Salinity and Heavy Metal Tolerance, and Phytoextraction Potential of Ranunculus sceleratus Plants from a Sandy Coastal Beach
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Experiments
2.2. Plant Establishment and Cultivation
2.3. Treatments
2.4. Termination of Experiments and Measurements
2.5. Data Analysis
3. Results
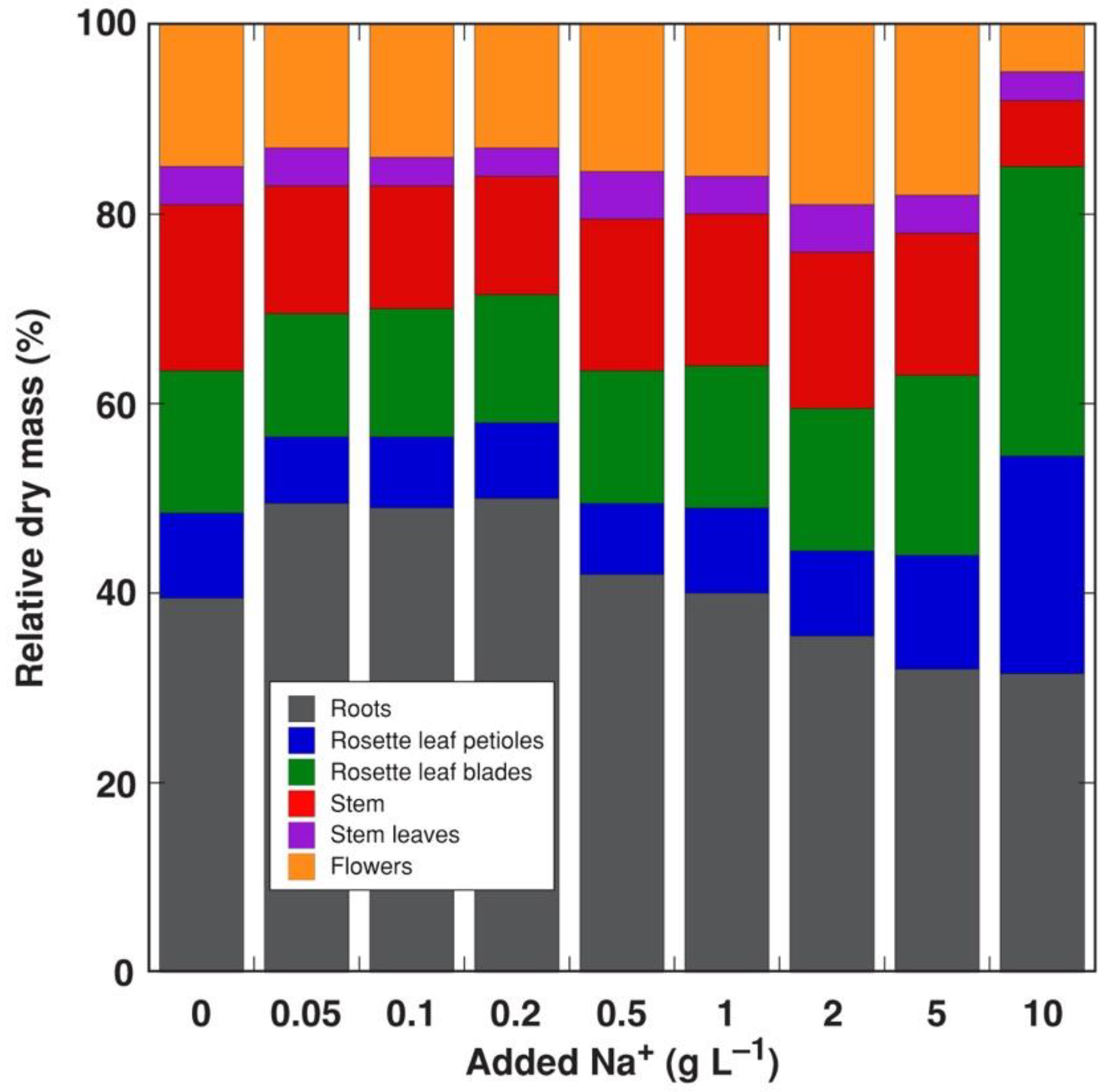
3.1. Experiment 1: NaCl Gradient
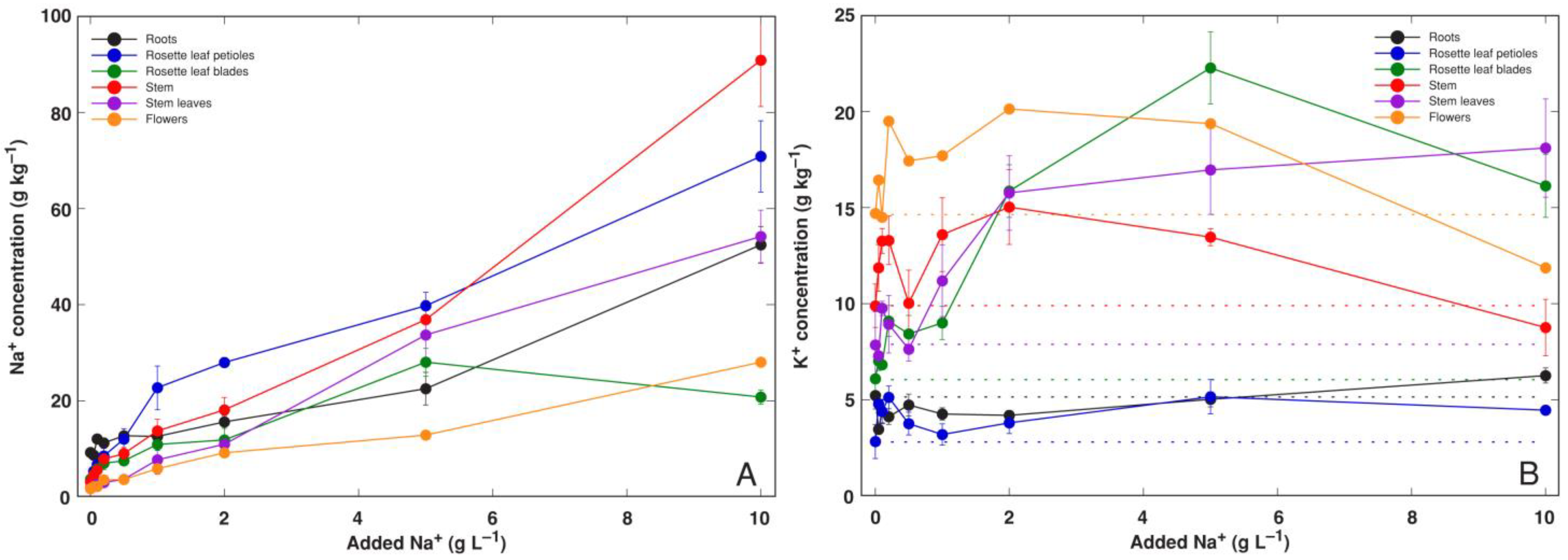
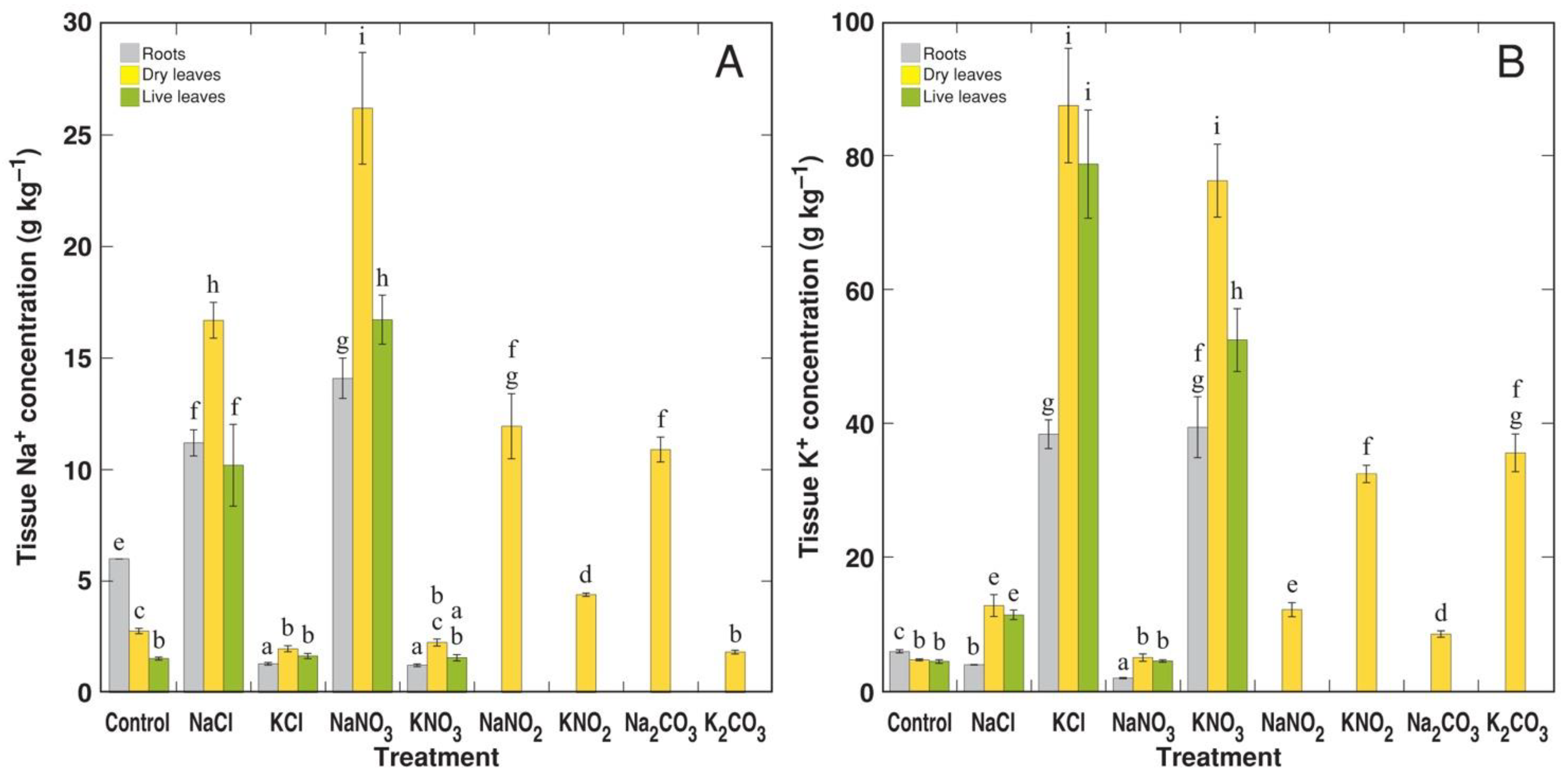
3.2. Experiment 2: Na+ and K+ Salts
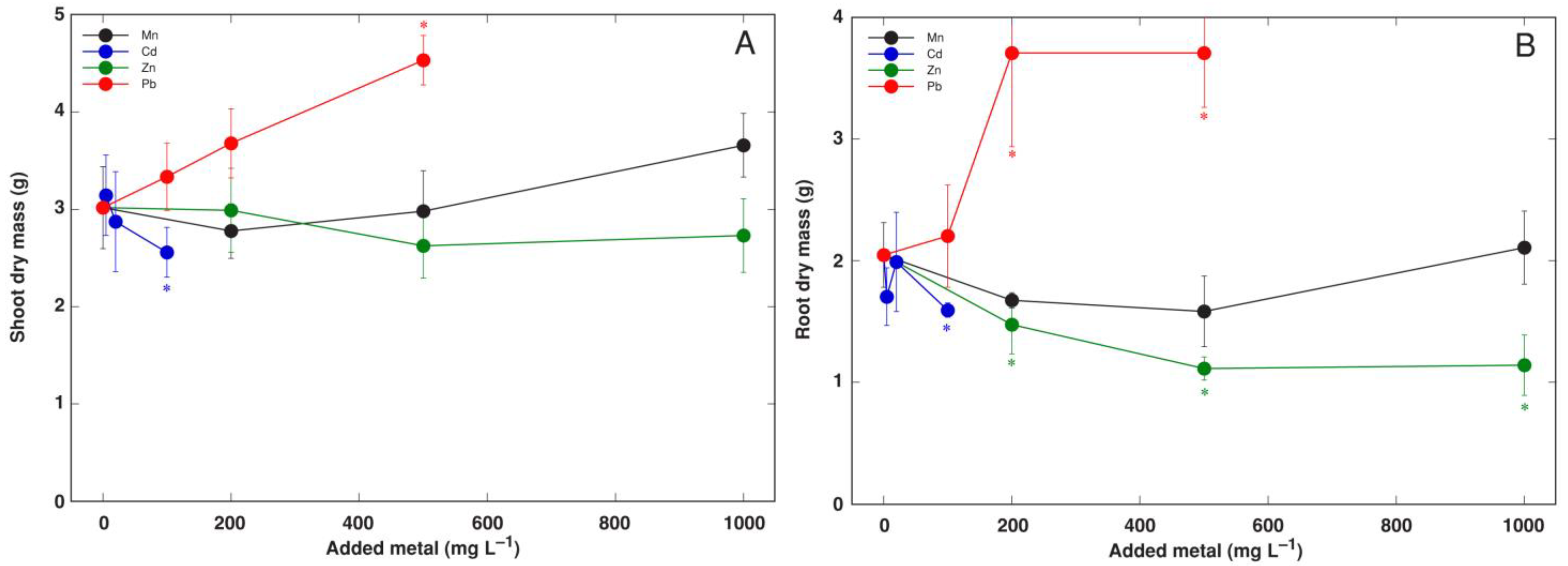
3.3. Experiment 3: Heavy Metals
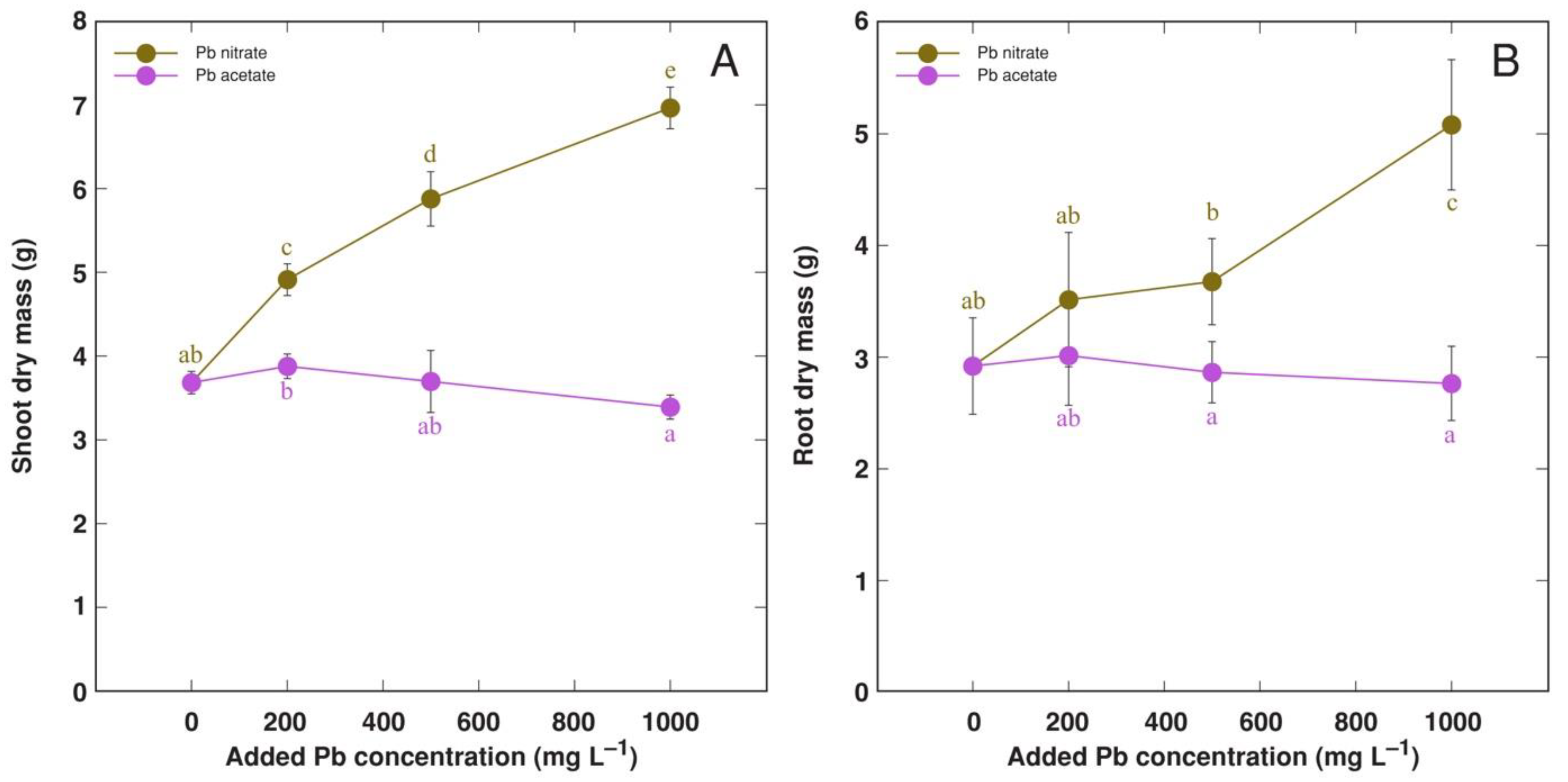
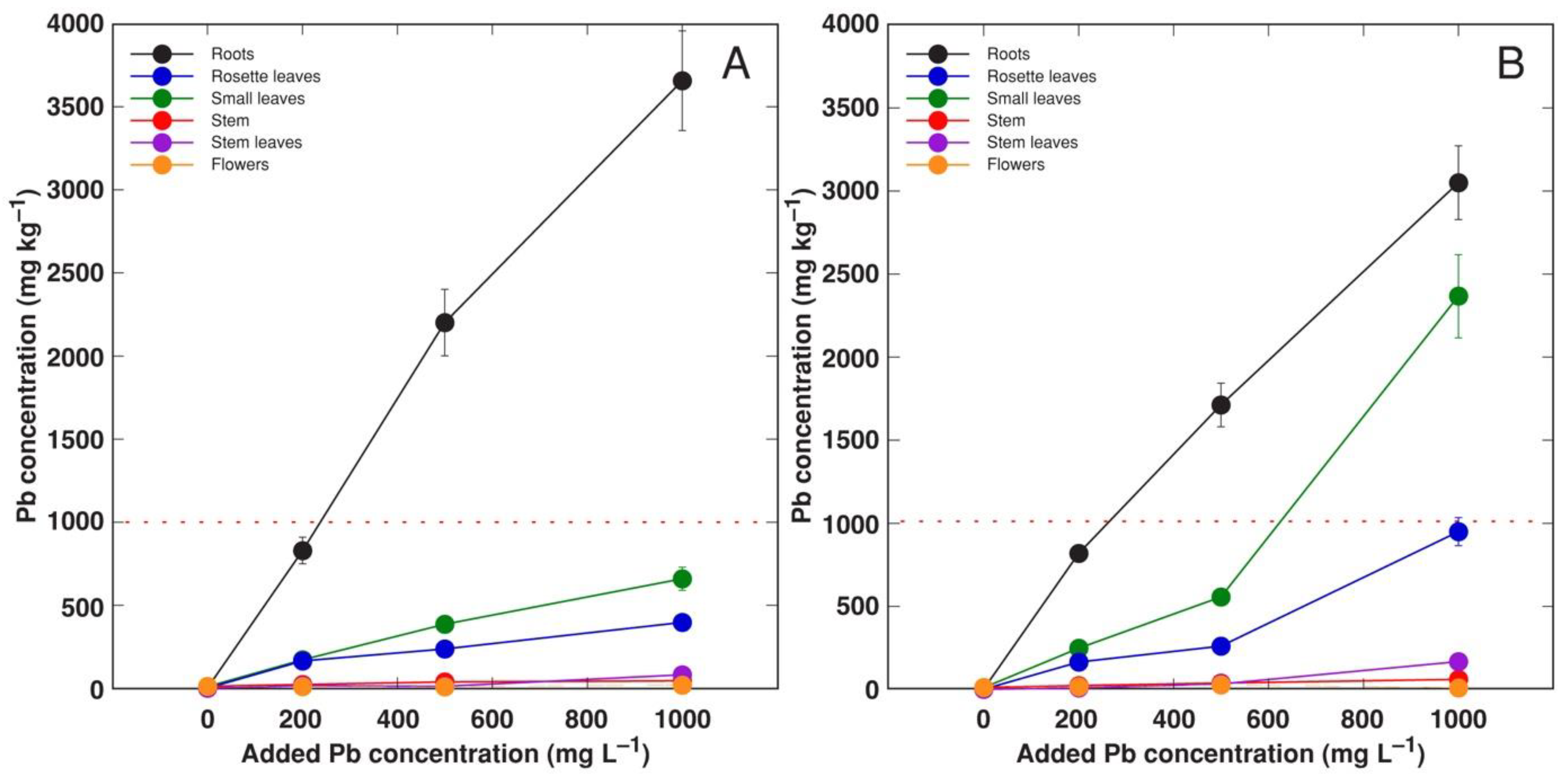
3.4. Experiment 4: Forms of Pb Salts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Padmavathiamma, P.K.; Li, L.Y. Phytoremediation technology, hyper-accumulation metals in plants. Water Air Soil Pollut. 2007, 184, 105–126. [Google Scholar] [CrossRef]
- Mayerová, M.; Petrová, Š.; Madaras, M.; Lipavský, J.; Šimon, T.; Vaněk, T. Non-enhanced phytoextraction of cadmium, zinc, and lead by high-yielding crops. Environ. Sci. Pollut. Res. 2017, 24, 14706–14716. [Google Scholar] [CrossRef]
- Mikołajczak, P.; Borowiak, K.; Niedzielski, P. Phytoextraction of rare earth elements in herbaceous plant species growing close to roads. Environ. Sci. Pollut. Res. 2017, 24, 14091–14103. [Google Scholar] [CrossRef]
- Goolsby, E.W.; Mason, C.M. Toward a more physiologically and evolutionarily relevant definition of metal hyperaccumulation in plants. Front. Plant Sci. 2015, 6, 33. [Google Scholar] [CrossRef]
- Favas, P.C.J.; Pratas, J.; Varun, M.; D’Souza, R.; Paul, M.S. Phytoremediation of soils contaminated with metals and metalloids at mining areas, potential of native flora. In Environmental Risk Assessment of Soil Contamination; Hernandez-Soriano, M.C., Ed.; Intech Open: London, UK, 2014; pp. 485–517. [Google Scholar]
- Yang, W.; Li, H.; Zhang, T.; Sen, L.; Ni, W. Classification and identification of metal-accumulating plant species by cluster analysis. Environ. Sci. Pollut. Res. 2014, 21, 10626–10637. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Ye, Z. Metal accumulation and tolerance in wetland plants. Front. Biol. China 2009, 4, 282–288. [Google Scholar] [CrossRef]
