprotective mechanisms of rutin for spinal cord injury through anti-oxidation and anti-inflammation and inhibition of p38 mitogen activated protein kinase pathway. Neural. Regen. Res. 2018, 13, 128–134. [Google Scholar]
- Yao, S.; Wang, L.; Chen, Q.; Lu, T.; Pu, X.; Luo, C. The effect of mild hypothermia plus rutin on the treatment of spinal cord injury and inflammatory factors by repressing TGF-β/smad pathway. Acta Cir. Bras. 2021, 36, e360307. [Google Scholar] [CrossRef]
- Wu, J.; Maoqiang, L.; Fan, H.; Zhenyu, B.; Qifang, H.; Xuepeng, W.; Liulong, Z. Rutin attenuates neuroinflammation in spinal cord injury rats. J. Surg. Res. 2016, 203, 331–337. [Google Scholar] [CrossRef]
- Lim, E.Y.; Lee, C.; Kim, Y.T. The Antinociceptive Potential of Camellia japonica Leaf Extract, (-)-Epicatechin, and Rutin against Chronic Constriction Injury-Induced Neuropathic Pain in Rats. Antioxidants 2022, 11, 410. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Q.; Yang, Z.; Li, R.; Huang, Z.; Huang, Z.; Liu, J.; Wu, X.; Lin, J.; Wu, X. Trihydroxyethyl Rutin Provides Neuroprotection in Rats with Cervical Spinal Cord Hemi-Contusion. Front. Neurosci. 2021, 15, 759325. [Google Scholar] [CrossRef]
- Chen, M.-M.; Qin, J.; Chen, S.-J.; Yao, L.-M.; Zhang, L.-Y.; Yin, Z.-Q.; Liao, H. Quercetin promotes motor and sensory function recovery following sciatic nerve-crush injury in C57BL/6J mice. J. Nutr. Biochem. 2017, 46, 57–67. [Google Scholar] [CrossRef]
- Schültke, E.; Kendall, E.; Kamencic, H.; Ghong, Z.; Griebel, R.; Juurlink, B. Quercetin promotes functional recovery following acute spinal cord injury. J. Neurotrauma 2003, 20, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Sauerbeck, A.; Schonberg, D.L.; Laws, J.L.; McTigue, D.M. Systemic iron chelation results in limited functional and histological recovery after traumatic spinal cord injury in rats. Exp. Neurol. 2013, 248, 53–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, L.G.; Garrick, J.M.; Roquè, P.J.; Pellacani, C. Mechanisms of Neuroprotection by Quercetin: Counteracting Oxidative Stress and More. Oxid. Med. Cell. Longev. 2016, 2016, 2986796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juurlink, B.H.; Paterson, P.G. Review of oxidative stress in brain and spinal cord injury: Suggestions for pharmacological and nutritional management strategies. J. Spinal Cord Med. 1998, 21, 309–334. [Google Scholar] [CrossRef]
- Çiftçi, U.; Delen, E.; Vural, M.; Uysal, O.; Coşan, D.T.; Baydemir, C.; Doğaner, F. Efficiacy of resveratrol and quercetin after experimental spinal cord injury. Ulus Travma. Acil. Cerrahi. Derg. 2016, 22, 423–431. [Google Scholar]
- Song, Y.; Liu, J.; Zhang, F.; Zhang, J.; Shi, T.; Zeng, Z. Antioxidant effect of quercetin against acute spinal cord injury in rats and its correlation with the p38MAPK/iNOS signaling pathway. Life Sci. 2013, 92, 1215–1221. [Google Scholar] [CrossRef]
- Schültke, E.; Kamencic, H.; Skihar, V.; Griebel, R.; Juurlink, B. Quercetin in an animal model of spinal cord compression injury: Correlation of treatment duration with recovery of motor function. Spinal Cord. 2010, 48, 112–117. [Google Scholar] [CrossRef] [Green Version]
