Abstract
Interactions between metals and microbes are critical in geomicrobiology and vital in microbial ecophysiological processes. Methane-oxidizing bacteria (MOB) and ammonia-oxidizing microorganisms (AOM) are key members in aerobic environments to start the C and N cycles. Ammonia and methane are firstly oxidized by copper-binding metalloproteins, monooxygenases, and diverse iron and copper-containing enzymes that contribute to electron transportation in the energy gain pathway, which is evolutionally connected between MOB and AOM. In this review, we summarized recently updated insight into the diverse physiological pathway of aerobic ammonia and methane oxidation of different MOB and AOM groups and compared the metabolic diversity mediated by different metalloenzymes. The elevation of iron and copper concentrations in ecosystems would be critical in the activity and growth of MOB and AOM, the outcome of which can eventually influence the global C and N cycles. Therefore, we also described the impact of various concentrations of metal compounds on the physiology of MOB and AOM. This review study could give a fundamental strategy to control MOB and AOM in diverse ecosystems because they are significantly related to climate change, eutrophication, and the remediation of contaminated sites for detoxifying pollutants.
1. Introduction
Nitrogen is an essential element for all living life on our planet, as it is a component of nucleic acids and proteins and constitutes most of the atmosphere, around 80%. The nitrogen in the ecosystem is cycled by various biological processes such as nitrogen fixation, nitrification, denitrification, assimilation, and ammonification. These processes include anaerobic nitrate reduction to ammonium (DNRA), denitrification of anaerobic methane oxidation (DAMO), and dissimilatory nitrate reduction to ammonium (DNRA) [1]. Nitrification is a vital process of the global biogeochemical nitrogen cycle. It plays a significant role in fertilizer loss in industrial agriculture, eutrophication, and the production of greenhouse gas N2O, which has a very long residence time in the atmosphere (120 years). Furthermore, it contributes to ozone destruction by reacting with the atomic oxygen to form nitric oxide (NO) in the atmosphere [2]. On the other hand, nitrification is essential for efficient sewage treatment. The aerobic oxidation of ammonia initiates nitrification by ammonia-oxidizing microorganisms (AOM), which is mediated by three distinct groups of aerobic autotrophic ammonia oxidizers: (i) ammonia-oxidizing bacteria (AOB), (ii) ammonia oxidizing-archaea (AOA), and (iii) complete ammonia-oxidizing bacteria (Comammox) [1]. Ammonia is also oxidized by the anammox (anaerobic ammonium oxidation) process in the anaerobic system [3] and also oxidized by the heterotrophic and fungal nitrification process [4]. However, these oxidation processes involve completely different physiological pathways compared to the canonical aerobic oxidation in AOM (see details below). Hence, in this review, we focused on canonical aerobic ammonia oxidation, which is physiologically comparable to the aerobic methane oxidation pathway.
Carbon dioxide (CO2) is the most prevalent greenhouse gas accounting for 95% of all emissions. The following two gases are methane (CH4) and nitrous oxide (N2O), which have a substantial impact on the climate [2]. Despite having atmospheric concentrations of ~1800 parts per billion (ppb) for CH4 and ~330 ppb for N2O [5], respectively, they are 25 and 300 times more effective at absorbing infrared light than CO2. Anaerobic decomposition of organic matter produces CH4 and later transforms it into CO2, increasing total atmospheric CO2 concentration. As a source of energy, methanotrophs (MOB; aerobic methane-oxidizing bacteria) are primarily responsible for the enzymatic oxidation of CH4. The aerobic and anaerobic methanotrophic reactions consume about 35% (0.6 Gt, gigatonne), and 18% (0.3 Gt) of the global CH4 production per year, respectively [6]. Canonical aerobic methane oxidation is performed by certain bacteria that combine molecular oxygen with CH4 to produce methanol (CH3OH), then the methanol is oxidized into formaldehyde (CH2O), and then finally oxidized to CO2 via formate (CH2O2) using either the serine or ribulose monophosphate pathway (Figure 1 and details see below) [7]. An anaerobic pathway of reverse methanogenesis is believed to oxidize methane by the ANME group of archaea [6,8], Methanosarcinales and Methanomicrobiales, which are closely associated with sulfate-reducing gamma-proteobacteria [9].
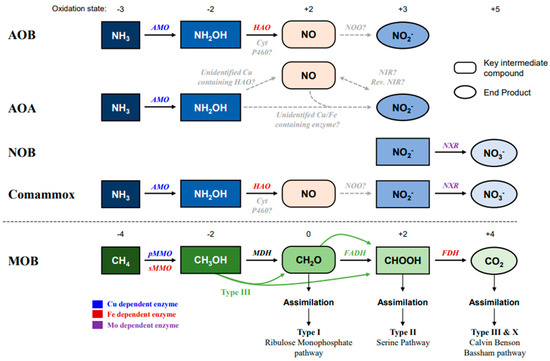
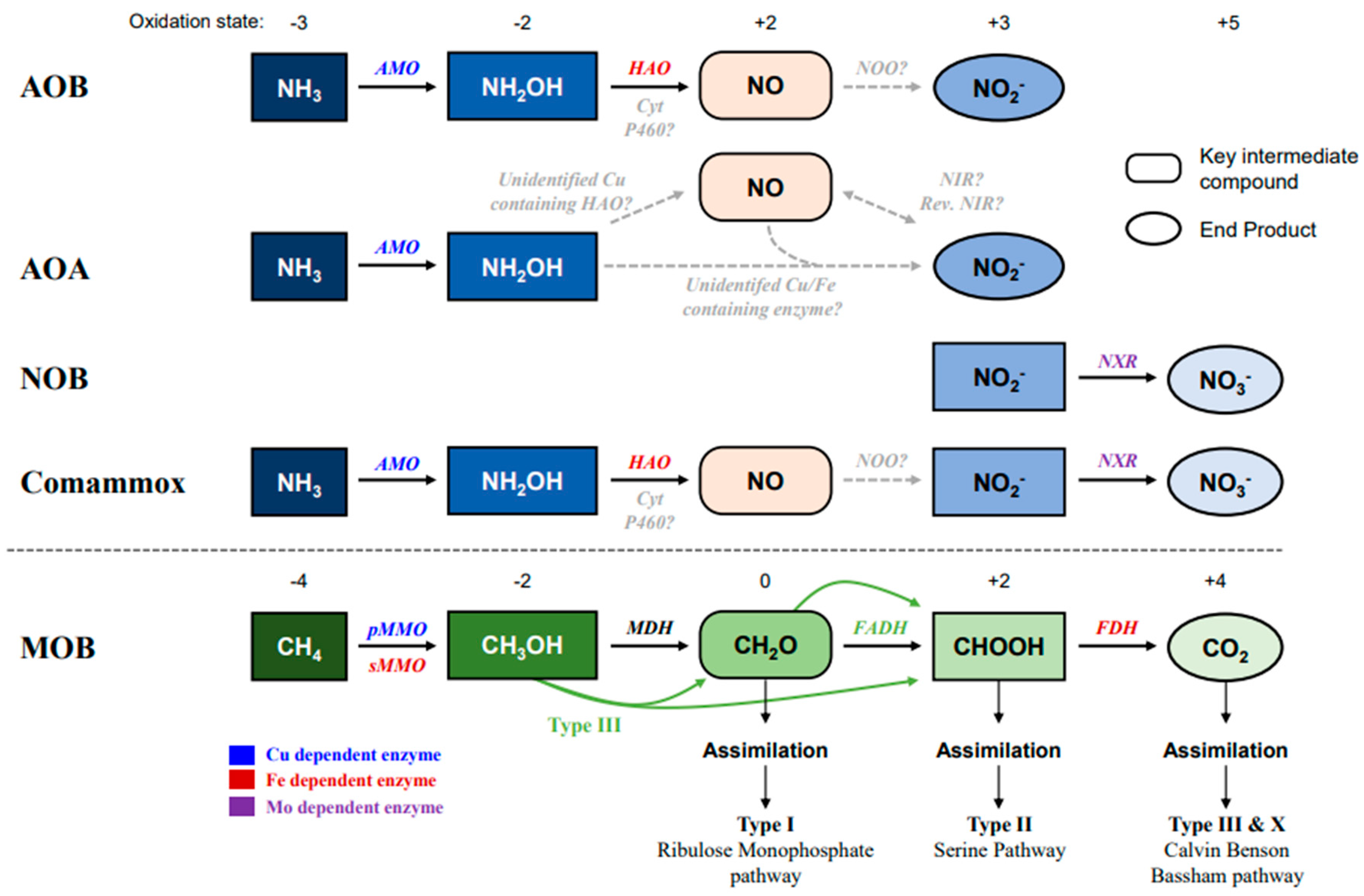
Figure 1.
Pathway of ammonia and methane oxidation by ammonia oxidizers (AMO: AOA, AOB, and Comammox), NOB, and methane oxidizer (MOB). The blue color indicates the oxidation of ammonia (NH3), and the green color indicates methane oxidation. Black straight arrows indicate identified reactions, and grey dotted arrows show unknown enzyme activity or unidentified pathways. Enzymes at the above arrows with different colors indicate different metal dependencies. The oxidation states are at the top of the pathways. Abbreviations: AMO, Ammonia monooxygenase; HAO, Hydroxylamine oxidoreductase; NXR, Nitrite oxidoreductase; pMMO, Particulate methane monooxygenase; sMMO, Soluble methane monooxygenase; MDH, methanol dehydrogenase; FADH, formaldehyde dehydrogenase; FDH, format dehydrogenase.
Interactions between microbes and trace metals such as copper (Cu), zinc (Zn), cobalt (Co), molybdenum (Mo), selenium (Se), manganese (Mn), and iron (Fe) are important in nature. Metals can influence microbial growth, activity, and survival directly (such as making metalloenzyme; see below) or indirectly [10]; thus, it is not unusual that they deal with them, sometimes to their use (bioavailable), often to their harm (toxic), when present at high enough concentrations [11]. Microorganisms can bind metal ions and transport them into the cells for various purposes, such as electron donors or acceptors in metabolic activities [12]. Most enzyme classes contain metalloenzymes, which are metal-bound proteins having a labile coordination site. The metal ion with a substrate-compatible shape is usually located in the active site of metalloenzymes. Therefore, as with all enzymes, the shape of the active site is crucial. Despite the intricacy of organic chemistry reactions, metal ions can execute such reactions that are challenging to achieve. The Irving-Williams stability series defines the order of affinity for essential divalent cations. In addition, the required concentration of trace metal can be calculated by the speciation of metals in solution, based on equilibrium stability constants (K) which is the strength of the interaction between metals and the ligands that come together to form the complex [13].
