Involvement of M1/M2 Macrophage Polarization in Reparative Dentin Formation
Abstract
1. Introduction
2. Materials and Methods
2.1. Induction of M0 Macrophages into M1 and M2 Macrophages
2.2. Preparation of the Conditioned Medium from the M2 Macrophages
2.3. Cell Isolation and Maintenance
2.4. Odontoblastic Differentiation
2.5. Quantitative RT-PCR
2.6. Direct Pulp Capping Model
2.7. Staining Procedures
2.8. Statistical Analysis
3. Results
3.1. Emergence of the M1 Macrophages in the Rat Dental Pulp Tissue after the Direct Pulp Capping
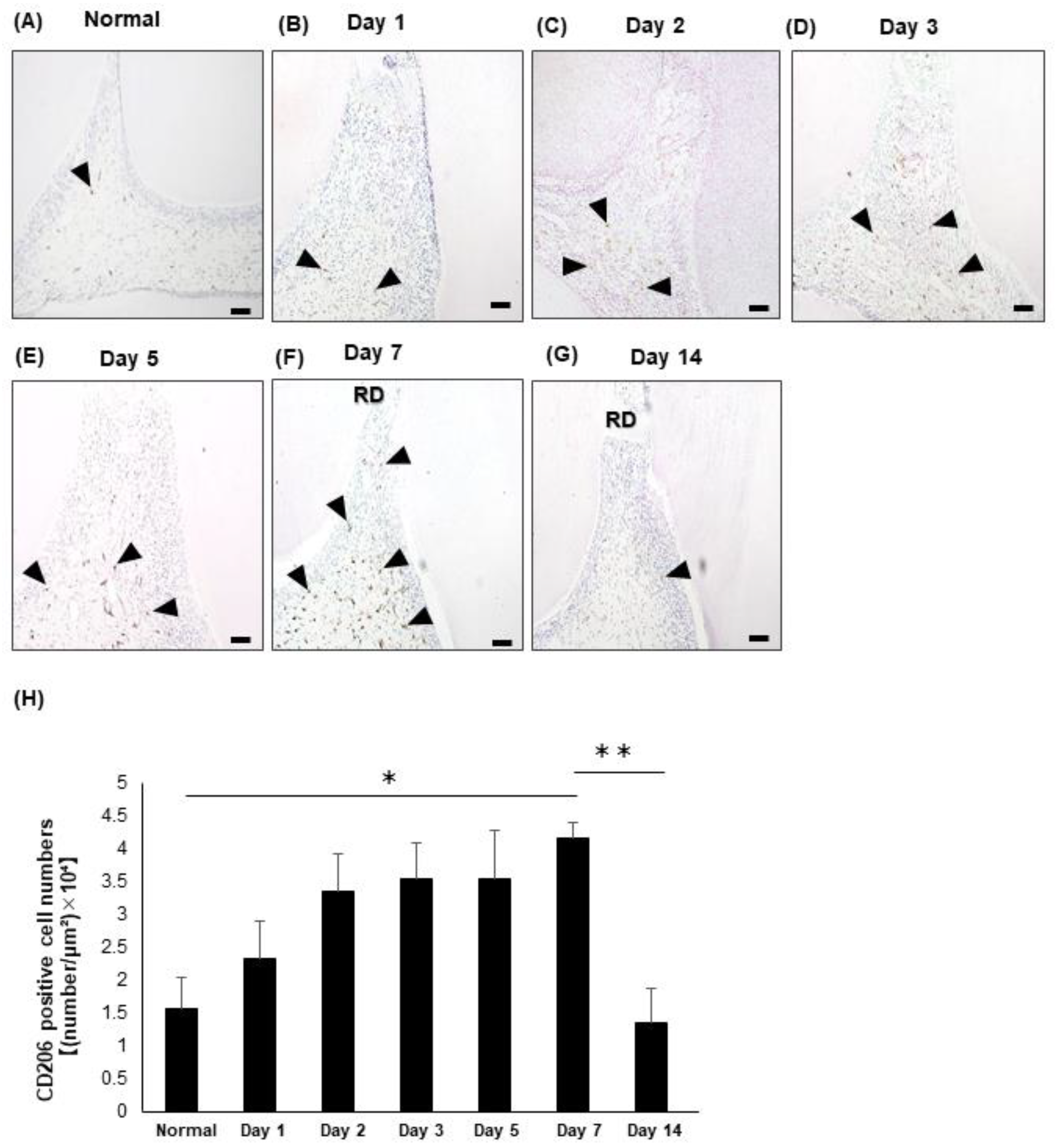
3.2. Emergence of the M2 Macrophages in the Rat Dental Pulp Tissue after the Direct Pulp Capping
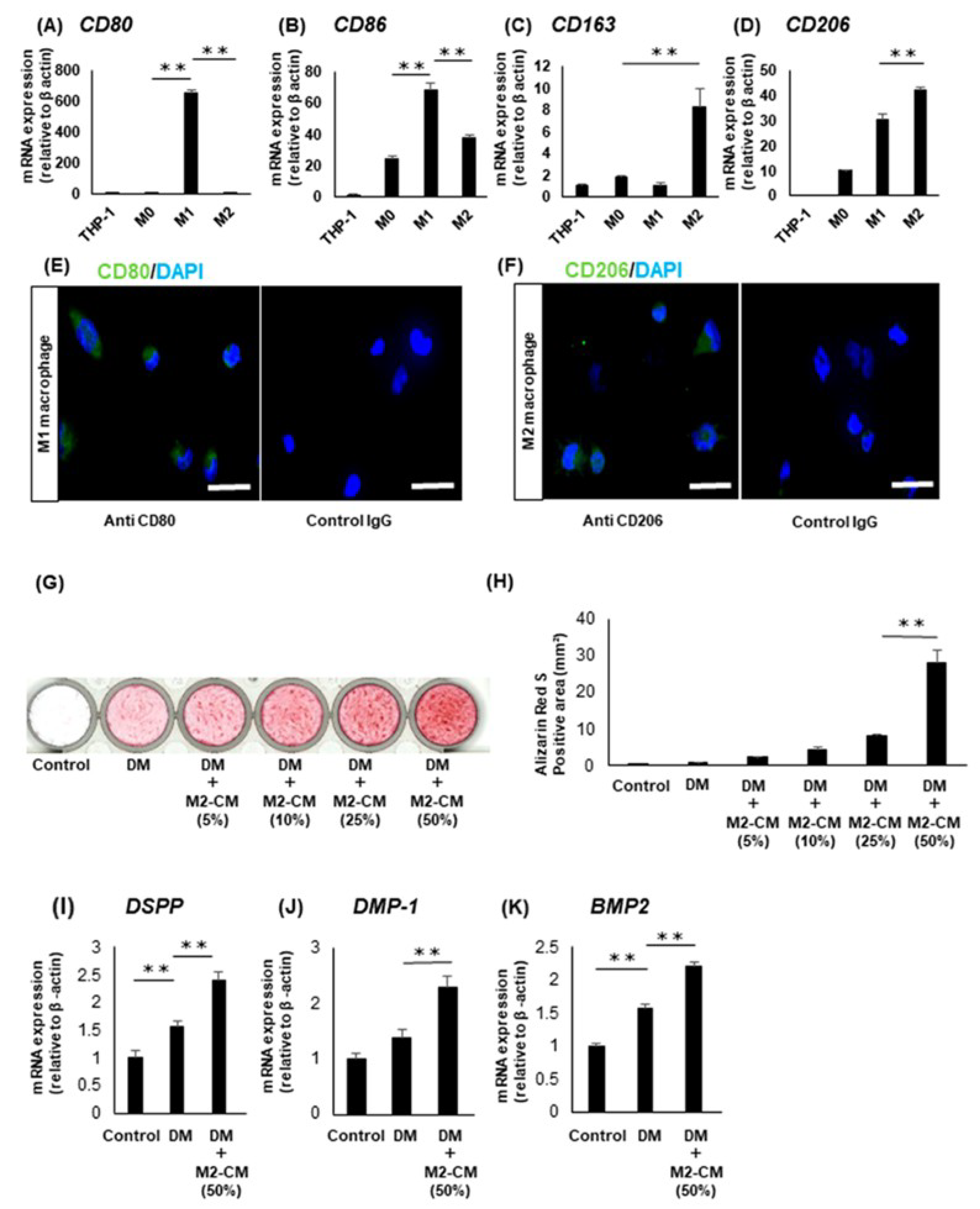
3.3. Effects of the Macrophages on the Odontoblastic Differentiation of the DPSC Clones
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goldberg, M.; Farges, J.C.; Lacerda-Pinheiro, S.; Six, N.; Jegat, N.; Decup, F.; Septier, D.; Carrouel, F.; Durand, S.; Chaussain-Miller, C.; et al. Inflammatory and immunological aspects of dental pulp repair. Pharmacol. Res. 2008, 58, 137–147. [Google Scholar] [CrossRef]
- Tang, W.; Wu, Y.; Smales, R.J. Identifying and reducing risks for potential fractures inendodontically treated teeth. J. Endod. 2010, 36, 609–617. [Google Scholar] [CrossRef]
- Yoshino, K.; Ito, K.; Kuroda, M.; Sugihara, N. Prevalence of vertical root fracture as the reason for tooth extraction in dental clinics. Clin. Oral Investig. 2015, 19, 1405–1409. [Google Scholar] [CrossRef]
- Zhu, C.; Ju, B.; Ni, R. Clinical outcome of direct pulp capping with MTA or calcium hydroxide: A systematic review and meta-analysis. Int. J. Clin. Exp. Med. 2015, 8, 17055–17060. [Google Scholar]
- Hilton, T.J.; Ferracane, J.L.; Mancl, L.; Northwest Practice-based Research Collaborative in Evidence-based Dentistry (NWP). Comparison of CaOH with MTA for direct pulp capping: A PBRN randomized clinical trial. J. Dent. Res. 2013, 92 (Suppl. 7), 16S–22S. [Google Scholar] [CrossRef]