- Kissoon, L.T.T.; Jacob, D.L.; Otte, M.L. Multi-element accumulation near Rumex crispus roots under wetland and dryland conditions. Environ. Pollut. 2010, 158, 1834–1841. [Google Scholar] [CrossRef]
- Teuchies, J.; Jacobs, S.; Oosterlee, L.; Bervoets, L.; Meire, P. Role of plants in metal cycling in a tidal wetland: Implications for phytoremediation. Sci. Total Environ. 2013, 445–446, 146–154. [Google Scholar] [CrossRef]
- Bonanno, G.; Vymazal, J.; Cirelli, G.L. Translocation; accumulation and bioindication of trace elements in wetland plants. Sci. Total Environ. 2018, 631–632, 252–261. [Google Scholar] [CrossRef]
- Nikalje, G.C.; Suprasanna, P. Coping with metal toxicity—Cues from halophytes. Front. Plant Sci. 2018, 9, 777. [Google Scholar] [CrossRef]
- Aziz, I.; Mujeeb, A. Halophytes for phytoremediation of hazardous metal(oid)s: A terse review on metal tolerance, bio-indication and hyperaccumulation. J. Hazard. Mater. 2022, 424, 127309. [Google Scholar] [CrossRef]
- Sruthi, P.; Shackira, A.M.; Puthur, J.T. Heavy metal detoxification mechanisms in halophytes, an overview. Wetl. Ecol. Manag. 2017, 25, 129–148. [Google Scholar] [CrossRef]
- Anjum, N.A.; Duarte, B.; Caçador, I.; Sleimi, N.; Duarte, A.C.; Pereira, E. Biophysical and biochemical markers of metal/metalloid impacts in salt marsh halophytes and their implications. Front. Environ. Sci. 2016, 4, 24. [Google Scholar] [CrossRef]
- Smulders, M.J.M.; Horton, R.F. Ethylene promotes elongation growth and auxin promotes radial growth in Ranunculus sceleratus petioles. Plant Physiol. 1991, 96, 806–811. [Google Scholar] [CrossRef][Green Version]
- He, J.B.; Bögemann, G.M.; van de Steeg, H.M.; Rijnders, J.G.H.M.; Voesenek, L.A.C.J.; Blom, C.W.P.M. Survival tactics of Ranunculus species in river floodplains. Oecologia 1999, 118, 1–8. [Google Scholar] [CrossRef]
- Laime, B. 1310 Salicornia and other annuals colonising mud and sand. In European Union Protected Habitats in Latvia. Interpretation Manual; Auniņš, A., Ed.; Latvian Fund for Nature, Ministry of Environmental Protection and Regional Development: Riga, Latvia, 2013; pp. 52–54. [Google Scholar]
- Rove, I. 1640 Boreal Baltic sandy beaches with perennial vegetation. In European Union Protected Habitats in Latvia. Interpretation Manual; Auniņš, A., Ed.; Latvian Fund for Nature, Ministry of Environmental Protection and Regional Development: Riga, Latvia, 2013; pp. 58–61. [Google Scholar]