Therefore, the microbes must deal with enough metal atoms to satisfy the protein requirements; however, not all metals are bioavailable. In the environmental system, metals are found in various forms, including the hydrated free ion, inorganic complexes (with ligands such as Cl−, OH−, CO32−), organic complexes (with simple organic molecules of biogenic or anthropogenic origin, and with natural organic matter, NOM), as well as in colloidal and other solid phase forms. It is well known that ligand concentration, temperature, pH, and redox state determine the partitioning (bioavailability and toxicity) of metals among the different forms [14,15]. In the natural environment, metals are usually present in complex and colloidal forms and are rarely found in free form [16]. The concentration of free metal ions can be decreased in the presence of chelating agents such as various organics and ethylenediaminetetraacetic acid (EDTA) or due to pH fluctuations. In addition, the stability of metalloenzymes can be affected by buffer complexations with metals. The free metal ion concentration in environmental systems is the best predictor of both bioaccumulation and toxicity of cationic metals [17,18]. Therefore, the bioavailability and toxicity of metals for microbes are dependent on (i) the ionic strength of a medium, (ii) the presence of organic matter, (iii) pH, (iv) redox potential, and (v) valence state. All these factors may favor the formation of different metal species with high or low bioavailability and toxicity [19].
Although MOB and AOM are substrate-specific, preferring CH4 and NH3, respectively, they can interact with one another in various ways [20]. Such interactions include the influence of ammonium on methane oxidation and MOB growth [21]. Moreover, MOB and AOB also share many physiological, structural, and ecological characteristics, including reliance on monooxygenase reactions catalyzed by the copper-containing membrane-bound monooxygenase superfamily (Figure 1 and see details below) [22], intracellular membrane systems, sensitivity to the same inhibitors, possession of hydroxylamine oxidoreductase systems, and the ability to grow in oxic environments [23]. Methane monooxygenase (MMO) in methanotrophs and ammonia monooxygenase (AMO) in ammonia oxidizers have evolved to be functionally identical, and they are capable of oxidizing both methane and ammonia [24]. Furthermore, interactions between methanotrophs and AOM and the effects of carbon and nitrogen cycles have rarely been studied in complex natural ecosystems because they are the critical member of global carbon and nitrogen cycles, respectively [23].
Copper is one of the important trace metals involved in various fundamental and specialized physiological processes, including electron transfer, oxygen transport, superoxide detoxification, denitrification, and ammonia and methane oxidation. Similar to other critical trace metals such as iron, copper is found in small amounts in the ocean, and is heavily complexed by organic ligands, which reduce the inorganic dissolved free metal [25]. It is suggested that Fe promotes the growth of various marine N-cycling microorganisms in a substantial section of oceans [26]. However, it is yet to be determined if metal availability influences the biological niche separation of AOM and MOB in the environmental systems. Therefore, it is crucial to know how AOM and MOB are affected by the fluctuations in metal concentrations, especially Cu and Fe, which will be covered in this review paper.
Collectively, since they are necessary for the enzymes involved in ammonia and methane oxidation, Cu and Fe are critically important for the growth and activity of microbes. However, high metal concentrations above the cell capacity could be toxic for microbes. In this review, we focused on aerobic ammonia and methane oxidation pathways mediated by metals and the impact of various metal concentrations. Especially Cu and Fe, on ammonia and methane oxidizers in in vitro and in situ systems, and different metal uptake strategies.
2. Ammonia and Methane Oxidation Pathways Mediated by Metal Compounds
Diverse ammonia- and methane-oxidizing microorganisms interact with each other in various ecosystems by coupling the oxidation reaction. They tend to utilize and sometimes compete with two different compounds, ammonia and methane, through copper-containing monooxygenase, which ultimately links the global carbon and nitrogen cycles.
2.1. Copper and Iron in the Ammonia-Oxidizing Pathway
Ammonia is oxidized to hydroxylamine (NH2OH) by ammonia monooxygenase (AMO) in all AOM, and then NH2OH is further oxidized to nitric oxide (NO) by hydroxylamine oxidoreductase (HAO) or cytochrome P460, which has been reported to be involved in the bacterial ammonia-oxidizing process (Figure 1) [27]. However, the exact mechanism of NO oxidation is still not identified, and some candidate proteins, such as a red copper protein oxidoreductase (NOO) in AOB and comammox, or reverse nitrite reductase (NIR) in all AOM, are assumed to be involved in this process. Comammox further oxidizes nitrite to nitrate (NO3−) by nitrate oxidoreductase (NXR; molybdenum containing enzyme) such as other nitrite-oxidizing bacterial (NOB) strains. However, ammonia oxidation mechanisms in AOA from NH2OH to NO2− are still mysterious, and several unknown enzymes are supposed to be involved in this (Figure 1). The comammox strain N. inopinata carries hao gene encoding octa-heme cytochrome c protein which resembles the HAO protein of AOB. This hao gene shares a genomic locus with three other genes, including haoB gene coding putative membrane protein and two genes for tetra-heme c-type cytochromes, which resemble cytochrome c554 (CycA) and cytochrome cm552 (CycB) of AOB. Moreover, HAO, CycA, and CycB form the hydroxylamine ubiquinone reduction module (HURM) in AOB, which transfers electrons from hydroxylamine to the quinone pool.
Abundant vital proteins in AOM, notably the AMO, which accomplishes the energy-gaining step in ammonia oxidation, require copper as a cofactor [28,29]. Plastocyanins are copper-containing proteins that mediate electron transfer, encoded in high numbers by AOA. In addition, a high number of multi-copper oxidases and blue copper-proteins resemble genes detected in AOA genomes [28,29,30,31,32]. While a much higher number of Fe-based enzymes and fewer Cu-containing proteins are used in the ammonia-oxidizing system of the AOB mediated the electron transport system, which comprises heme-containing proteins such as HAO and CycB [29]. HAO and CycB have no homologs in AOA, and it is still unclear how these enzymes were replaced. However, Cu-containing plastocyanin is thought to operate as an electron carrier in place of CycB [33]. As a result, AOA should rely on Cu-containing enzymes more than their bacterial counterparts, necessitating complex regulatory mechanisms to respond to rapidly changing local Cu concentrations [34]. On the other hand, AOB uses more Fe-based enzymes and fewer Cu-proteins [35]. Therefore, AOB may have a lower Cu requirement.
As described above, the bioavailable free Cu2+ in the natural environment is substantially less than total copper because the Cu2+ speciation is dominated by complexation by dissolved organic carbon [36,37,38]. Therefore, the bioavailability of the free Cu2+ is highly dependent on complexation with organic matter. It has been identified that reducing copper bioavailability by metal-complexing organic compounds is a significant cause of AOA growth inhibition. AOA is considered much more sensitive than AOB to increased and decreased copper concentrations, and copper is a more critical trace element for AOA for ammonia oxidation and growth systems [34,39]. Therefore, it leads to the disproportion of AOA and AOB abundance in municipal activated sludge because of the different sensitivity of AOA and AOB to organic compounds [39]. Iron (Fe) is a vital element for AOB growth as it is related to energy generation and the functioning of enzymes in the electron transport chain, such as cytochrome c, which contains heme-c groups and needs to chelate with ferrous iron for active regions formation [40,41]. However, metals can also cause bio-accumulative toxicity because of their non-biodegradability, and accumulation in AOB leads to damage from reactive oxygen species (ROS) compounds; thus, too high concentration of Fe will also cause inhibition of ammonia oxidation (Figure 2). It has recently been found that a marine AOA strain N. maritimus has a lower Fe′ uptake rate (free Fe′ half-saturation constant; Kin) in comparison to a marine AOB strain, a Nitrosococcus oceani strain C-107, and marine microorganisms [42]. Furthermore, the number of hypothetical Fe-binding sites in genome-predicted proteomes was significantly greater in marine AOB compared to AOA, and it is accorded that utilization of cytochromes burdens AOB with a significant additional Fe demand [42]. Contrariwise, a more significantly enriched Cu-binding protein in AOA than AOB is that AOA has an unusually high number of Cu proteins, including plastocyanins and multi-copper oxidases with a putative role in electron transport, as described above.
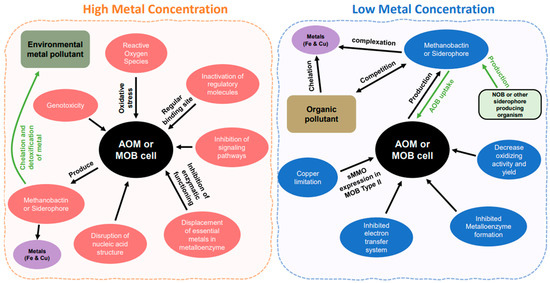
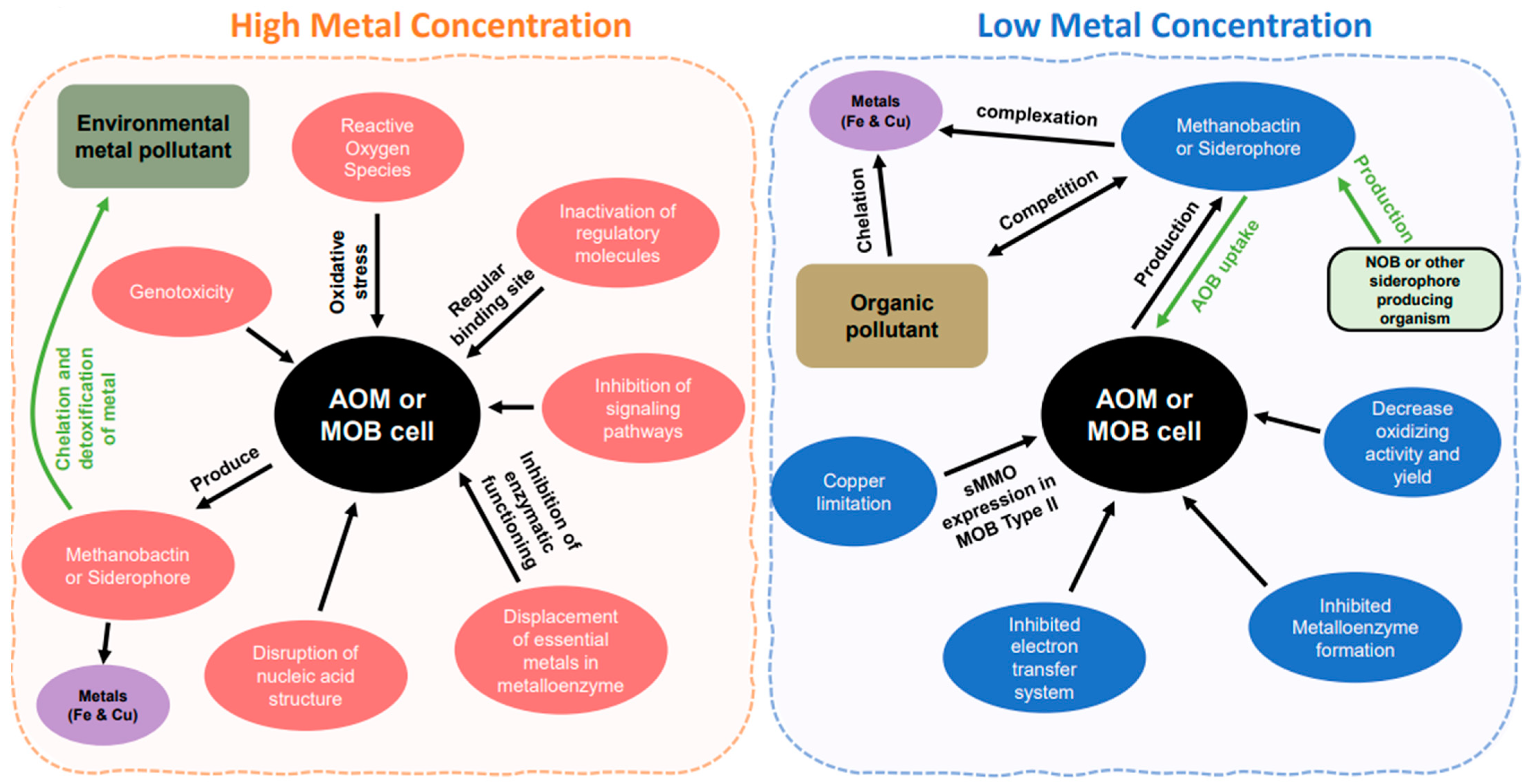
Figure 2.