- Farsi, N.; Alamoudi, N.; Balto, K.; Al Mushayt, A. Clinical assessment of mineral trioxide aggregate (MTA) as direct pulp capping in young permanent teeth. J. Clin. Pediatr. Dent. 2006, 31, 72–76. [Google Scholar] [CrossRef]
- Wynn, T.A.; Vannella, K.M. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity 2016, 44, 450–462. [Google Scholar] [CrossRef]
- Gordon, S. Alternative activation of macrophages. Nat. Rev. Immunol. 2003, 3, 23–35. [Google Scholar] [CrossRef]
- Tarique, A.A.; Logan, J.; Thomas, E.; Holt, P.G.; Sly, P.D.; Fantino, E. Phenotypic, functional, and plasticity features of classical and alternatively activated human macrophages. Am. J. Respir. Cell Mol. Biol. 2015, 53, 676–688. [Google Scholar] [CrossRef]
- Xing, Z. Current understanding of macrophage type 1 cytokine responses during intracellular infections. Histol. Histopathol. 2000, 15, 199–205. [Google Scholar] [CrossRef]
- Yang, L.; Xiao, L.; Gao, W.; Huang, X.; Wei, F.; Zhang, Q.; Xiao, Y. Macrophages at Low-Inflammatory Status Improved Osteogenesis via Autophagy Regulation. Tissue Eng. Part A 2021. [Google Scholar] [CrossRef]
- Sica, A.; Erreni, M.; Allavena, P.; Porta, C. Macrophage polarization in pathology. Cell. Mol. Life Sci. 2015, 72, 4111–4126. [Google Scholar] [CrossRef]
- Mantovani, A.; Sica, A.; Sozzani, S.; Allavena, P.; Vecchi, A.; Locati, M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004, 25, 677–686. [Google Scholar] [CrossRef]
- Zhu, L.; Fu, X.; Chen, X.; Han, X.; Dong, P. M2 macrophages induce EMT through the TGF-β/Smad2 signaling pathway. Cell Biol. Int. 2017, 41, 960–968. [Google Scholar] [CrossRef]
- Vega-Galaviz, D.; Vecchyo-Tenorio, G.D.; Alcántara-Suárez, R.; Méndez-García, L.A.; Sánchez-Del Real, A.L.; Villalobos-Molina, R.; Fragoso, J.M.; León-Cabrera, S.; Ostoa-Saloma, P.; Pérez-Tamayo, R.; et al. M2 macrophage immunotherapy abolishes glucose intolerance by increasing IL-10 expression and AKT activation. Immunotherapy 2020, 12, 9–24. [Google Scholar] [CrossRef]
- Rőszer, T. Understanding the Mysterious M2 Macrophage through Activation Markers and Effector Mechanisms. Mediat. Inflamm. 2015, 2015, 816460. [Google Scholar] [CrossRef]