- Hill, M.O.; Ellenberg, H.H. Ellenberg’s Indicator Values for British Plants; ECOFACT Research Report. Technical Annex; Institute of Terrestrial Ecology: Wallingford, UK, 1999; Volume 2. [Google Scholar]
- Tyler, T.; Herbertsson, L.; Olofsson, J.; Olsson, P.A. Ecological indicator and traits values for Swedish vascular plants. Ecol. Indic. 2021, 120, 106923. [Google Scholar] [CrossRef]
- Ievinsh, G.; Ieviņa, S.; Andersone-Ozola, U.; Samsone, I. Leaf sodium; potassium and electrolyte accumulation capacity of plant species from salt-affected coastal habitats of the Baltic Sea: Towards a definition of Na hyperaccumulation. Flora 2021, 274, 151748. [Google Scholar] [CrossRef]
- Zuo, S.; Wan, K.; Ma, S. Environmental restoration effects of Ranunculus sceleratus L. in a eutrophic sewage system. Biochem. Syst. Ecol. 2014, 55, 34–40. [Google Scholar] [CrossRef]
- Farahat, E.A.; Galal, T.M. Trace metal accumulation by Ranunculus sceleratus, implications for phytostabilization. Environ. Sci. Pollut. Res. 2018, 25, 4214–4222. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Tripathi, S.; Sirohi, R.; Kim, S.H.; Ngo, H.H.; Pandey, A. Uptake and mobilization of heavy metals through phytoremediation process from native plant species growing on complex pollutants: Antioxidant enzymes and photosynthetic pigments response. Environ. Technol. Innov. 2021, 23, 101629. [Google Scholar] [CrossRef]
- Khalifa, S.M.; Badr, B.M. Habitats, phytochemical metabolites and phytoremediation potential of Ranunculus sceleratus L. Sebha Univ. J. Pure Appl. Sci. 2021, 20, 20–24. [Google Scholar]
- Losfeld, G.; L’Huillier, L.; Fogliani, B.; Mc Coy, S.; Grison, C.; Jaffré, T. Leaf-age and soil-plant relationships: Key factors for reporting trace-elements hyperaccumulation by plants and design applications. Environ. Sci. Pollut. Res. 2015, 22, 5620–5632. [Google Scholar] [CrossRef] [PubMed]
- Muszyńska, E.; Labudda, M. Dual role of metallic trace elements in stress biology—From negative to beneficial impact on plants. Int. J. Mol. Sci. 2019, 20, 3117. [Google Scholar] [CrossRef]
- Corso, M.; de la Torre, V.S.G. Biomolecular approaches to understanding metal tolerance and hyperaccumulation in plants. Metallomics 2020, 12, 840–859. [Google Scholar] [CrossRef]