Metal effects between the high and low concentrations. The arrows indicate the direction to the cell or from the cell. In high concentration, pink color is showing the effect of high concentration of metal within the cell. Green and purple color showing metals outside the cell or in the environment. In low concentration, blue color is indicating the disruption within the cell. Brown color represent metals in the environment and green color shows microorganisms other than ammonia and methane oxidizers.
There are no studies that directly examined the role of the trace metal copper (Cu) and iron (Fe) in comammox bacteria growth. The iron requirement for a comammox strain may be the same with AOB because the ammonia oxidation and electron transfer machinery system are significantly similar. Furthermore, unlike canonical Nitrospira nitrite-oxidizing bacteria (NOB), the copper resistance proteins CopCD in comammox genome are highly similar to the homologs in beta-proteobacterial AOB and are located close to the AMO. CopAB is also detected in comammox Nitrospira, but it is not common for AOB. These proteins confer higher Cu2+ tolerance or increased Cu2+ uptake and [43], therefore, comammox Nitrospira may have higher requirements for copper than canonical Nitrospira NOB. Comammox may benefit from surviving in iron-limited environments because comammox genomes contain genes of the cytochrome c biogenesis system I, which has hemes with higher iron affinity [43]. In contrast, canonical Nitrospira NOB uses the cytochrome c biogenesis system II, which requires less energy for cytochrome synthesis [44]. Fe also participates as Fe-S clusters, utilized for electron transport and catalysis [45]. As bacterial nitrifiers have a high iron requirement for their Fe-S cluster- and heme-containing enzymes [43], this could provide an essential competitive advantage for comammox Nitrospira than canonical NOB Nitrospira in iron-limited environments [46]. Interestingly, the performance of comammox Nitrospira remained unaffected in a copper-limited wastewater system, and it even requires less copper than AOB [43]. Nonetheless, comammox Nitrospira may still need copper from natural environmental concentrations [47].
Other known ammonia oxidation processes include anammox [48], heterotrophic, and fungal nitrification process. Anaerobic anammox oxidation reaction include conversion of NO2− and NH4+ to N2 and 2H2O, which is carried out in the anammoxosome, a membrane-bound compartment inside the cytoplasm. Despite the fact that anammox reaction can occur in two different ways, both use the same intermediate chemical, NH2OH, which is reduced from NO2−, and then interact with ammonia and are subsequently converted to hydrazine. Hydrazine is finally oxidized to N2 in the periplasm [49,50]. Anammox bacteria are known to have high ammonium, and nitrite affinity which make them suitable for substrate conversion at low concentrations [3]. Moreover, anammox is an essential process in the WWTP to remove nitrogen compounds. Anammox bacteria anaerobically convert ammonium and nitrite directly to N2, in the absence of aeration and other electron donors, which distinguish this process from heterotrophic denitrification. Anammox and aerobic AOM commonly co-exist in WWTPs systems, as nitrite is produced by the canonical ammonia oxidation, which can be utilized by anammox. However, due to their different oxygen and nitrogen sensitivity and activity, they need mutual cooperation for stable and efficient performance [51,52,53].
Heterotrophic nitrification is another method of oxidizing ammonia carried by heterotrophic nitrifiers, but the physiology and biochemistry of the relevant pathways is poorly known. Some heterotrophic ammonia oxidizers have an AMO system, but there are also non-AMO containing types of heterotrophic ammonia oxidizers [4]. Furthermore, heterotrophic nitrifiers use organic compounds as their energy source, in contrast to chemolithotrophic nitrifiers. One more ammonia oxidation process is fungal nitrification which is not identified clearly yet. AMO has not been identified in the fungal oxidation system [4].
2.2. Copper and Iron in the Methane-Oxidizing Pathways
Methane is the sole source of carbon and energy in methanotrophs. Methane-oxidizing bacteria and anaerobic methane-oxidizing archaea spontaneously oxidize methane [54]. There are three main groups of aerobic methanotrophs: type I, gamma-proteobacteria, which are found in the families Methylococcaceae and Methylothermaceae encode particulate methane monooxygenase (pMMO); type II, alpha-proteobacteria, which are found in the families Methylocystaceae and Beijerinckiaceae encode both pMMO and soluble methane monooxygenase (sMMO); and type III, Verrucomicrobia, which are members of the family Methylacidiphilaceae encode pMMO. Finally, Methylomirabilis oxyfera, a member of the NC10 phylum and the only known anaerobic denitrifying methanotrophic bacteria [55], utilizes an intra-aerobic pathway through the reduction in nitrite via a unique oxygen-producing pathway [56]. In order to use methane for energy and cell assimilation, methanotroph enzymes oxidize it to carbon dioxide through a series or particular pathways of linked reactions (Figure 1) [57].
The first step of methane oxidation on all known aerobic methanotrophs is started by methane monooxygenase (MMO; pMMO or sMMO), a crucial enzyme to oxidize methane (CH4) into methanol (CH3OH). The next step is the oxidation of methanol to formaldehyde (CH2O), which can either be converted to biomass or further oxidized to formate and carbon dioxide (Figure 1) [58]. pMMO is found in almost all methanotrophs and is located in intracytoplasmic membranes (ICMs). The sMMO and pMMO are mixed function oxidases that incorporate one oxygen atom into methanol and the other into H2O. These enzymes require two electrons and two protons to function [58]. It has been reported that MOB cultures expressing pMMO typically show a higher affinity for methane than cells that express sMMO [59]. In a classical aerobic MOB system, a cytochrome bc1 complex contains three heme groups and one Fe2S2 cluster, which is the electron donor to pMMO [60], based on similarities of pMMO to AMO in the AOB system. Therefore, even though pMMO only has two mono-copper sites [61], Fe is also required for pMMO activity [62]. However, the type 2 alpha-proteobacterial pMMO acquires electrons from the ubiquinol (Q8H2) through NADH oxidation [45]. In contrast, in gamma-proteobacterial methanotrophs, the oxidation of methanol to formaldehyde is also regarded as pMMO activity. Furthermore, it has been demonstrated that cells using pMMO for growth display higher growth yield, suggesting that the pMMO is the more efficient system for methane oxidation [63]. Interestingly, several MOB strains create sMMO that contains Fe under copper-limiting circumstances, while when copper is abundant, MOB expresses pMMO [64]. In the case of sMMO, it contains a di-Fe active site cluster in the hydroxylase component and an additional Fe2S2 cluster in a reductase subunit [64], and electrons would be donated by NAHD [65]. After oxidation of CH4 to CH3OH, methanol dehydrogenase (MDH) oxidizes CH3OH to formaldehyde (CH2O) using cytochrome cL and cH proteins as electron acceptors, which is oxidized by cytochrome aa3, that is heme-copper protein which contains two heme and copper [66]. MDH (MxaF-MDH) contains Ca2+ as a catalytic cofactor but recently discovered XoxF-MDH is present in yeast, fungi, and non-methylotrophic bacteria, depending on rare earth elements (REEs). In subsequent steps, oxidation from CH2O to CO2 is complex, and multiple pathways depend separately on the different methanotroph types [59]. During these steps, most enzymes primarily rely on Fe, with minor requirements of Cu, Ca, Mo, and Zn ions. In particular, the final step in the conversion of CHOOH to CO2, which contains the Fe-S cluster, is catalyzed by the membrane-associated enzyme formate dehydrogenase (FDH). The step from CH2O to CHOOH in M. capsulatus Bath is mediated by either a membrane-bound cytochrome-linked formaldehyde dehydrogenase (DL-FADH) which is mediated by high Cu (>1 µmol of Cu per mg of cell protein) or a soluble NAD(P)+-linked enzyme (N-FADH), which is mediated by low Cu [67]. As an electron acceptor, cytochrome bc1 (with Fe) is required for FADH. Therefore, Cu and Fe are the critical metal compounds that activate methane oxidation.
2.3. Ammonia Oxidation by MOB and Methane Oxidation by AOM
Many connections exist between methanotrophic and nitrifying microorganisms. Methane monooxygenase (MMO) is tightly linked to AMO, although inefficiently, it can oxidize ammonia to hydroxylamine [68]. Since ammonia (NH4+) and methane (CH4) are structurally similar, AMO in nitrifiers may also oxidize methane, but the oxidation activity is less efficient than MMO in methanotrophs [1,69]. CH4 is a nonpolar molecule as it has a symmetric tetrahedral geometrical shape with four identical C-H bonds. Ammonium ion (NH4+) is a positively charged ion (cation) and is also nonpolar in nature based on its tetrahedral structure. On the other hand, ammonia is a polar molecule, its polarity is induced by the electronegativity difference between N (3.04) and H (2.2) atoms. Interestingly, it has not been clearly identified yet which form of ammonia (NH4+ vs. NH3) is the actual substrate for AOM, but recently it has been proposed by a kinetic experiment that ammonia (NH3) rather than ammonium ion (NH4+) is the actual substrate for all AOM [70]. Nevertheless, the pMMO and AMO enzymes are located in the periplasmic membrane; therefore, the methane and ammonia could be active without passive transportation. However, sMMO is located in the cytoplasmic space, so methane should be transported into the cytoplasm for the sMMO (see above).