- Funes, S.C.; Rios, M.; Escobar-Vera, J.; Kalergis, A.M. Implications of macrophage polarization in autoimmunity. Immunology 2018, 154, 186–195. [Google Scholar] [CrossRef]
- Kang, M.; Huang, C.C.; Lu, Y.; Shirazi, S.; Gajendrareddy, P.; Ravindran, S.; Cooper, L.F. Bone regeneration is mediated by macrophage extracellular vesicles. Bone 2020, 141, 115627. [Google Scholar] [CrossRef]
- Nakai, K. Multiple roles of macrophage in skin. J. Dermatol. Sci. 2021, 104, 2–10. [Google Scholar] [CrossRef]
- Yoshida, S.; Wada, N.; Hasegawa, D.; Miyaji, H.; Mitarai, H.; Tomokiyo, A.; Hamano, S.; Maeda, H. Semaphorin 3A Induces Odontoblastic Phenotype in Dental Pulp Stem Cells. J. Dent. Res. 2016, 95, 1282–1290. [Google Scholar] [CrossRef]
- Yoshida, S.; Sugii, H.; Itoyama, T.; Kadowaki, M.; Hasegawa, D.; Tomokiyo, A.; Hamano, S.; Ipposhi, K.; Yamashita, K.; Maeda, H. Development of a novel direct dental pulp-capping material using 4-META/MMA-TBB resin with nano hydroxyapatite. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 130, 112426. [Google Scholar] [CrossRef] [PubMed]
- Mizumachi, H.; Yoshida, S.; Tomokiyo, A.; Hasegawa, D.; Hamano, S.; Yuda, A.; Sugii, H.; Serita, S.; Mitarai, H.; Koori, K.; et al. Calcium-sensing receptor-ERK signaling promotes odontoblastic differentiation of human dental pulp cells. Bone 2017, 101, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Koori, K.; Maeda, H.; Fujii, S.; Tomokiyo, A.; Kawachi, G.; Hasegawa, D.; Hamano, S.; Sugii, H.; Wada, N.; Akamine, A. The roles of calcium-sensing receptor and calcium channel in osteogenic differentiation of undifferentiated periodontal ligament cells. Cell Tissue Res. 2014, 357, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Chomczynslci, P.; Sacchi, N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol chloroform extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef]
- Biswas, S.K.; Mantovani, A. Macrophage plasticity and interaction with lymphocyte subsets: Cancer as a paradigm. Nat. Immunol. 2010, 11, 889–896. [Google Scholar] [CrossRef]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef]