- Manara, A.; Fasani, E.; Furini, A.; DalCorso, G. Evolution of the metal hyperaccumulation and hypertolerance traits. Plant Cell Environ. 2020, 43, 2969–2986. [Google Scholar] [CrossRef] [PubMed]
- Angulo-Bejarrano, P.I.; Puente-Rivera, J.; Cruz-Ortega, R. Metal and metalloid toxicity in plants: And overview on molecular aspects. Plants 2021, 10, 635. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.-S.; Guan, Y.-H.; Lin, X.-J.; Xu, X.-J.; Xiao, L.; Wang, H.-H.; Meng, S. Comparative understanding of metal hyperaccumulation in plants: A mini review. Environ. Geochem. Health 2021, 43, 1599–1607. [Google Scholar] [CrossRef] [PubMed]
- Sytar, O.; Ghosh, S.; Malinska, H.; Zivcak, M.; Brestic, M. Physiological and molecular mechanisms of metal accumulation and hyperaccumulation. Physiol. Plant 2021, 173, 148–166. [Google Scholar]
- Seregin, I.V.; Kozhevnikova, A.D. Low-molecular-weight ligands in plants: Role in metal homeostasis and hyperaccumulation. Photosynth. Res. 2021, 150, 51–96. [Google Scholar]
- Ernst, W.H.O. Evolution of heavy metal tolerance in higher plants. For. Snow Landsc. Res. 2006, 80, 251–274. [Google Scholar]
- Baumbach, H.; Hellwig, F.H. Genetic differentiation of metallicolous and non-metallicolous Armeria maritima (Mill.) Willd. taxa (Plumbaginaceae) in Central Europe. Plant Syst. Evol. 2007, 269, 245–258. [Google Scholar] [CrossRef]
- Purmale, L.; Jēkabsone, A.; Andersone-Ozola, U.; Karlsons, A.; Osvalde, A.; Ievinsh, G. Comparison of in vitro and in planta heavy metal tolerance and accumulation potential of different Armeria maritima accessions from a dry coastal meadow. Plants 2022, 11, 2104. [Google Scholar] [CrossRef] [PubMed]
- Pollard, A.J.; Reeves, R.D.; Baker, A.J.M. Facultative hyperaccumulation of heavy metals and metaloids. Plant Sci. 2014, 217–218, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Bothe, H.; Słomka, A. Divergent biology of facultative heavy metal plants. J. Plant Physiol. 2017, 219, 45–61. [Google Scholar] [CrossRef]
- Dou, X.; Dai, H.; Skuza, L.; Wei, S. Bidens pilosa L. hyperaccumulating Cd with different species in soil and the role of EDTA on the hyperaccumulation. Environ. Sci. Pollut. Res. 2019, 26, 25668–25675. [Google Scholar] [CrossRef]