In the initial phase of aerobic oxidation, Cu-containing monooxygenase (MMO) enzymes combine a single oxygen atom from O2 into CH4 or NH3 (see above) to produce methanol from CH4 and hydroxylamine from NH3 (Figure 1) [23]. Both of these microbes can co-oxidize various substrates and are blocked by the same chemicals [23]. Methanotrophs have been demonstrated to engage in soil nitrification in nutrient-limited circumstances [71]; however, aerobic MOB cannot grow by either NH3 or NH2OH oxidation. Therefore, they must rely on methane (or methanol) oxidation for ammonia oxidation [23]. Although NH2OH is an intermediate of ammonia oxidation, it is highly toxic; therefore, both ammonia-oxidizers and MOB have to remove it quickly. In the bacterial ammonia oxidizer, electrons produced from hydroxylamine oxidation either by a two c-type cytochrome and hydroxylamine oxidoreductase (HAO) or cytochrome P460 to the quinone pool for energy generation and cell growth (see above and Figure 1) [27], but MOB lack this system. Therefore, the hydroxylamine is oxidized by detoxifying activity without generating electrons [72]. Many methanotrophs encode a HAO-like protein, and these HAO-like proteins carry out hydroxylamine oxidation in methanotrophs, but biochemical evidence has not yet been identified [73]. Up-regulation of HAO transcription in response to ammonia has been shown for several methanotrophic bacteria that oxidize ammonia faster than those not encoding HAO genes [71,74,75]. During the step of hydroxylamine oxidation by MOB, nitric oxide (NO) would be produced as a key intermediate, similar to AOM (Figure 1). Recently, it has been reported the catalytic properties of the HAO in thermophilic Verrucomicrobia methanotroph (MOB type III), and the HAO has a crucial function of rapid oxidation of NH2OH to NO and help methanotrophs thrive in environments where methane and ammonia coexist [76].
The other way around, methane oxidation by AOM is also reported in some AOB strains, Nitrosomonas and Nitrosococcus [77,78,79]. For example, in the 14CH4 trace experiment, the Nitrosomonas and Nitrosococcus AOB strains oxidized a significant amount of CH4 and incorporated 13C into cellular components. The ability of AOM to use both ammonia and methane could be a profitable strategy when one or the other is limited and cannot be used as an energy source. Since methane is nearly always present in natural environmental systems, the survival advantage of using either substrate is obvious. However, the precise methane oxidation mechanism of AOB has not been identified. Furthermore, the possibility of methane oxidation by other members of AOA, and comammox, has not been demonstrated yet.
2.4. Environmental Pollutants with Ammonia and Methane Oxidizer
In AOB-dominant conditions, organic micropollutants are bio-transformed to nitrogen-containing compounds, such as hydroxylamine, nitrite, and nitric oxide. Furthermore, In autotrophic reactors, the contaminant was quickly degraded with high efficiencies than in non-AOB-dominant microbial communities [80]. Micropollutants might activate AMO by positing into the hydrophobic pocket and reacting with the oxygen-activating site, allowing micropollutants to be oxidized in the presence of ammonia [81]. It has been reported that an AOA strain Nitrososphaera gargensis, could biotransform numerous micropollutants, including tertiary amines, mianserin, and ranitidine, during ammonia oxidation [82]. Furthermore, the heavy metal transport genes found in AOA genomes suggest that they are adaptable to heavy metal pollution [83].
Methanotrophs have also been reported to decompose various heavy metals and contaminants, including hydrocarbons, and halogenated organic compounds, in the presence of methane monooxygenase (MMO) [84,85,86]. Furthermore, it has been demonstrated that pMMO protein could have the activity to oxidize alkanes and alkenes, while soluble sMMO can oxidize aromatics, aliphatic compounds, and a wide range of organic compounds [87,88]. Therefore, it is worth revisiting the physiological pathway of ammonia and methane oxidation to verify which enzymatic function could connect to the pollutant degradation or biotransformation containing metal compounds.
3. Ammonia and Methane Oxidizer Reactions with Metal in Elevated Metal Concentrations
Metals are essential ecosystem components, and their biological availability is primarily determined by geological and biological processes [89]. As described above, bioavailable metals serve metabolic purposes as enzyme ingredients or structural functions such as maintaining the cell envelope. Accordingly, it would be challenging for microbes to uptake the bioavailable metal from the ecosystem in limited metal concentrations. Harmful metal effects are supplanting the enzymes-associated metals with similar structures or enzyme damage and inactivation by high concentrations (Figure 2) [90]. Therefore, different concentrations of metals may be key control factors for the microbial community and abundance, and as a result, the environmental system may exhibit microbial niche differentiation.
3.1. High Concentrations of Metals
As described above, the bioavailable free metal concentration is tightly affected by various factors. Total metal concentration is increased by the input of the environmental pollution that has become a problem, and it is a significant concern due to the adverse effects it is causing worldwide. Inorganic pollutants are being discarded in both the terrestrial and the aquatic environment due to the astounding increase in the use of heavy metals for anthropogenic activity, which is the primary cause of pollution. Heavy metal pollution is also caused by agricultural activity, such as pesticides, insecticides, fertilizers, etc. Since metals are non-biodegradable and remain persistent in the environment for a long time, they cannot be cleared. Therefore, heavy metals in the environmental system remain present for long periods until they are eluted to other compartments, and then the accumulated metal becomes more toxic to microbes. Excess high concentration of metals is toxic in (i) the displacement of essential metals from their regular binding sites of metalloenzyme, (ii) disruption of nucleic acid structure leads to inhibition of enzymatic functioning, (iii) genotoxicity, (iv) oxidation stress (production of ROS), and (v) inhibition of signaling pathways [91] (Figure 2). In other cases, organic ligands and metal complex formation may effectively reduce the metal concentration below a toxic threshold. Adding metal chelators may alleviate the harmful effects of metal because toxicity is related to the free metal concentration, which is critical for metal toxicity. For instance, if copper were not complexed in the environment, copper-sensitive microbes, many members of autotrophs, would be exposed to toxicity due to high copper concentrations. On the other way around, organic pollution could decrease metal bioavailability by forming a complex between metal and organic ligands, which inhibits the growth and activity of oligotrophs such as AOA in the various heterotrophic environments [39]. Therefore, the equilibrium between the predominance of chelators and metals determines the bioavailability of the metal concentration necessary for the growth of different microorganisms.
A high level of heavy metal compounds had a significant negative impact on both environmental nitrification activity and diversities of AOM. Therefore, several previous studies demonstrated the complexation of copper and other metals with organic matter in activated sludge that focuses on metal toxicity for microbially mediated processes [92]. It has revealed that increasing concentrations of metal (320X of original trace metal solution), including Cu and Fe, above the capacity of the cell, clearly decreased ammonia-oxidizing activity of both AOA and AOB strains, even though there were chelating agents in the media [39]. Furthermore, Nitrosomonas AOB was much more resistant to copper amendment than other tested AOA strains [39]. However, short-term laboratory microcosm experiments found that AOA were consistently more abundant than AOB, and there was no significant AOA community shift after treatment with different concentrations of Cu, either with or without Cu [93]. In another study, the abundance of AOA was lower than recovered AOB abundance after 2-year Zn exposure, and AOB played a significant role in the restoration of nitrification rather than AOA [94]. Therefore, these results indicated that the deleterious effect of metal compounds on the abundance, diversity, activity, and composition of ammonia oxidizers in the natural environment was closely related to the element types, contents, metal exposure times, ecophysiological conditions, etc.
Methanotrophic niches containing pMMO are found in soils, sediments, lakes, and oceans at a high concentration of copper. In contrast, excessive copper concentrations inhibit pMMO activity, resulting in the generation of hydrogen peroxide, which reversibly inhibits pMMO [95,96]. In the culture of Methylococcus capsulatus Bath, pMMO facilitates CH4 oxidation in the cytoplasm when Cu/cell ratios are high (increased from 0 to 55 µM of Cu), while sMMO is activated in the periplasmic membrane space when Cu/cell ratios are low (decreased to 0 µM of Cu) because of different expression patterns (see above). Any other metal ion except copper does not control this switch; however, precisely how copper regulates pMMO expression is still unknown. This copper switch is regulated by methanobactin (Mb), a chalkophore (such as a siderophore; see below). The chelation of copper forms the Cu-Mb complex, which is a copper uptaking strategy of MOB in copper-limited conditions [97]. On the other hand, Cu-chelating Mb also played a critical role in maintaining Cu homeostasis and protection against the potentially toxic effects of a high Cu concentration (M. trichosporium OB3b on 10 µM copper) (see Figure 2) [98]. In fact, the primary function of Mb is to bind and accumulate high-affinity Cu(I) atoms in the growth media when copper concentration is limited.
3.2. Low Concentrations of Metals
Various organic chemicals inhibit the growth of AOA and AOB in sterile-filtered wastewater. AOA was more susceptible to organic chemical inhibition than the tested AOB representative [99]. As described above, the copper complexation with organic compounds significantly inhibited AOA growth, implying that differences in copper requirements and acquisition methods between AOA and AOB likely explain their different sensitivity to organic compounds [39]. Furthermore, copper supplements significantly diminish AOA inhibition by organic compounds, allowing an AOA strain to flourish in municipal nitrifying activated sludge [39]. The effect of ammonia removal was also enhanced by copper addition in a copper-limited full-scale groundwater treatment bioreactor [47]. Therefore, a metal-restricted environment not only loses biological function but also reduces ammonia oxidation activity in various environmental systems.
The bioavailability limitation of divalent metals by complexation with organic matter could be explained by the stability constants (K) for various divalent metal-organic complexes (see above). Free copper (Cu2+), the dominant form of dissolved copper in oxic water, is situated at the top of the Irving-Williams Series, leading to its highest affinity for most environmental organic ligands [100]. Cysteine and histidine being highly inhibitory to AOA, have the highest K values with Cu2+. Organic matters composed of fresh and partially decomposed (e.g., amino acids, sugars, and peptides) and well-decomposed (e.g., humic acids) organic compounds can form tight complexes with Cu2+ [101,102,103]. Thus, the bioavailable form of copper compound (Cu2+) is limited in various environments because most Cu2+ (98–99%) have been found in complexes with organic matter [104,105,106]. Then the different enzyme affinities, together with the different quantities of bioavailable metals, create niche differentiation between various ammonia oxidizer types. Low copper bioavailability can only affect the growth and activity of microbes in the absence of siderophore and chalkophore systems. It is a significant constraint to ammonia and methane oxidation in urban wastewater treatment plants (WWTPs) [107], as well as in many other organic-rich ecosystems, and copper bioavailability is a crucial element in the niche difference between different groups of ammonia oxidizers [39]. Furthermore, when Cu2+ bioavailability is lower than the Cu2+-limiting threshold of AOA [106], microbial Cu2+ dependency can impact the nitrogen cycle [33]. In the WWTPs, metal compounds and other factors such as dissolved oxygen (DO) and sulfate lead to activity and community change of AOB, sulfate-reducing bacteria, anammox, and anaerobic heterotrophic bacteria. Therefore, these multiple factors should be considered together with controlling metal compounds to enhance the efficiency of wastewater treatment [108,109].
Higher pH increases metal binding to organic and inorganic soil particles, dissolved organic molecules, or dissolved minerals [110]. The reduction in copper and iron in these soil environments can affect the abundance and activity of AOM and MOB. In acidic environments, where copper is more bioavailable than iron, the physiological properties of AOA may provide a competitive advantage over AOB [111]. According to the findings of the soil community investigation, reduced pH to AOA strains are considerably more crucial to contributing to environmental nitrogen cycles [112,113]. N. viennensis, a soil AOA strain, had reduced ammonia oxidation and growth when Cu was limited, which was accompanied by the downregulation of the genes involved in metabolism for electron transport, carbon fixation, nucleotide, and lipid biosynthesis [34].