- Ocal, O.; Börcek, A.; Pasaoglu, O.; Gundogdu, A.; Kaplanoğlu, G.T.; Baykaner, M. Can quercetin be an option for treatment of spinal cord injury? An experimental study. Turk. Neurosurg. 2019, 29, 247–253. [Google Scholar] [CrossRef]
- Çevik, Ö.; Erşahin, M.; Şener, T.E.; Tinay, İ.; Tarcan, T.; Çetinel, Ş.; Şener, A.; Toklu, H.Z.; Şener, G. Beneficial effects of quercetin on rat urinary bladder after spinal cord injury. J. Surg. Res. 2013, 183, 695–703. [Google Scholar] [CrossRef]
- Firgany, A.E.-D.L.; Sarhan, N.R. Quercetin mitigates monosodium glutamate-induced excitotoxicity of the spinal cord motoneurons in aged rats via p38 MAPK inhibition. Acta Histochem. 2020, 122, 151554. [Google Scholar] [CrossRef]
- Muto, N.; Matsuoka, Y.; Arakawa, K.; Kurita, M.; Omiya, H.; Taniguchi, A.; Kaku, R.; Morimatsu, H. Quercetin attenuates neuropathic pain in rats with spared nerve injury. Acta Med. Okayama 2018, 72, 457–465. [Google Scholar] [PubMed]
- Zaragozá, C.; Villaescusa, L.; Monserrat, J.; Zaragozá, F.; Álvarez-Mon, M. Potential therapeutic anti-inflammatory and immunomodulatory effects of dihydroflavones, flavones, and flavonols. Molecules 2020, 25, 1017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, H.; Tang, H.-B.; Shan, L.-Q.; Liu, S.-C.; Huang, D.-G.; Chen, X.; Chen, Z.; Yang, M.; Yin, X.-H.; Yang, H. Quercetin prevents necroptosis of oligodendrocytes by inhibiting macrophages/microglia polarization to M1 phenotype after spinal cord injury in rats. J. Neuroinflamm. 2019, 16, 206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schültke, E.; Griebel, R.; Juurlink, B. Quercetin attenuates inflammatory processes after spinal cord injury in an animal model. Spinal Cord. 2010, 48, 857–861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Li, W.; Wang, M.; Lin, C.; Li, G.; Zhou, X.; Luo, J.; Jin, D. Quercetin reduces neural tissue damage and promotes astrocyte activation after spinal cord injury in rats. J. Cell. Biochem. 2018, 119, 2298–2306. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiong, M.; Wang, M.; Chen, H.; Li, W.; Zhou, X. Quercetin promotes locomotor function recovery and axonal regeneration through induction of autophagy after spinal cord injury. Clin. Exp. Pharmacol. Physiol. 2021, 48, 1642–1652. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Zhang, L.; Li, G.; Zhang, H. Combinatory effect of mesenchymal stromal cells transplantation and quercetin after spinal cord injury in rat. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 2876–2887. [Google Scholar]
- Nie, J.-X.; Meng, Q.-T.; Li, R.-B.; Yang, G.; Zhou, W.; Yu, H.-D.; Chen, B.; Jiang, L.; Shang, J.-B. Bone marrow stromal cells with quercetin show a synergistic effect on permeability reduction of blood-spinal cord barrier in rat model with spinal cord injury. Biomed. Res. 2017, 28, 338–344. [Google Scholar]
- Yang, Y.; Liu, X.; Wu, T.; Zhang, W.; Shu, J.; He, Y.; Tang, S.-J. Quercetin attenuates AZT-induced neuroinflammation in the CNS. Sci. Rep. 2018, 8, 6194. [Google Scholar] [CrossRef] [Green Version]