- Liu, J.; Mo, L.; Zhang, X.; Yao, S.; Wang, Y. Simultaneous accumulation of cadmium and manganese in Celosia argentea Linn. Int. J. Phytoremediat. 2018, 20, 1106–1112. [Google Scholar] [CrossRef] [PubMed]
- Abubakari, F.; Nkrumah, P.N.; Fernando, D.R.; Brown, G.K.; Erdkine, P.D.; Echevarria, G.; van der Ent, A. Incidence of hyperaccumulation and tissue-level distribution of manganese, cobalt, and zinc in the genus Gossia (Myrtaceae). Metallomics 2021, 13, mfam008. [Google Scholar] [CrossRef]
- Coakley, S.; Gahill, G.; Enright, A.-M.; O’Rourke, B.; Petti, C. Cadmium hyperaccumulation and translocation in Impatiens glandulifera: From foe to friend? Sustainability 2019, 11, 5018. [Google Scholar] [CrossRef]
- Moray, C.; Goolsby, E.W.; Bromham, L. The phylogenetic association between salt tolerance and heavy metal hyperaccumulation in angiosperms. Evol. Biol. 2016, 43, 119–130. [Google Scholar] [CrossRef]
- Reeves, R.D.; Baker, A.J.M.; Jaffré, T.; Erskine, P.D.; Echevarria, G.; van der Ent, A. A global database for plants that hyperaccumulate metal and metalloid trace elements. New Phytol. 2018, 218, 407–411. [Google Scholar] [CrossRef]
- Liang, H.-M.; Lin, T.-H.; Chiou, J.-M.; Yeh, K.-C. Model evaluation of the phytoextraction potential of heavy metal hyperaccumulators and non-hyperaccumulators. Environ. Pollut. 2009, 157, 1945–1952. [Google Scholar] [CrossRef] [PubMed]
- Ievinsh, G.; Andersone-Ozola, U.; Jēkabsone, A. Similar responses of relatively salt tolerant plants to Na and K during chloride salinity: Comparison of growth, water content and ion accumulation. Life 2022, 12, 1577. [Google Scholar] [CrossRef] [PubMed]
- Purmale, L.; Jēkabsone, A.; Andersone-Ozola, U.; Ievinsh, G. Salinity tolerance and ion accumulation potential in vitro and in planta of different Armeria maritima accessions from a dry coastal meadow. Plants 2022, 11, 2570. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hussain, T.; Feng, X.; Guo, K.; Chen, H.; Yang, C.; Liu, X. Comparative study on the resistance of Suaeda glauca and Suaeda salsa to drought, salt, and alkali stresses. Ecol. Eng. 2019, 140, 105593. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, W.; Yan, X.; Guo, J. Physiological and transcriptomic analyses of yellow horn (Xanthoceras sorbifolia) provide important insights into salt and saline-alkali stress tolerance. PLoS ONE 2020, 15, e0244365. [Google Scholar] [CrossRef]
