Connexin43 in Post-Surgical Peritoneal Adhesion Formation
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Ethics Approval
2.2. Ischaemic Button Model of Post-Surgical Peritoneal Adhesions
2.3. Studying the Role of Cx43 during Peritoneal Adhesion Formation
2.4. Peritoneal Lavage
2.5. Macroscopic Assessment of Peritoneal Adhesions
2.6. Histology and Immunostaining
2.7. Picro-Sirius Red (PSR) Staining
2.8. Brightfield and Confocal Microscopy
2.9. Microscopic Assessment–Inclusion and Exclusion Criteria
2.10. Extent of Inflammation
2.11. Fibrosis
2.12. Fibrosis Severity
2.13. Protein Levels of αSMA, Cx43 and TGF-β1
2.14. Statistical Analysis
3. Results
3.1. Adhesions Were Observed as Early as 6 h Post-Surgery
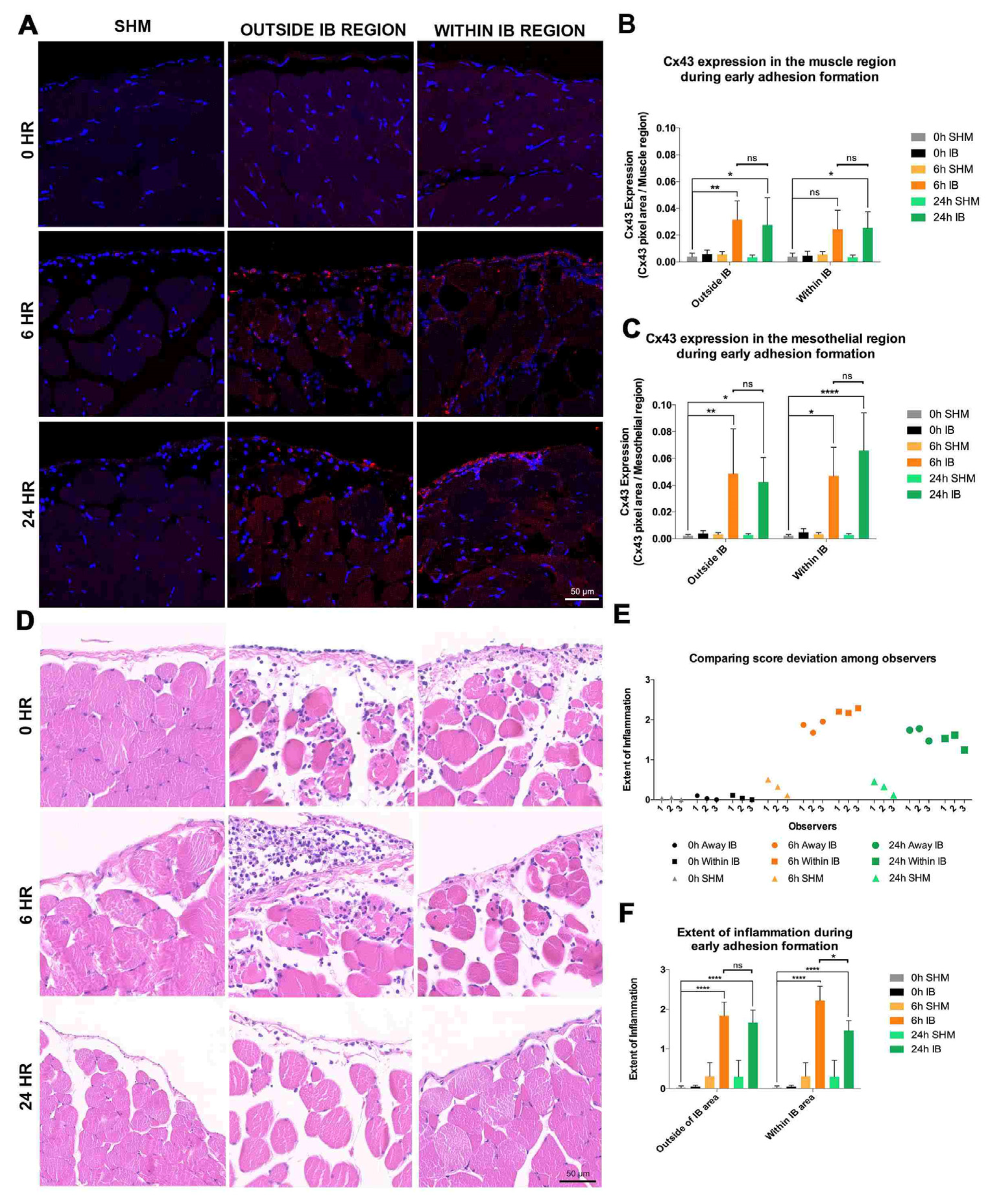
3.2. Significant Upregulation of Cx43 in IB during Early Adhesion Formation
3.3. Increased Inflammation within IB during Early Adhesion Formation
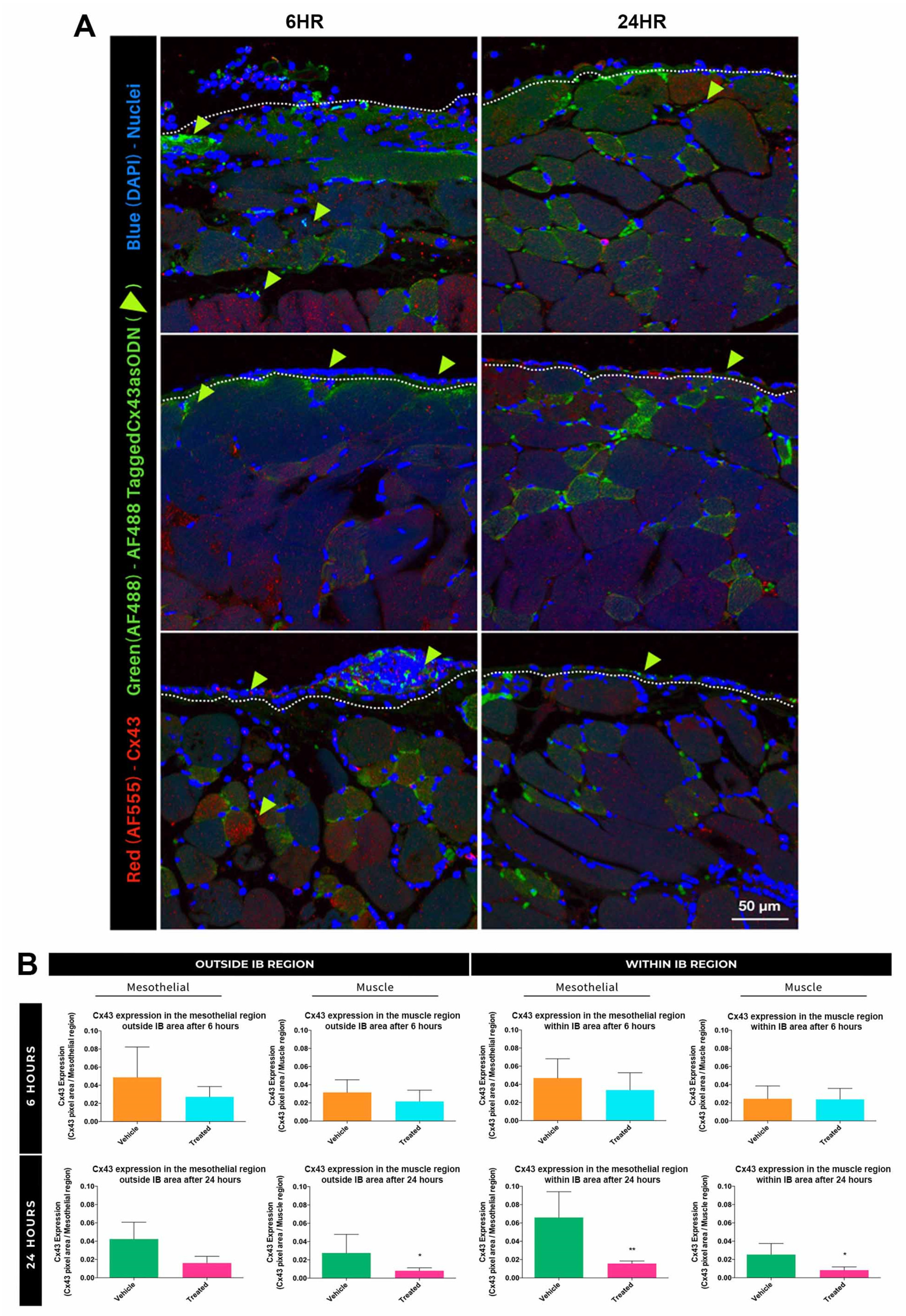
3.4. Treatment with Cx43asODN Significantly Reduced Elevation of Cx43 in the Mesothelial WIB Region
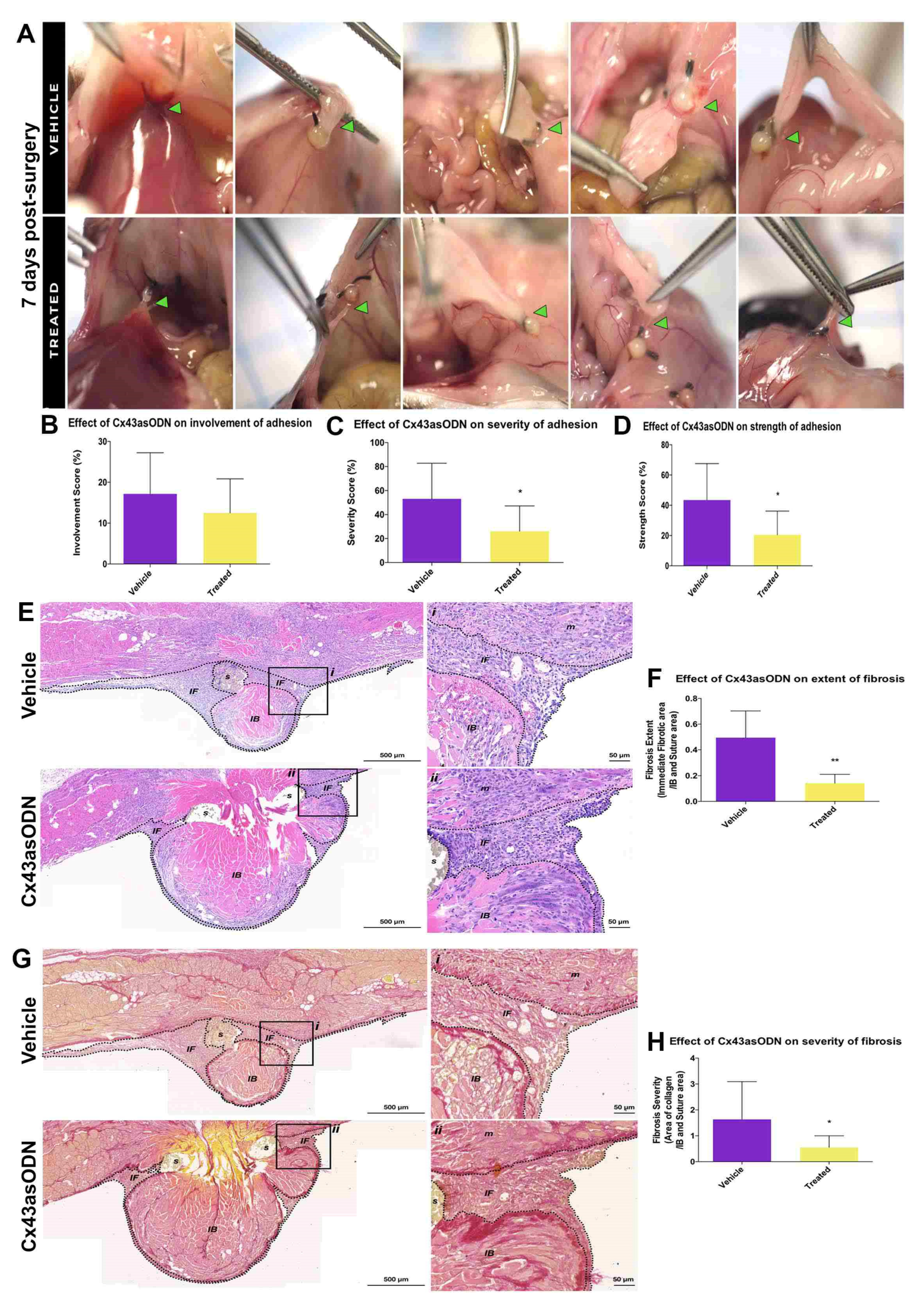