- Chanput, W.; Mes, J.J.; Savelkoul, H.F.; Wichers, H.J. Characterization of polarized THP-1 macrophages and polarizing ability of LPS and food compounds. Food Funct. 2013, 4, 266–276. [Google Scholar] [CrossRef]
- Genin, M.; Clement, F.; Fattaccioli, A.; Raes, M.; Michiels, C. M1 and M2 macrophages derived from THP-1 cells differentially modulate the response of cancer cells to etoposide. BMC Cancer 2015, 15, 577. [Google Scholar] [CrossRef]
- Atri, C.; Guerfali, F.Z.; Laouini, D. Role of Human Macrophage Polarization in Inflammation during Infectious Diseases. Int. J. Mol. Sci. 2018, 19, 1801. [Google Scholar] [CrossRef]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef]
- Varela, P.; Sartori, S.; Viebahn, R.; Salber, J.; Ciardelli, G. Macrophage immunomodulation: An indispensable tool to evaluate the performance of wound dressing biomaterials. J. Appl. Biomater. Funct. Mater. 2019, 17, 2280800019830355. [Google Scholar] [CrossRef] [PubMed]
- Arabpour, M.; Saghazadeh, A.; Rezaei, N. Anti-inflammatory and M2 macrophage polarization-promoting effect of mesenchymal stem cell-derived exosomes. Int. Immunopharmacol. 2021, 97, 107823. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Xu, X.H.; Jin, L. Macrophage Polarization in Physiological and Pathological Pregnancy. Front. Immunol. 2019, 10, 792. [Google Scholar] [CrossRef]
- Wang, L.X.; Zhang, S.X.; Wu, H.J.; Rong, X.L.; Guo, J. M2b macrophage polarization and its roles in diseases. J. Leukoc. Biol. 2019, 106, 345–358. [Google Scholar] [CrossRef]
- Zizzo, G.; Hilliard, B.A.; Monestier, M.; Cohen, P.L. Efficient clearance of early apoptotic cells by human macrophages requires M2c polarization and MerTK induction. J. Immunol. 2012, 189, 3508–3520. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, C.J.; Pinhal-Enfield, G.; Elson, G.; Cronstein, B.N.; Hasko, G.; Outram, S.; Leibovich, S.J. The adenosine-dependent angiogenic switch of macrophages to an M2-like phenotype is independent of interleukin-4 receptor alpha (IL-4Rα) signaling. Inflammation 2013, 36, 921–931. [Google Scholar] [CrossRef] [PubMed]