- Geng, G.; Wang, G.; Stevanato, P.; Lv, C.; Wang, Q.; Yu, L.; Wang, Y. Physiological and proteomic analysis of different molecular mechanisms of sugar beet response to acidic and alkaline pH environment. Front. Plant Sci. 2021, 12, 682799. [Google Scholar] [CrossRef]
- Yang, C.W.; Wang, P.; Li, C.Y.; Shi, D.C.; Wang, D.L. Comparison of effects of salt and alkali stresses on the growth and photosynthesis of wheat. Photosynthetica 2008, 46, 107–114. [Google Scholar] [CrossRef]
- Guo, R.; Shi, L.; Yan, G.; Zhong, X.; Gu, F.; Liu, Q.; Xia, X.; Li, H. Ionomic and metabolic responses to salt or alkaline salt stresses in maize (Zea mays L.) seedlings. BMC Plant Biol. 2017, 17, 41. [Google Scholar] [CrossRef]
- Cole, J.J.; Prairie, Y.T. Dissolved CO2 in freshwater systems. In Encyclopedia of Inland Waters; Elsevier: Amsterdam, The Netherlands, 2009; pp. 30–34. [Google Scholar]
- Poschenrieder, C.; Fernández, J.A.; Rubio, L.; Pérez, L.; Terés, J.; Barcélo, J. Transport and use of bicarbonate in plants: Current knowledge and challenges ahead. Int. J. Mol. Sci. 2018, 19, 1352. [Google Scholar] [CrossRef]
- Csitári, B.; Bedics, A.; Felföldi, T.; Boros, E.; Nagy, H.; Máthé, I.; Szkely, A.J. Anion-type modulates the effect of salt stress on saline lake bacteria. Extremophiles 2022, 26, 12. [Google Scholar] [CrossRef]
- Crawford, N.M. Nitrate: Nutrient and signal for plant growth. Plant Cell 1995, 7, 859–868. [Google Scholar]
- Bharucha, F.R.; Dubash, P.J. The problem of nitrophily. Vegetatio 1951, 3, 183–194. [Google Scholar] [CrossRef]
- Moreau, D.; Milard, G.; Munier-Jolain, N. A plant nitrophily index based on plant leaf area response to soil nitrogen availability. Agron. Sustain. Dev. 2013, 33, 809–815. [Google Scholar] [CrossRef]
- Xu, G.; Fan, X.; Miller, A.J. Plant nitrogen assimilation and use efficiency. Annu. Rev. Plant Biol. 2012, 63, 153–182. [Google Scholar] [CrossRef]
- Liu, X.; Hu, B.; Chu, C. Nitrogen assimilation in plants: Current status and future perspectives. J. Genet. Genom. 2022, 49, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Kotur, Z.; Siddiqi, Y.M.; Glass, A.D.M. Characterization of nitrite uptake in Arabidopsis thaliana: Evidence for a nitrite-specific transporter. New Phytol. 2013, 200, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Maia, L.B.; Moura, J.J.G. How biology handles nitrite. Chem. Rev. 2014, 114, 5273–5357. [Google Scholar]