When copper is limited, MOB releases the Mb, which is a class of copper-binding metallophores known as chalkophores mediating microbial copper homeostasis, functioning similar to siderophores which are involved in iron homeostasis (see below in details) [114]. Under metal-limited conditions, various microbes tend to uptake the metal compounds needed for the active site of the key functional protein, such as MMO and FDH in MOB [95,115]. As previously mentioned, variable copper concentrations could control the differential expression of pMMO and sMMO.
4. Chalkophores and Siderophore
Some microorganisms produce siderophores, which are high-affinity Fe binding ligands that increase Fe bioavailability. The siderophore-complexed Fe is subsequently transported into the cytoplasmic space of the cell [114]. Various types of siderophore are transported into cytoplasmic space by siderophore-specific membrane channels or by reduction in Fe-chelates, and it provides a competitive advantage in iron-poor niches of the environments [26]. Ammonia oxidizers, especially bacterial ammonia oxidizers, AOB and comammox, are known for their significant Fe requirement because many cytochromes and heme-containing enzymes are necessary for the energy metabolism (see above). However, the AOB strain Nitrosomonas europaea lacks a genome-based system for producing its own siderophores. Instead, it encodes a large number of genes for iron acquisition enabling efficient scavenging and uptake of various forms of iron in iron-limited environments [35]. Interestingly, oligotrophic AOB strain Nitrosomonas eutropha and marine AOB strain Nitrosomonas oceani have the aerobactin biosynthesis pathway in the genome (Table 1) [116,117]. In vitro investigation on siderophore production by any AOM have not yet been conducted. In contrast to N. europaea, which possesses multiple classes of σ factor/anti-σ factor/TonB-dependent outer membrane (OM) siderophore transporter as well as regulatory genes [118], N. eutropha has only a single ferric uptake regulator gene. Thus, N. eutropha likely relies on different mechanisms compare to N. europaea to regulate iron uptake. Interestingly, the Fe3+-siderophore transporter of N. europaea responded to iron limitation, which is generally repressed under iron-replete conditions. N. eutropha contains conserved genes encoding the energy-transducing TonB-protein complex but lacks an ABC transporter for Fe3+/siderophores. Therefore, N. europaea may utilize siderophores produced by other organisms in its environmental consortium. The ability of the strain to survive in an iron-limited environment without costly secretion of reduced carbon and siderophore could be advantageous. For instance, NOB Nitrobacter and Nitrospira can produce siderophores, and that N. europaea presumably uses these siderophores, hence the AOB N. europaea and NOB strains commonly live together as a consortium in ecosystems [119]. However, smaller genetic inventories for iron transport and the presence of putative siderophore biosynthesis genes in other AOB such as N. eutropha and N. oceanii indicate that they possess different iron acquisition strategies. It has been recently reported that marine AOA strain N. maritimus can utilize strongly chelated Fe to the siderophore desferrioxamine B mesylate (DFB), using an extracellular reductive uptake strategy, even though the AOA strain lacks an endogenous siderophores biosynthesis and transporter system (Table 1) [26].

Table 1.
Siderophore and chalkophore of ammonia and methane oxidizer.
As described, chalkophores are high affinity copper-complexing agents that are secreted by particular bacteria and form solid Cu complexes [115,123]. Most methanotrophs produce Mb to facilitate copper uptake, maintain Cu homeostasis, and protect against the toxic effects of high Cu concentration (M. trichosporium OB3b on 10 µM Cu) [98]. Mb chelate and scavenge Cu2+ or Cu+ from the environment and shuttle them into the bacterial cells. In MOB, metals are transported into the cell by passive diffusion or active transport through TonB-dependent metal transport or other metal transport proteins; in the case of the Mb-Cu complex are transported by the active transporter systems [124]. Therefore, methanotrophic activity in nature may be controlled and influenced significantly by the capacity of Mb to acquire Cu even from insoluble surroundings such as minerals [125]. As mentioned above, Mb maintains Cu homeostasis and protects the cells from metal toxicity caused by the high concentration of Cu as well as other metals. For example, Mb has the ability to bind Au(III), Hg(II), although its binding constant is 15 parts lower in magnitude than copper [114]. However, it has not been verified if interactions between Mb and these metal compounds are relevant to the activity of methanotrophs. Mb is also related to binding other metals such as Fe(III), Ni(II), Zn(II), Au(III), Ag(I), Pb(II), Mn(II), Cd(II), and Co(II), but the binding constants of all these metals examined were less than Cu(II) except Ag(I) and Au(III) [121]. Conversely, some groups of siderophore could bind other metals rather than Fe with relatively high affinity, however, the copper-binding capacity is not regarded as copper uptake. Therefore, it might be a biologically relevant function of several siderophores [114].
5. Conclusions and Future Study
High concentrations of metals in the environment are introduced from metal-containing inorganic pollutions produced by anthropogenic activity, which has a substantial impact on microbial communities and alters their activity. Understanding carbon and nitrogen cycling at the molecular level may also allow insight into how microorganisms are adapting and benefiting from various metal concentrations at the ecosystem level. This is an important step toward mitigating the further release of greenhouse gases containing carbon and nitrogen into the atmosphere. Aerobic methane and ammonia oxidizers (MOB and AOM) are critical members of the carbon and nitrogen cycle in aerobic environments. Their metabolic capacity is significantly affected by metal compounds. Our review concluded that copper and iron metals are essential for various fundamental and specialized physiological processes in MOB and AOM. They are vital in the functioning of enzymes involved in the oxidation processes, which facilitate the growth and activity of microbes in various environments. On the other hand, high metal concentrations are considered toxic for microbial communities. However, further studies are still needed to address the unsolved question in our knowledge of trace metal physiology. For example, (i) comprehending the uptake system of metal or complex of metal-ligands by AOM, (ii) the ammonia oxidation activity affected by methanobactin on AOM growing culture in limited or excess metal conditions, (iii) co-metabolism of methane oxidation by AOA and comammcox with different metal concentrations, and (iv) relationships between metal bioavailability and fluxes of greenhouse gases produced by MOB and AOM. This review study could provide essential information for future works, not only to answer these fundamental questions regarding the ecology and physiology of MOB and AOM in diverse ecosystems but also for comprehending and managing C and N cycles in nature.
Author Contributions
Conceptualization, M.-Y.J.; writing—original draft preparation, H.A. and M.-Y.J.; writing—review and editing, H.A., M.-J.K., A.F. and M.-Y.J.; figure and table preparation, M.-J.K. and M.-Y.J.; supervision, M.-Y.J.; All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Research Facilities and Equipment Center of Korea Basic Science Institute (2020R1A6C101A188) and Research Institute for Basic Sciences (RIBS) of Jeju National University (2019R1A6A1A10072987), funded by the Ministry of Education. And supported by the National Research Foundation of Korea (NRF-2021R1C1C1008303 and NRF-2022R1A4A503144711), funded by MSIT.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare that they have no relevant financial interests.
Abbreviations
| AOM | Ammonia-oxidizing microorganisms |
| MOB | Methane-oxidizing microorganism |
| Comammox | Complete ammonia-oxidizing bacteria |
| NOB | Nitrite-oxidizing bacteria |
| Fe | Iron |
| Cu | Copper |
| CH4 | Methane |
| NH4+ | Ammonium |
| NH3 | Ammonia |
| AMO | Ammonia monooxygenase |
| HAO | Hydroxylamine oxidoreductase |
| pMMO | Partial membrane monooxygenase |
| sMMO | soluble membrane monooxygenase |
References
- Kuypers, M.M.M.; Marchant, H.K.; Kartal, B. The microbial nitrogen-cycling network. Nat. Rev. Microbiol. 2018, 16, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Montzka, S.A.; Dlugokencky, E.J.; Butler, J.H. Non-CO2 greenhouse gases and climate change. Nature 2011, 476, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Strous, M.; Kuenen, J.G.; Jetten, M.S. Key physiology of anaerobic ammonium oxidation. Appl. Environ. Microbiol. 1999, 65, 3248–3250. [Google Scholar] [CrossRef] [PubMed]
- Martikainen, P.J. Heterotrophic nitrification–An eternal mystery in the nitrogen cycle. Soil Biol. Biochem. 2022, 168, 108611. [Google Scholar] [CrossRef]
- Smith, K. Changing views of nitrous oxide emissions from agricultural soil: Key controlling processes and assessment at different spatial scales. Eur. J. Soil Sci. 2017, 68, 137–155. [Google Scholar] [CrossRef]