- Jaynes, J.M.; Sable, R.; Ronzetti, M.; Bautista, W.; Knotts, Z.; Abisoye-Ogunniyan, A.; Li, D.; Calvo, R.; Dashnyam, M.; Singh, A.; et al. Mannose receptor (CD206) activation in tumor-associated macrophages enhances adaptive and innate antitumor immune responses. Sci. Transl. Med. 2020, 12, eaax6337. [Google Scholar] [CrossRef]
- Gensel, J.C.; Zhang, B. Macrophage activation and its role in repair and pathology after spinal cord injury. Brain Res. 2015, 1619, 1–11. [Google Scholar] [CrossRef]
- Camilleri, J.; Montesin, F.E.; Brady, K.; Sweeney, R.; Curtis, R.V.; Ford, T.R. The constitution of mineral trioxide aggregate. Dent. Mater. 2005, 21, 297–303. [Google Scholar] [CrossRef]
- Yamashita, K.; Tomokiyo, A.; Ono, T.; Ipposhi, K.; Alhasan, M.A.; Tsuchiya, A.; Hamano, S.; Sugii, H.; Yoshida, S.; Itoyama, T.; et al. Mineral trioxide aggregate immersed in sodium hypochlorite reduce the osteoblastic differentiation of human periodontal ligament stem cells. Sci. Rep. 2021, 11, 22091. [Google Scholar] [CrossRef]
- Kunert, M.; Lukomska-Szymanska, M. Bio-Inductive Materials in Direct and Indirect Pulp Capping—A Review Article. Materials 2020, 13, 1204. [Google Scholar] [CrossRef] [PubMed]
- Paula, A.B.; Laranjo, M.; Marto, C.M.; Paulo, S.; Abrantes, A.M.; Casalta-Lopes, J.; Marques-Ferreira, M.; Botelho, M.F.; Carrilho, E. Direct Pulp Capping: What is the Most Effective Therapy?—Systematic Review and Meta-Analysis. J. Evid. Based Dent. Pract. 2018, 18, 298–314. [Google Scholar] [CrossRef] [PubMed]
- Chicarelli, L.P.G.; Webber, M.B.F.; Amorim, J.P.A.; Rangel, A.; Camilotti, V.; Sinhoreti, M.A.C.; Mendonça, M.J. Effect of Tricalcium Silicate on Direct Pulp Capping: Experimental Study in Rats. Eur. J. Dent. 2021, 15, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Cao, L.; Fan, M.; Xu, Q. Direct Pulp Capping with Calcium Hydroxide or Mineral Trioxide Aggregate: A Meta-analysis. J. Endod. 2015, 41, 1412–1417. [Google Scholar] [CrossRef] [PubMed]