- Ezzine, M.; Debouba, M.; Ghorbel, M.H.; Gouia, H. Ion uptake and structural modifications induced by nitrogen source in tomato (Solanum lycopersicum Mill. Cv. Ibiza F1). C. R. Biol. 2011, 334, 526–534. [Google Scholar] [CrossRef]
- Zsoldos, F.; Vashegyi, Á.; Pécsváradi, A.; Haunold, E.; Herger, P. Unhibition of ion uptake and growth of wheat and rice exposed to nitrite at low pH. Cereal Res. Commun. 1997, 25, 35–42. [Google Scholar] [CrossRef]
- Samater, A.H.; Van Cleemput, O.; Ertebo, T. Influence of the presence of nitrite and nitrate in soil on maize biomass production, nitrogen immobilization and nitrogen recover. Biol. Fertil. Soils 1998, 27, 211–218. [Google Scholar] [CrossRef]
- Sharma, P.; Ahmad, M.; Rathee, S.; Singh, H.P.; Batish, D.R.; Kohli, R.K. Bridging the gap: Linking morpho-functional traits’ plasticity with hyperaccumulation. Environ. Monit. Assess. 2021, 193, 762. [Google Scholar] [CrossRef] [PubMed]





| Experiment No. | Description | Treatments (Final Concentrations in Substrate) | Duration of Experiment |
|---|---|---|---|
| 1 | Effect of NaCl gradient | NaCl (equivalent to 0.05, 0.1, 0.2, 0.5, 1, 2, 5, 10 g L−1 Na+) | Treatment phase 3 weeks, cultivation phase 5 weeks |
| 2 | Effect of different Na+ and K+ salts | NaCl, NaNO3, NaNO2, Na2CO3 (equivalent to 4.0 g L−1 Na+); KCl, KNO3, KNO2, K2CO3 (equivalent to 6.8 g L−1 K+) | Treatment phase 2 weeks, cultivation phase 5 weeks |
| 3 | Comparison of heavy metal tolerance and accumulation | MnSO4 (equivalent to 200, 500, 1000 mg L−1 Mn), CdSO4 (equivalent to 5, 20, 100 mg L−1 Cd), ZnSO4 (equivalent to 200, 500, 1000 mg L−1 Zn), Pb(NO3)2 (equivalent to 100, 200, 500 mg L−1 Pb) | Treatment phase 2 weeks, cultivation phase 5 weeks |
| 4 | Comparison of Pb nitrate and acetate | Pb(NO3)2, Pb(CH3COO)2 (equivalent to 200, 500, 1000 mg L−1 Pb) | Treatment phase 2 weeks, cultivation phase 7 weeks |
| Na+ (g L−1) | Roots | Rosette Leaf Petioles | Rosette Leaf Blades | Stem | Stem Leaves | Flowers |
|---|---|---|---|---|---|---|
| 0.05 | 174 | 107 | 93 | 91 | 43 | 43 |
| 0.1 | 121 | 68 | 59 | 56 | 27 | 22 |
| 0.2 | 96 | 43 | 35 | 39 | 15 | 18 |
| 0.5 | 25 | 24 | 15 | 18 | 7 | 7 |