- Thauer, R.K. Anaerobic oxidation of methane with sulfate: On the reversibility of the reactions that are catalyzed by enzymes also involved in methanogenesis from CO2. Curr. Opin. Microbiol. 2011, 14, 292–299. [Google Scholar] [CrossRef]
- Stein, L.Y. Methane Oxidation. In Encyclopedia of Astrobiology; Gargaud, M., Irvine, W.M., Amils, R., Cleaves, H.J., Pinti, D., Cernicharo Quintanilla, J., Viso, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 1–4. [Google Scholar]
- Scheller, S.; Goenrich, M.; Boecher, R.; Thauer, R.K.; Jaun, B. The key nickel enzyme of methanogenesis catalyses the anaerobic oxidation of methane. Nature 2010, 465, 606–608. [Google Scholar] [CrossRef]
- Scheller, S.; Yu, H.; Chadwick, G.L.; McGlynn, S.E.; Orphan, V.J. Artificial electron acceptors decouple archaeal methane oxidation from sulfate reduction. Science 2016, 351, 703–707. [Google Scholar] [CrossRef]
- Fashola, M.O.; Ngole-Jeme, V.M.; Babalola, O.O. Heavy Metal Pollution from Gold Mines: Environmental Effects and Bacterial Strategies for Resistance. Int. J. Environ. Res. Public Health 2016, 13, 1047. [Google Scholar] [CrossRef]
- Gadd, G.M. Metals, minerals and microbes: Geomicrobiology and bioremediation. Microbiology 2010, 156, 609–643. [Google Scholar] [CrossRef]
- Ehrlich, H. Microbes and metals. Appl. Microbiol. Biotechnol. 1997, 48, 687–692. [Google Scholar] [CrossRef]
- Miličević, A.; Branica, G.; Raos, N. Irving-Williams order in the framework of connectivity index 3 χv enables simultaneous prediction of stability constants of bivalent transition metal complexes. Molecules 2011, 16, 1103–1112. [Google Scholar] [CrossRef] [PubMed]
- Morel, F.M.; Hering, J.G. Principles and Applications of Aquatic Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 1993. [Google Scholar]
- Buffle, J.; Wilkinson, K.J.; Van Leeuwen, H.P. Chemodynamics and bioavailability in natural waters. Environ. Sci. Technol. 2009, 43, 7170–7174. [Google Scholar] [CrossRef]
- Buffle, J.; Altmann, R.S.; Filella, M.; Tessier, A. Complexation by natural heterogeneous compounds: Site occupation distribution functions, a normalized description of metal complexation. Geochim. Cosmochim. Acta 1990, 54, 1535–1553. [Google Scholar] [CrossRef]
- Zhao, L.; Qiu, G.; Anderson, C.W.N.; Meng, B.; Wang, D.; Shang, L.; Yan, H.; Feng, X. Mercury methylation in rice paddies and its possible controlling factors in the Hg mining area, Guizhou province, Southwest China. Environ. Pollut. 2016, 215, 1–9. [Google Scholar] [CrossRef]
- Anderson, R.; Vinikour, W.; Brower, J. The distribution of Cd, Cu, Pb and Zn in the biota of two freshwater sites with different trace metal inputs. Ecography 1978, 1, 377–384. [Google Scholar] [CrossRef]
- Bjerregaard, P.; Andersen, C.B.; Andersen, O. Ecotoxicology of metals—sources, transport, and effects on the ecosystem. In Handbook on the Toxicology of Metals; Elsevier: Amsterdam, The Netherlands, 2022; pp. 593–627. [Google Scholar]
- Mohammadi, R.; Shahrokhian, S. In-situ fabrication of nanosheet arrays on copper foil as a new substrate for binder-free high-performance electrochemical supercapacitors. J. Electroanal. Chem. 2017, 802, 48–56. [Google Scholar] [CrossRef]
- Xia, X.; Gao, Y. Methane from microbial hydrogenolysis of sediment organic matter before the great oxidation event. Nat. Commun. 2021, 12, 5032. [Google Scholar] [CrossRef]
- Daebeler, A.; Bodelier, P.L.; Yan, Z.; Hefting, M.M.; Jia, Z.; Laanbroek, H.J. Interactions between Thaumarchaea, Nitrospira and methanotrophs modulate autotrophic nitrification in volcanic grassland soil. ISME J. 2014, 8, 2397–2410. [Google Scholar] [CrossRef]
- Stein, L.Y.; Roy, R.; Dunfield, P.F. Aerobic methanotrophy and nitrification: Processes and connections. eLS 2012. [Google Scholar] [CrossRef]
- Zheng, Y.; Huang, R.; Wang, B.; Bodelier, P.; Jia, Z. Competitive interactions between methane-and ammonia-oxidizing bacteria modulate carbon and nitrogen cycling in paddy soil. Biogeosciences 2014, 11, 3353–3368. [Google Scholar] [CrossRef]
- Srivastava, P.; Kowshik, M. Mechanisms of metal resistance and homeostasis in haloarchaea. Archaea 2013, 2013, 732864. [Google Scholar] [CrossRef]
- Shafiee, R.T.; Snow, J.T.; Zhang, Q.; Rickaby, R.E.M. Iron requirements and uptake strategies of the globally abundant marine ammonia-oxidising archaeon, Nitrosopumilus maritimus SCM1. ISME J. 2019, 13, 2295–2305. [Google Scholar] [CrossRef]
- Klotz, M.G.; Stein, L.Y. Nitrifier genomics and evolution of the nitrogen cycle. FEMS Microbiol. Lett. 2008, 278, 146–156. [Google Scholar] [CrossRef]
- Kerou, M.; Offre, P.; Valledor, L.; Abby, S.S.; Melcher, M.; Nagler, M.; Weckwerth, W.; Schleper, C. Proteomics and comparative genomics of Nitrososphaera viennensis reveal the core genome and adaptations of archaeal ammonia oxidizers. Proc. Natl. Acad. Sci. USA 2016, 113, E7937–E7946. [Google Scholar] [CrossRef] [PubMed]
- Walker, C.B.; de la Torre, J.R.; Klotz, M.G.; Urakawa, H.; Pinel, N.; Arp, D.J.; Brochier-Armanet, C.; Chain, P.S.; Chan, P.P.; Gollabgir, A.; et al. Nitrosopumilus maritimus genome reveals unique mechanisms for nitrification and autotrophy in globally distributed marine crenarchaea. Proc. Natl. Acad. Sci. USA 2010, 107, 8818–8823. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.Y.; Kim, J.G.; Sinninghe Damste, J.S.; Rijpstra, W.I.; Madsen, E.L.; Kim, S.J.; Hong, H.; Si, O.J.; Kerou, M.; Schleper, C.; et al. A hydrophobic ammonia-oxidizing archaeon of the Nitrosocosmicus clade isolated from coal tar-contaminated sediment. Environ. Microbiol. Rep. 2016, 8, 983–992. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Amin, S.A.; Lundeen, R.A.; Heal, K.R.; Martens-Habbena, W.; Turkarslan, S.; Urakawa, H.; Costa, K.C.; Hendrickson, E.L.; Wang, T.; et al. Stress response of a marine ammonia-oxidizing archaeon informs physiological status of environmental populations. ISME J. 2018, 12, 508–519. [Google Scholar] [CrossRef]
- Santoro, A.E.; Dupont, C.L.; Richter, R.A.; Craig, M.T.; Carini, P.; McIlvin, M.R.; Yang, Y.; Orsi, W.D.; Moran, D.M.; Saito, M.A. Genomic and proteomic characterization of “Candidatus Nitrosopelagicus brevis”: An ammonia-oxidizing archaeon from the open ocean. Proc. Natl. Acad. Sci. USA 2015, 112, 1173–1178. [Google Scholar] [CrossRef]
- Gorman-Lewis, D.; Martens-Habbena, W.; Stahl, D.A. Cu (II) adsorption onto ammonia-oxidizing bacteria and archaea. Geochim. Cosmochim. Acta 2019, 255, 127–143. [Google Scholar] [CrossRef]
- Reyes, C.; Hodgskiss, L.H.; Kerou, M.; Pribasnig, T.; Abby, S.S.; Bayer, B.; Kraemer, S.M.; Schleper, C. Genome wide transcriptomic analysis of the soil ammonia oxidizing archaeon Nitrososphaera viennensis upon exposure to copper limitation. ISME J. 2020, 14, 2659–2674. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Vajrala, N.; Hauser, L.; Sayavedra-Soto, L.A.; Arp, D.J. Iron nutrition and physiological responses to iron stress in Nitrosomonas europaea. Arch. Microbiol. 2006, 186, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Morel, F.M.; Price, N.M. The biogeochemical cycles of trace metals in the oceans. Science 2003, 300, 944–947. [Google Scholar] [CrossRef] [PubMed]
- Whitby, H.; Posacka, A.M.; Maldonado, M.T.; Van Den Berg, C.M. Copper-binding ligands in the NE Pacific. Mar. Chem. 2018, 204, 36–48. [Google Scholar] [CrossRef]
- Hoffmann, S.R.; Shafer, M.M.; Armstrong, D.E. Strong colloidal and dissolved organic ligands binding copper and zinc in rivers. Environ. Sci. Technol. 2007, 41, 6996–7002. [Google Scholar] [CrossRef]
- Gwak, J.H.; Jung, M.Y.; Hong, H.; Kim, J.G.; Quan, Z.X.; Reinfelder, J.R.; Spasov, E.; Neufeld, J.D.; Wagner, M.; Rhee, S.K. Archaeal nitrification is constrained by copper complexation with organic matter in municipal wastewater treatment plants. ISME J. 2020, 14, 335–346. [Google Scholar] [CrossRef]
- Abendroth, J.; Buchko, G.W.; Liew, F.N.; Nguyen, J.N.; Kim, H.J. Structural Characterization of Cytochrome c’ β-Met from an Ammonia-Oxidizing Bacterium. Biochemistry 2022, 61, 563–574. [Google Scholar] [CrossRef]
- Kim, H.J.; Zatsman, A.; Upadhyay, A.K.; Whittaker, M.; Bergmann, D.; Hendrich, M.P.; Hooper, A.B. Membrane tetraheme cytochrome c(m552) of the ammonia-oxidizing Nitrosomonas europaea: A ubiquinone reductase. Biochemistry 2008, 47, 6539–6551. [Google Scholar] [CrossRef][Green Version]
- Shafiee, R.T.; Diver, P.J.; Snow, J.T.; Zhang, Q.; Rickaby, R.E. Marine ammonia-oxidising archaea and bacteria occupy distinct iron and copper niches. ISME Commun. 2021, 1, 1. [Google Scholar] [CrossRef]
- Palomo, A.; Pedersen, A.G.; Fowler, S.J.; Dechesne, A.; Sicheritz-Ponten, T.; Smets, B.F. Comparative genomics sheds light on niche differentiation and the evolutionary history of comammox Nitrospira. ISME J. 2018, 12, 1779–1793. [Google Scholar] [CrossRef]
- Feissner, R.E.; Richard-Fogal, C.L.; Frawley, E.R.; Loughman, J.A.; Earley, K.W.; Kranz, R.G. Recombinant cytochromes c biogenesis systems I and II and analysis of haem delivery pathways in Escherichia coli. Mol. Microbiol. 2006, 60, 563–577. [Google Scholar] [CrossRef] [PubMed]
- Glass, J.B.; Orphan, V.J. Trace metal requirements for microbial enzymes involved in the production and consumption of methane and nitrous oxide. Front. Microbiol. 2012, 3, 61. [Google Scholar] [CrossRef] [PubMed]