- Karami, A.; Fakhri, S.; Kooshki, L.; Khan, H. Polydatin: Pharmacological Mechanisms, Therapeutic Targets, Biological Activities, and Health Benefits. Molecules 2022, 27, 647. [Google Scholar] [CrossRef]
- Liu, J.-b.; Tang, T.-s.; Yang, H.-l. Antioxidation of quercetin against spinal cord injury in rats. Chin. J. Traumatol. 2006, 9, 303–307. [Google Scholar] [PubMed]
- Çivi, S.; Emmez, G.; Dere, Ü.A.; Börcek, A.; Emmez, H. Effects of quercetin on chronic constriction nerve injury in an experimental rat model. Acta Neurochir. 2016, 158, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Kong, B.; Gu, J.W.; Kuang, Y.Q.; Cheng, L.; Yang, W.T.; Xia, X.; Shu, H.F. Anti-apoptotic and anti-oxidative roles of quercetin after traumatic brain injury. Cell. Mol. Neurobiol. 2014, 34, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Lv, R.; Du, L.; Zhang, L.; Zhang, Z. Polydatin attenuates spinal cord injury in rats by inhibiting oxidative stress and microglia apoptosis via Nrf2/HO-1 pathway. Life Sci. 2019, 217, 119–127. [Google Scholar] [CrossRef]
- Zhao, H.; Mei, X.; Yang, D.; Tu, G. Resveratrol inhibits inflammation after spinal cord injury via SIRT-1/NF-κB signaling pathway. Neurosci. Lett. 2021, 762, 136151. [Google Scholar] [CrossRef]
- Schültke, E.; Griebel, R.; Juurlink, B. Quercetin administration after spinal cord trauma changes S-100β levels. Can. J. Neurol. Sci. 2010, 37, 223–228. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.; Choonara, Y.; Modi, G.; Naidoo, D.; Pillay, V. Cur (Que) min: A neuroactive permutation of Curcumin and Quercetin for treating spinal cord injury. Med. Hypotheses 2014, 82, 437–441. [Google Scholar] [CrossRef]
- Figueira, I.; Menezes, R.; Macedo, D.; Costa, I.; Nunes dos Santos, C. Polyphenols beyond barriers: A glimpse into the brain. Curr. Neuropharmacol. 2017, 15, 562–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nimgampalle, M.; Banavath, H.N.; Chakravarthy, H.; Saxena, A.; Devanathan, V. Novel inhibitors of Rho-kinase mediated neuroinflammatory pathways and their potential application in recovery of injured spinal cord. J. Biomol. Struct. Dyn. 2020, 38, 4669–4686. [Google Scholar] [CrossRef]
- Chen, F.; Hu, M.; Shen, Y.; Zhu, W.; Cao, A.; Ni, B.; Qian, J.; Yang, J. Isorhamnetin promotes functional recovery in rats with spinal cord injury by abating oxidative stress and modulating M2 macrophages/microglia polarization. Eur. J. Pharmacol. 2021, 895, 173878. [Google Scholar] [CrossRef]
- Jiang, W.; Huang, Y.; Han, N.; He, F.; Li, M.; Bian, Z.; Liu, J.; Sun, T.; Zhu, L. Quercetin suppresses NLRP3 inflammasome activation and attenuates histopathology in a rat model of spinal cord injury. Spinal Cord. 2016, 54, 592–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, W.; Pei, B.; Li, Z.; Ou, X.L.; Sun, T.; Zhu, Z. PLGA-PEG-PLGA hydrogel with NEP1-40 promotes the functional recovery of brachial plexus root avulsion in adult rats. PeerJ 2021, 9, e12269. [Google Scholar] [CrossRef] [PubMed]