- Serita, S.; Tomokiyo, A.; Hasegawa, D.; Hamano, S.; Sugii, H.; Yoshida, S.; Mizumachi, H.; Mitarai, H.; Monnouchi, S.; Wada, N.; et al. Transforming growth factor-β-induced gene product-h3 inhibits odontoblastic differentiation of dental pulp cells. Arch. Oral Biol. 2017, 78, 135–143. [Google Scholar] [CrossRef]
- Jin, S.; He, D.; Luo, D.; Wang, Y.; Yu, M. A Biomimetic Hierarchical Nanointerface Orchestrates Macrophage Polarization and Mesenchymal Stem Cell Recruitment to Promote Endogenous Bone Regeneration. ACS Nano 2019, 13, 6581–6595. [Google Scholar] [CrossRef]
- Li, X.; He, X.T.; Kong, D.Q.; Xu, X.Y.; Wu, R.X.; Sun, L.-J.; Tian, B.-M.; Chen, F.-M. M2 Macrophages Enhance the Cementoblastic Differentiation of Periodontal Ligament Stem Cells via the Akt and JNK Pathways. Stem Cells 2019, 37, 1567–1580. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kadowaki, M.; Yoshida, S.; Itoyama, T.; Tomokiyo, A.; Hamano, S.; Hasegawa, D.; Sugii, H.; Kaneko, H.; Sugiura, R.; Maeda, H. Involvement of M1/M2 Macrophage Polarization in Reparative Dentin Formation. Life 2022, 12, 1812. https://doi.org/10.3390/life12111812
Kadowaki M, Yoshida S, Itoyama T, Tomokiyo A, Hamano S, Hasegawa D, Sugii H, Kaneko H, Sugiura R, Maeda H. Involvement of M1/M2 Macrophage Polarization in Reparative Dentin Formation. Life. 2022; 12(11):1812. https://doi.org/10.3390/life12111812
Chicago/Turabian StyleKadowaki, Masataka, Shinichiro Yoshida, Tomohiro Itoyama, Atsushi Tomokiyo, Sayuri Hamano, Daigaku Hasegawa, Hideki Sugii, Hiroshi Kaneko, Risa Sugiura, and Hidefumi Maeda. 2022. "Involvement of M1/M2 Macrophage Polarization in Reparative Dentin Formation" Life 12, no. 11: 1812. https://doi.org/10.3390/life12111812
APA StyleKadowaki, M., Yoshida, S., Itoyama, T., Tomokiyo, A., Hamano, S., Hasegawa, D., Sugii, H., Kaneko, H., Sugiura, R., & Maeda, H. (2022). Involvement of M1/M2 Macrophage Polarization in Reparative Dentin Formation. Life, 12(11), 1812. https://doi.org/10.3390/life12111812