- Daims, H.; Lücker, S.; Wagner, M. A new perspective on microbes formerly known as nitrite-oxidizing bacteria. Trends Microbiol. 2016, 24, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Koike, K.; Smith, G.J.; Yamamoto-Ikemoto, R.; Lucker, S.; Matsuura, N. Distinct comammox Nitrospira catalyze ammonia oxidation in a full-scale groundwater treatment bioreactor under copper limited conditions. Water Res. 2022, 210, 117986. [Google Scholar] [CrossRef] [PubMed]
- Strous, M.; Fuerst, J.A.; Kramer, E.H.; Logemann, S.; Muyzer, G.; van de Pas-Schoonen, K.T.; Webb, R.; Kuenen, J.G.; Jetten, M.S. Missing lithotroph identified as new planctomycete. Nature 1999, 400, 446–449. [Google Scholar] [CrossRef]
- Van De Graaf, A.A.; De Bruijn, P.; Robertson, L.A.; Jetten, M.S.; Kuenen, J.G. Metabolic pathway of anaerobic ammonium oxidation on the basis of 15N studies in a fluidized bed reactor. Microbiology 1997, 143, 2415–2421. [Google Scholar] [CrossRef]
- Ni, S.-Q.; Zhang, J. Anaerobic ammonium oxidation: From laboratory to full-scale application. Biomed Res. Int. 2013, 2013, 469360. [Google Scholar] [CrossRef]
- Wang, H.; Yu, G.; He, W.; Du, C.; Deng, Z.; Wang, D.; Yang, M.; Yang, E.; Zhou, Y.; Sanjaya, E.H. Enhancing autotrophic nitrogen removal with a novel dissolved oxygen-differentiated airlift internal circulation reactor: Long-term operational performance and microbial characteristics. J. Environ. Manag. 2021, 296, 113271. [Google Scholar] [CrossRef]
- Chen, H.; Tu, Z.; Wu, S.; Yu, G.; Du, C.; Wang, H.; Yang, E.; Zhou, L.; Deng, B.; Wang, D. Recent advances in partial denitrification-anaerobic ammonium oxidation process for mainstream municipal wastewater treatment. Chemosphere 2021, 278, 130436. [Google Scholar] [CrossRef]
- Zekker, I.; Raudkivi, M.; Artemchuk, O.; Rikmann, E.; Priks, H.; Jaagura, M.; Tenno, T. Mainstream-sidestream wastewater switching promotes anammox nitrogen removal rate in organic-rich, low-temperature streams. Environ. Technol. 2021, 42, 3073–3082. [Google Scholar] [CrossRef]
- Cui, M.; Ma, A.; Qi, H.; Zhuang, X.; Zhuang, G. Anaerobic oxidation of methane: An “active” microbial process. MicrobiologyOpen 2015, 4, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.-D.; Wu, H.-S.; Gao, Z.-Q. Distribution and environmental significance of nitrite-dependent anaerobic methane-oxidising bacteria in natural ecosystems. Appl. Microbiol. Biotechnol. 2015, 99, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Versantvoort, W.; Guerrero-Cruz, S.; Speth, D.R.; Frank, J.; Gambelli, L.; Cremers, G.; van Alen, T.; Jetten, M.S.M.; Kartal, B.; Op den Camp, H.J.M.; et al. Comparative Genomics of Candidatus Methylomirabilis Species and Description of Ca. Methylomirabilis Lanthanidiphila. Front. Microbiol. 2018, 9, 1672. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, K.K.; Goswami, G.; Das, D. Biotransformation of Methane and Carbon Dioxide Into High-Value Products by Methanotrophs: Current State of Art and Future Prospects. Front. Microbiol. 2021, 12, 636486. [Google Scholar] [CrossRef] [PubMed]
- Semrau, J.D.; DiSpirito, A.A.; Yoon, S. Methanotrophs and copper. FEMS Microbiol. Rev. 2010, 34, 496–531. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, O.P.; Smith, T.J.; Dandare, S.U.; Parwin, K.S.; Singh, H.; Loh, H.X.; Cunningham, M.R.; Williams, P.N.; Nichol, T.; Subramanian, A.; et al. Metal(loid) speciation and transformation by aerobic methanotrophs. Microbiome 2021, 9, 156. [Google Scholar] [CrossRef] [PubMed]
- Zahn, J.A.; DiSpirito, A.A. Membrane-associated methane monooxygenase from Methylococcus capsulatus (Bath). J. Bacteriol. Res. 1996, 178, 1018–1029. [Google Scholar] [CrossRef]
- Ross, M.O.; MacMillan, F.; Wang, J.; Nisthal, A.; Lawton, T.J.; Olafson, B.D.; Mayo, S.L.; Rosenzweig, A.C.; Hoffman, B.M. Particulate methane monooxygenase contains only mononuclear copper centers. Science 2019, 364, 566–570. [Google Scholar] [CrossRef]
- Takeguchi, M.; Ohashi, M.; Okura*, I. Role of iron in particulate methane monooxygenase from Methylosinus trichosporium OB3b. Biometals 1999, 12, 123–129. [Google Scholar] [CrossRef]
- Leak, D.J.; Dalton, H. Growth yields of methanotrophs. Appl. Microbiol. Biotechnol. 1986, 23, 470–476. [Google Scholar] [CrossRef]
- Semrau, J.D.; DiSpirito, A.A.; Gu, W.; Yoon, S. Metals and methanotrophy. Appl. Environ. Microbiol. 2018, 84, e02289-17. [Google Scholar] [CrossRef] [PubMed]
- Merkx, M.; Kopp, D.A.; Sazinsky, M.H.; Blazyk, J.L.; Muller, J.; Lippard, S.J. Dioxygen Activation and Methane Hydroxylation by Soluble Methane Monooxygenase: A Tale of Two Irons and Three Proteins. Angew. Chem. Int. Ed. 2001, 40, 2782–2807. [Google Scholar] [CrossRef]
- Hanson, R.S.; Hanson, T.E. Methanotrophic bacteria. Microbiol. Rev. 1996, 60, 439–471. [Google Scholar] [CrossRef] [PubMed]
- Zahn, J.A.; Bergmann, D.J.; Boyd, J.M.; Kunz, R.C.; DiSpirito, A.A. Membrane-associated quinoprotein formaldehyde dehydrogenase from Methylococcus capsulatus Bath. J. Bacteriol. Res. 2001, 183, 6832–6840. [Google Scholar] [CrossRef]
- Stein, L.Y.; Yoon, S.; Semrau, J.D.; Dispirito, A.A.; Crombie, A.; Murrell, J.C.; Vuilleumier, S.; Kalyuzhnaya, M.G.; Op den Camp, H.J.; Bringel, F.; et al. Genome sequence of the obligate methanotroph Methylosinus trichosporium strain OB3b. J. Bacteriol. 2010, 192, 6497–6498. [Google Scholar] [CrossRef]
- Arp, D.J.; Stein, L.Y. Metabolism of inorganic N compounds by ammonia-oxidizing bacteria. Crit. Rev. Biochem. Mol. Biol. 2003, 38, 471–495. [Google Scholar] [CrossRef]
- Jung, M.Y.; Sedlacek, C.J.; Kits, K.D.; Mueller, A.J.; Rhee, S.K.; Hink, L.; Nicol, G.W.; Bayer, B.; Lehtovirta-Morley, L.; Wright, C.; et al. Ammonia-oxidizing archaea possess a wide range of cellular ammonia affinities. ISME J. 2022, 16, 272–283. [Google Scholar] [CrossRef]
- Mohammadi, S.S.; Pol, A.; van Alen, T.; Jetten, M.S.; Op den Camp, H.J. Ammonia oxidation and nitrite reduction in the verrucomicrobial methanotroph Methylacidiphilum fumariolicum SolV. Front. Microbiol. 2017, 8, 1901. [Google Scholar] [CrossRef]
- Duine, J.A.; Frank, J., Jr. Studies on methanol dehydrogenase from Hyphomicrobium X. Isolation of an oxidized form of the enzyme. Biochem. J. 1980, 187, 213–219. [Google Scholar] [CrossRef]
- Stein, L.Y.; Klotz, M.G. Nitrifying and denitrifying pathways of methanotrophic bacteria. Biochem. Soc. Trans. 2011, 39, 1826–1831. [Google Scholar] [CrossRef]
- Poret-Peterson, A.T.; Graham, J.E.; Gulledge, J.; Klotz, M.G. Transcription of nitrification genes by the methane-oxidizing bacterium, Methylococcus capsulatus strain Bath. ISME J. 2008, 2, 1213–1220. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.A.; Nyerges, G.; Kozlowski, J.A.; Poret-Peterson, A.T.; Stein, L.Y.; Klotz, M.G. Model of the molecular basis for hydroxylamine oxidation and nitrous oxide production in methanotrophic bacteria. FEMS Microbiol. Lett. 2011, 322, 82–89. [Google Scholar] [CrossRef]
- Versantvoort, W.; Pol, A.; Jetten, M.S.M.; van Niftrik, L.; Reimann, J.; Kartal, B.; Op den Camp, H.J.M. Multiheme hydroxylamine oxidoreductases produce NO during ammonia oxidation in methanotrophs. Proc. Natl. Acad. Sci. USA 2020, 117, 24459–24463. [Google Scholar] [CrossRef]
- Jones, R.D.; Morita, R.Y. Methane Oxidation by Nitrosococcus oceanus and Nitrosomonas europaea. Appl. Environ. Microbiol. 1983, 45, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Hyman, M.R.; Wood, P.M. Methane oxidation by Nitrosomonas europaea. Biochem. J. 1983, 212, 31–37. [Google Scholar] [CrossRef]
- Ward, B.B. Kinetic studies on ammonia and methane oxidation by Nitrosococcus oceanus. Arch. Microbiol. 1987, 147, 126–133. [Google Scholar] [CrossRef]
- Su, Q.; Schittich, A.R.; Jensen, M.M.; Ng, H.; Smets, B.F. Role of Ammonia Oxidation in Organic Micropollutant Transformation during Wastewater Treatment: Insights from Molecular, Cellular, and Community Level Observations. Environ. Sci. Technol. 2021, 55, 2173–2188. [Google Scholar] [CrossRef]
- Helbling, D.E.; Johnson, D.R.; Honti, M.; Fenner, K. Micropollutant biotransformation kinetics associate with WWTP process parameters and microbial community characteristics. Environ. Sci. Technol. 2012, 46, 10579–10588. [Google Scholar] [CrossRef]
- Men, Y.; Han, P.; Helbling, D.E.; Jehmlich, N.; Herbold, C.; Gulde, R.; Onnis-Hayden, A.; Gu, A.Z.; Johnson, D.R.; Wagner, M. Biotransformation of two pharmaceuticals by the ammonia-oxidizing archaeon Nitrososphaera gargensis. Environ. Sci. Technol. 2016, 50, 4682–4692. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, J.M.; Cui, G.J.; Nunoura, T.; Takaki, Y.; Li, W.L.; Li, J.; Gao, Z.M.; Takai, K.; Zhang, A.Q. Genomics insights into ecotype formation of ammonia-oxidizing archaea in the deep ocean. Environ. Microbiol. 2019, 21, 716–729. [Google Scholar] [CrossRef]
- Burrows, K.J.; Cornish, A.; Scott, D.; Higgins, I.J. Substrate specificities of the soluble and particulate methane mono-oxygenases of Methylosinus trichosporium OB3b. Microbiology 1984, 130, 3327–3333. [Google Scholar] [CrossRef]
- Colby, J.; Stirling, D.I.; Dalton, H. The soluble methane mono-oxygenase of Methylococcus capsulatus (Bath). Its ability to oxygenate n-alkanes, n-alkenes, ethers, and alicyclic, aromatic and heterocyclic compounds. Biochem. J. 1977, 165, 395–402. [Google Scholar] [CrossRef]