| 1 | 13 | 23 | 11 | 14 | 8 | 6 |
| 2 | 8 | 14 | 6 | 9 | 6 | 5 |
| 5 | 5 | 8 | 6 | 7 | 7 | 3 |
| 10 | 5 | 7 | 2 | 9 | 5 | 3 |
| Metal | Concentration (mg L−1) | Roots | Leaves | Stem | Flowers |
|---|---|---|---|---|---|
| Mn | 200 | 3.3 | 18.3 | 6.0 | 3.6 |
| 500 | 2.5 | 14.1 | 5.4 | 3.6 | |
| 1000 | 1.5 | 7.5 | 3.2 | 2.0 | |
| Cd | 5 | 20.0 | 1.8 | 0.10 | 0.33 |
| 20 | 6.9 | 1.0 | 0.08 | 0.38 | |
| 100 | 6.8 | 0.8 | 0.05 | 0.24 | |
| Zn | 200 | 19.7 | 6.7 | 2.8 | 1.76 |
| 500 | 11.7 | 11.0 | 1.9 | 0.96 | |
| 1000 | 8.2 | 7.0 | 2.3 | 0.44 | |
| Pb | 100 | 6.0 | 2.3 | 0.16 | 0.03 |
| 200 | 3.9 | 1.7 | 0.12 | 0.01 | |
| 500 | 2.9 | 1.4 | 0.09 | 0.02 |
| Salt | Concentration (mg L−1) | Roots | Small Leaves | Rosette Leaves | Stem | Stem Leaves | Flowers |
|---|---|---|---|---|---|---|---|
| Pb nitrate | 200 | 4.2 | 0.87 | 0.83 | 0.12 | 0.09 | 0.06 |
| 500 | 4.0 | 0.77 | 0.48 | 0.08 | 0.03 | 0.02 | |
| 1000 | 3.7 | 0.66 | 0.40 | 0.05 | 0.08 | 0.02 | |
| Pb acetate | 200 | 4.1 | 1.25 | 1.83 | 0.12 | 0.05 | 0.09 |
| 500 | 3.4 | 1.11 | 0.52 | 0.08 | 0.07 | 0.06 | |
| 1000 | 3.1 | 2.37 | 0.95 | 0.06 | 0.17 | 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ievinsh, G.; Landorfa-Svalbe, Z.; Andersone-Ozola, U.; Karlsons, A.; Osvalde, A. Salinity and Heavy Metal Tolerance, and Phytoextraction Potential of Ranunculus sceleratus Plants from a Sandy Coastal Beach. Life 2022, 12, 1959. https://doi.org/10.3390/life12121959
Ievinsh G, Landorfa-Svalbe Z, Andersone-Ozola U, Karlsons A, Osvalde A. Salinity and Heavy Metal Tolerance, and Phytoextraction Potential of Ranunculus sceleratus Plants from a Sandy Coastal Beach. Life. 2022; 12(12):1959. https://doi.org/10.3390/life12121959
Chicago/Turabian StyleIevinsh, Gederts, Zaiga Landorfa-Svalbe, Una Andersone-Ozola, Andis Karlsons, and Anita Osvalde. 2022. "Salinity and Heavy Metal Tolerance, and Phytoextraction Potential of Ranunculus sceleratus Plants from a Sandy Coastal Beach" Life 12, no. 12: 1959. https://doi.org/10.3390/life12121959
APA StyleIevinsh, G., Landorfa-Svalbe, Z., Andersone-Ozola, U., Karlsons, A., & Osvalde, A. (2022). Salinity and Heavy Metal Tolerance, and Phytoextraction Potential of Ranunculus sceleratus Plants from a Sandy Coastal Beach. Life, 12(12), 1959. https://doi.org/10.3390/life12121959