- Paramos-de-Carvalho, D.; Martins, I.; Cristóvão, A.M.; Dias, A.F.; Neves-Silva, D.; Pereira, T.; Chapela, D.; Farinho, A.; Jacinto, A.; Saúde, L. Targeting senescent cells improves functional recovery after spinal cord injury. Cell Rep. 2021, 36, 109334. [Google Scholar] [CrossRef] [PubMed]
| Quercetin and Derivatives | Study Type | Cell Line/Animal Type | Mechanism | Reference |
|---|---|---|---|---|
| Quercetin | In vivo | Sprague-Dawley rats | ↓ROS, ↓NLRP3, ↓TNF-α, ↓IL-1β | [111] |
| ↓neuropathic pain, ↓satellite glial cells, ↑ability to walk, ↑Cldn5, ↑Ocln, ↑Tjp1 | [81,87,98] | |||
| ↑SOD, ↑GSH, ↓polymorphonuclearleukocyte infiltration, | [85] | |||
| ↓iNOS, ↓p38MAPK, ↑SOD, ↑MDA | [86] | |||
| ↓MDA, ↓NO | [112] | |||
| ↓GFAP, ↓neuropathic pain | [91] | |||
| ↓TNF-α, ↓IL-1β, ↓IL-6, ↓ IL-8 | [92] | |||
| ↓STAT1, ↓NF-κB | [93] | |||
| ↓MPO | [94] | |||
| ↑5-HT-positvie nerve fibers, ↑BDNF | [95] | |||
| ↓GFAP, ↓phosphorylation of Akt, ↓mTOR, | [96] | |||
| ↑IL-4, ↑IL-10, ↑TGF, ↓IL-6, ↓IL-1 | [97] | |||
| C57BL/6J mice | ↑neuronal intrinsic growth capacity, ↑functional recovery, | [80] | ||
| Wistar albino rats | ↓MDA, ↓NO, ↓caspase-3, ↑SOD, ↑GSH | [89] | ||
| Quercetin 3,4′-O-β-D-diglucoside | In vivo | Sprague-Dawley rats | ↓MDA, ↓NO, ↑total antioxidant levels | [88] |
| Quercetin-3-O-glucuronide | In vivo | Sprague-Dawley rats | ↓MDA, ↓IL-1, ↓IL-6, ↓TNFα, ↓INFɣ, ↓caspase-3 activity, ↑SOD, ↓p38 MAPK | [90] |
| Rutin (Quercetin-3-Rutinoside) | In vivo | Sprague-Dawley rats | ↑MAPK | [78] |
| ↓MIP-2, ↓MMP-9, ↓p Akt, ↓p38MAPK | [74] | |||
| ↓cell death, ↓IL-1β, ↓TNF-α, ↓p38MAPK | [75] | |||
| ↓TGF-β/Smad, | [76] | |||
| ↓MPO, ↓MDA, ↓ROS, ↓TNF-α | [77] | |||
| 3′,4′,7-trihydroxyethylrutin | In vivo | Sprague-Dawley rats | ↓size of coronal, sagittal, and transversal lesions | [79] |
| Combination of quercetin and curcumin | In vivo | Wistar albino rats | ↓S-100β, ↓p38MAPK, ↑Fe2+-chelation, ↑Fe2+-clearance, ↓6-OHDA, ↑CAT | [106,107] |
| Luteolin | In vivo | Wistar albino rats | ↓activity of caspase-1, ↓ROCK2 | [109] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fakhri, S.; Gravandi, M.M.; Abdian, S.; Moradi, S.Z.; Echeverría, J. Quercetin Derivatives in Combating Spinal Cord Injury: A Mechanistic and Systematic Review. Life 2022, 12, 1960. https://doi.org/10.3390/life12121960
Fakhri S, Gravandi MM, Abdian S, Moradi SZ, Echeverría J. Quercetin Derivatives in Combating Spinal Cord Injury: A Mechanistic and Systematic Review. Life. 2022; 12(12):1960. https://doi.org/10.3390/life12121960
Chicago/Turabian StyleFakhri, Sajad, Mohammad Mehdi Gravandi, Sadaf Abdian, Seyed Zachariah Moradi, and Javier Echeverría. 2022. "Quercetin Derivatives in Combating Spinal Cord Injury: A Mechanistic and Systematic Review" Life 12, no. 12: 1960. https://doi.org/10.3390/life12121960
APA StyleFakhri, S., Gravandi, M. M., Abdian, S., Moradi, S. Z., & Echeverría, J. (2022). Quercetin Derivatives in Combating Spinal Cord Injury: A Mechanistic and Systematic Review. Life, 12(12), 1960. https://doi.org/10.3390/life12121960