- Green, J.; Dalton, H. Substrate specificity of soluble methane monooxygenase: Mechanistic implications. Biol. Chem. 1989, 264, 17698–17703. [Google Scholar] [CrossRef]
- Sirajuddin, S.; Rosenzweig, A.C. Enzymatic oxidation of methane. Biochemistry 2015, 54, 2283–2294. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Vu, D.Q.; Bui, T.P.L.; Mai, T.L.A.; Jensen, L.S.; de Neergaard, A. Organic matter and water management strategies to reduce methane and nitrous oxide emissions from rice paddies in Vietnam. Agric. Ecosyst. Environ. 2014, 196, 137–146. [Google Scholar] [CrossRef]
- Ehrlich, H.L.; Newman, D.K.; Kappler, A. Ehrlich’s Geomicrobiology; Dekker, D., Ed.; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Vázquez, M.N.; Guerrero, Y.R.; González, L.M.; de la Noval, W.T. Brassinosteroids and plant responses to heavy metal stress. An overview. Open J. Metal. 2013, 3, 34–41. [Google Scholar] [CrossRef]
- Banfalvi, G. (Ed.) Cellular Effects of Heavy Metals; Springer: Dordrecht, The Netherlands, 2011; pp. XIV, 348. [Google Scholar]
- Principi, P.; Villa, F.; Bernasconi, M.; Zanardini, E. Metal toxicity in municipal wastewater activated sludge investigated by multivariate analysis and in situ hybridization. Water Res. 2006, 40, 99–106. [Google Scholar] [CrossRef]
- Subrahmanyam, G.; Hu, H.-W.; Zheng, Y.-M.; Gattupalli, A.; He, J.-Z.; Liu, Y.-R. Response of ammonia oxidizing microbes to the stresses of arsenic and copper in two acidic alfisols. Appl. Soil Ecol. 2014, 77, 59–67. [Google Scholar] [CrossRef]
- Mertens, J.; Broos, K.; Wakelin, S.A.; Kowalchuk, G.A.; Springael, D.; Smolders, E. Bacteria, not archaea, restore nitrification in a zinc-contaminated soil. ISME J. 2009, 3, 916–923. [Google Scholar] [CrossRef]
- Balasubramanian, R.; Smith, S.M.; Rawat, S.; Yatsunyk, L.A.; Stemmler, T.L.; Rosenzweig, A.C. Oxidation of methane by a biological dicopper centre. Nature 2010, 465, 115–119. [Google Scholar] [CrossRef]
- Yu, S.S.-F.; Chen, K.H.-C.; Tseng, M.Y.-H.; Wang, Y.-S.; Tseng, C.-F.; Chen, Y.-J.; Huang, D.-S.; Chan, S.I. Production of high-quality particulate methane monooxygenase in high yields from Methylococcus capsulatus (Bath) with a hollow-fiber membrane bioreactor. J. Bacteriol. Res. 2003, 185, 5915–5924. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, R.; Kenney, G.E.; Rosenzweig, A.C. Dual pathways for copper uptake by methanotrophic bacteria. Biol. Chem. 2011, 286, 37313–37319. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, R.; Rosenzweig, A.C. Copper methanobactin: A molecule whose time has come. Curr. Opin. Chem. Biol. 2008, 12, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Prosser, J.I.; Nicol, G.W. Archaeal and bacterial ammonia-oxidisers in soil: The quest for niche specialisation and differentiation. Trends Microbiol. 2012, 20, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Berg, J.M. Principles of Bioinorganic Chemistry; University Science Books: Sausalito, CA, USA, 1994. [Google Scholar]
- Yang, R.; Van den Berg, C.M. Metal complexation by humic substances in seawater. Environ. Sci. Technol. 2009, 43, 7192–7197. [Google Scholar] [CrossRef]
- Shank, G.C.; Skrabal, S.A.; Whitehead, R.F.; Kieber, R.J. Strong copper complexation in an organic-rich estuary: The importance of allochthonous dissolved organic matter. Mar. Chem. 2004, 88, 21–39. [Google Scholar] [CrossRef]
- Voelker, B.M.; Kogut, M.B. Interpretation of metal speciation data in coastal waters: The effects of humic substances on copper binding as a test case. Mar. Chem. 2001, 74, 303–318. [Google Scholar] [CrossRef]
- Sanders, J. The effect of pH on the total and free ionic concentrations of mnganese, zinc and cobalt in soil solutions. Open J. Soil Sci. 1983, 34, 315–323. [Google Scholar] [CrossRef]
- Coale, K.H.; Bruland, K.W. Copper complexation in the Northeast Pacific. Limnol. Oceanogr. 1988, 33, 1084–1101. [Google Scholar] [CrossRef]
- Jacquot, J.E.; Horak, R.E.; Amin, S.A.; Devol, A.H.; Ingalls, A.E.; Armbrust, E.V.; Stahl, D.A.; Moffett, J.W. Assessment of the potential for copper limitation of ammonia oxidation by Archaea in a dynamic estuary. Mar. Chem. 2014, 162, 37–49. [Google Scholar] [CrossRef]
- Yang, Y.; Herbold, C.W.; Jung, M.-Y.; Qin, W.; Cai, M.; Du, H.; Lin, J.-G.; Li, X.; Li, M.; Gu, J.-D. Survival strategies of ammonia-oxidizing archaea (AOA) in a full-scale WWTP treating mixed landfill leachate containing copper ions and operating at low-intensity of aeration. Water Res. 2021, 191, 116798. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yang, M.; Liu, K.; Yang, E.; Chen, J.; Wu, S.; Xie, M.; Wang, D.; Deng, H.; Chen, H. Insights into the synergy between functional microbes and dissolved oxygen partition in the single-stage partial nitritation-anammox granules system. Bioresour. Technol. 2022, 347, 126364. [Google Scholar] [CrossRef]
- Chen, H.; Wu, J.; Liu, B.; Li, Y.Y.; Yasui, H. Competitive dynamics of anaerobes during long-term biological sulfate reduction process in a UASB reactor. Bioresour. Technol. 2019, 280, 173–182. [Google Scholar] [CrossRef]
- Villaverde, P.; Gondar, D.; Antelo, J.; Lopez, R.; Fiol, S.; Arce, F. Influence of pH on copper, lead and cadmium binding by an ombrotrophic peat. Eur. J. Soil Sci. 2009, 60, 377–385. [Google Scholar] [CrossRef]
- Reyes, C.; Hodgskiss, L.H.; Baars, O.; Kerou, M.; Bayer, B.; Schleper, C.; Kraemer, S.M. Copper limiting threshold in the terrestrial ammonia oxidizing archaeon Nitrososphaera viennensis. Res. Microbiol. 2020, 171, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Leininger, S.; Urich, T.; Schloter, M.; Schwark, L.; Qi, J.; Nicol, G.W.; Prosser, J.I.; Schuster, S.C.; Schleper, C. Archaea predominate among ammonia-oxidizing prokaryotes in soils. Nature 2006, 442, 806–809. [Google Scholar] [CrossRef]
- Zhang, L.-M.; Hu, H.-W.; Shen, J.-P.; He, J.-Z. Ammonia-oxidizing archaea have more important role than ammonia-oxidizing bacteria in ammonia oxidation of strongly acidic soils. ISME J. 2012, 6, 1032–1045. [Google Scholar] [CrossRef]
- Kenney, G.E.; Rosenzweig, A.C. Chalkophores. Annu. Rev. Biochem. 2018, 87, 645. [Google Scholar] [CrossRef]
- El Ghazouani, A.; Baslé, A.; Gray, J.; Graham, D.W.; Firbank, S.J.; Dennison, C. Variations in methanobactin structure influences copper utilization by methane-oxidizing bacteria. Proc. Natl. Acad. Sci. USA 2012, 109, 8400–8404. [Google Scholar] [CrossRef]
- Klotz, M.G.; Arp, D.J.; Chain, P.S.; El-Sheikh, A.F.; Hauser, L.J.; Hommes, N.G.; Larimer, F.W.; Malfatti, S.A.; Norton, J.M.; Poret-Peterson, A.T.; et al. Complete genome sequence of the marine, chemolithoautotrophic, ammonia-oxidizing bacterium Nitrosococcus oceani ATCC 19707. Appl. Environ. Microbiol. 2006, 72, 6299–6315. [Google Scholar] [CrossRef]
- Franza, T.; Mahe, B.; Expert, D. Erwinia chrysanthemi requires a second iron transport route dependent of the siderophore achromobactin for extracellular growth and plant infection. Mol. Microbiol. 2005, 55, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Chain, P.; Lamerdin, J.; Larimer, F.; Regala, W.; Lao, V.; Land, M.; Hauser, L.; Hooper, A.; Klotz, M.; Norton, J.; et al. Complete genome sequence of the ammonia-oxidizing bacterium and obligate chemolithoautotroph Nitrosomonas europaea. J. Bacteriol. 2003, 185, 2759–2773. [Google Scholar] [CrossRef] [PubMed]
- Koch, H.; Lücker, S.; Albertsen, M.; Kitzinger, K.; Herbold, C.; Spieck, E.; Nielsen, P.H.; Wagner, M.; Daims, H. Expanded metabolic versatility of ubiquitous nitrite-oxidizing bacteria from the genus Nitrospira. Proc. Natl. Acad. Sci. USA 2015, 112, 11371–11376. [Google Scholar] [CrossRef]
- Peng, P.; Gu, W.; DiSpirito, A.A.; Semrau, J.D. Multiple Mechanisms for Copper Uptake by Methylosinus trichosporium OB3b in the Presence of Heterologous Methanobactin. mBio 2022, 13, e0223922. [Google Scholar] [CrossRef]
- Choi, D.W.; Do, Y.S.; Zea, C.J.; McEllistrem, M.T.; Lee, S.W.; Semrau, J.D.; Pohl, N.L.; Kisting, C.J.; Scardino, L.L.; Hartsel, S.C.; et al. Spectral and thermodynamic properties of Ag(I), Au(III), Cd(II), Co(II), Fe(III), Hg(II), Mn(II), Ni(II), Pb(II), U(IV), and Zn(II) binding by methanobactin from Methylosinus trichosporium OB3b. J. Inorg. Biochem. 2006, 100, 2150–2161. [Google Scholar] [CrossRef] [PubMed]
- Matsen, J.B.; Yang, S.; Stein, L.Y.; Beck, D.; Kalyuzhnaya, M.G. Global Molecular Analyses of Methane Metabolism in Methanotrophic Alphaproteobacterium, Methylosinus trichosporium OB3b. Part I: Transcriptomic Study. Front. Microbiol. 2013, 4, 40. [Google Scholar] [CrossRef] [PubMed]
- Hakemian, A.S.; Tinberg, C.E.; Kondapalli, K.C.; Telser, J.; Hoffman, B.M.; Stemmler, T.L.; Rosenzweig, A.C. The copper chelator methanobactin from Methylosinus trichosporium OB3b binds copper(I). J. Am. Chem. Soc. 2005, 127, 17142–17143. [Google Scholar] [CrossRef]
- Gu, W.; Haque, M.F.U.; Baral, B.S.; Turpin, E.A.; Bandow, N.L.; Kremmer, E.; Flatley, A.; Zischka, H.; DiSpirito, A.A.; Semrau, J.D. A TonB-Dependent Transporter Is Responsible for Methanobactin Uptake by Methylosinus trichosporium OB3b. Appl. Environ. Microbiol. 2016, 82, 1917–1923. [Google Scholar] [CrossRef]
- Knapp, C.W.; Fowle, D.A.; Kulczycki, E.; Roberts, J.A.; Graham, D.W. Methane monooxygenase gene expression mediated by methanobactin in the presence of mineral copper sources. Proc. Natl. Acad. Sci. USA 2007, 104, 12040–12045. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).