3.5. Treatment with Cx43asODN Significantly Reduced Adhesion Strength and Severity at 7 Days Post-Surgery
3.6. Cx43asODN Treatment Significantly Reduced Fibrosis and Severity of Adhesions
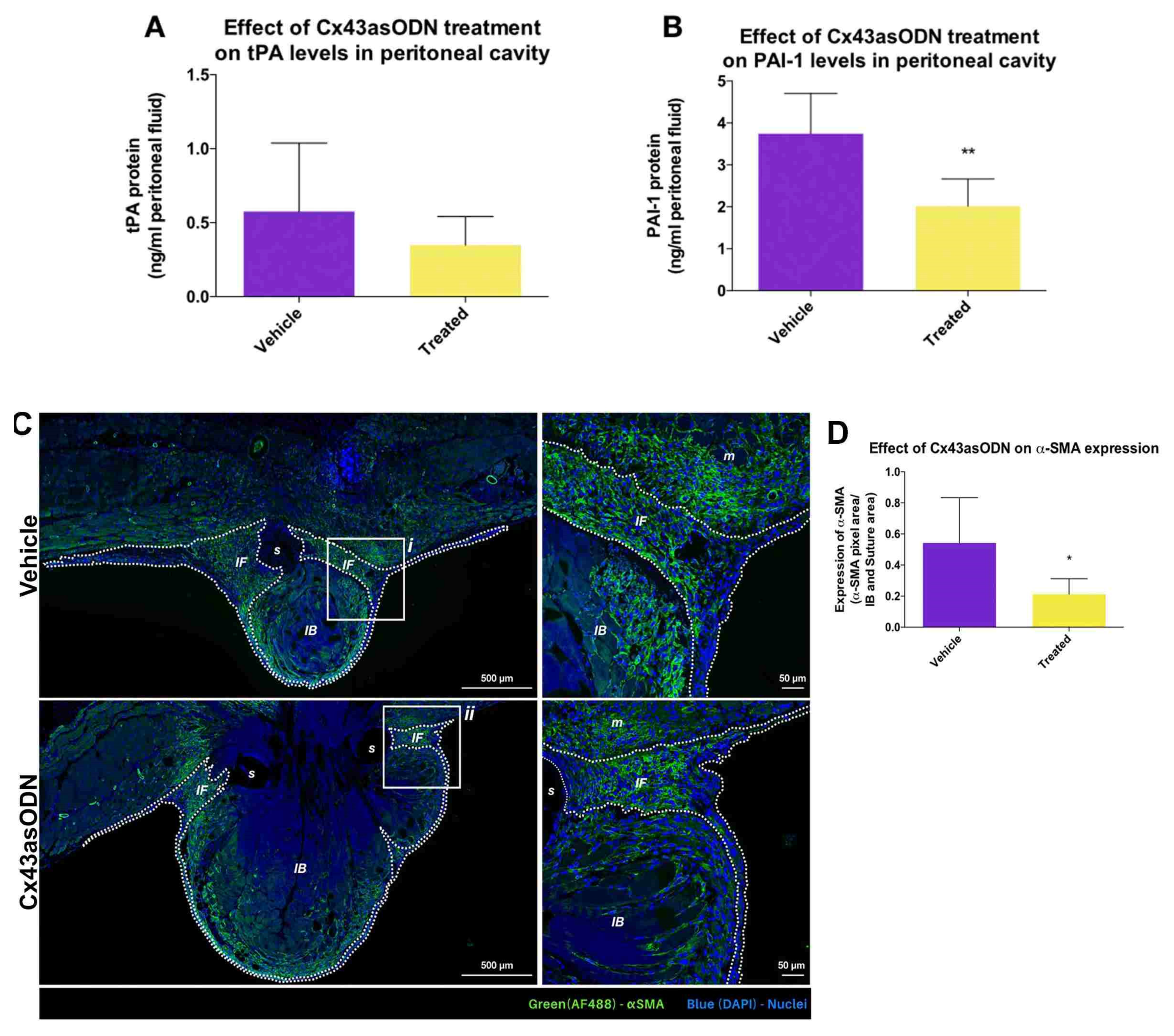
3.7. Effect of Cx43asODN Treatment on the Plasminogen Activating Activity
3.8. Treatment with Cx43asODN Significantly Reduced Fibroblast Activation
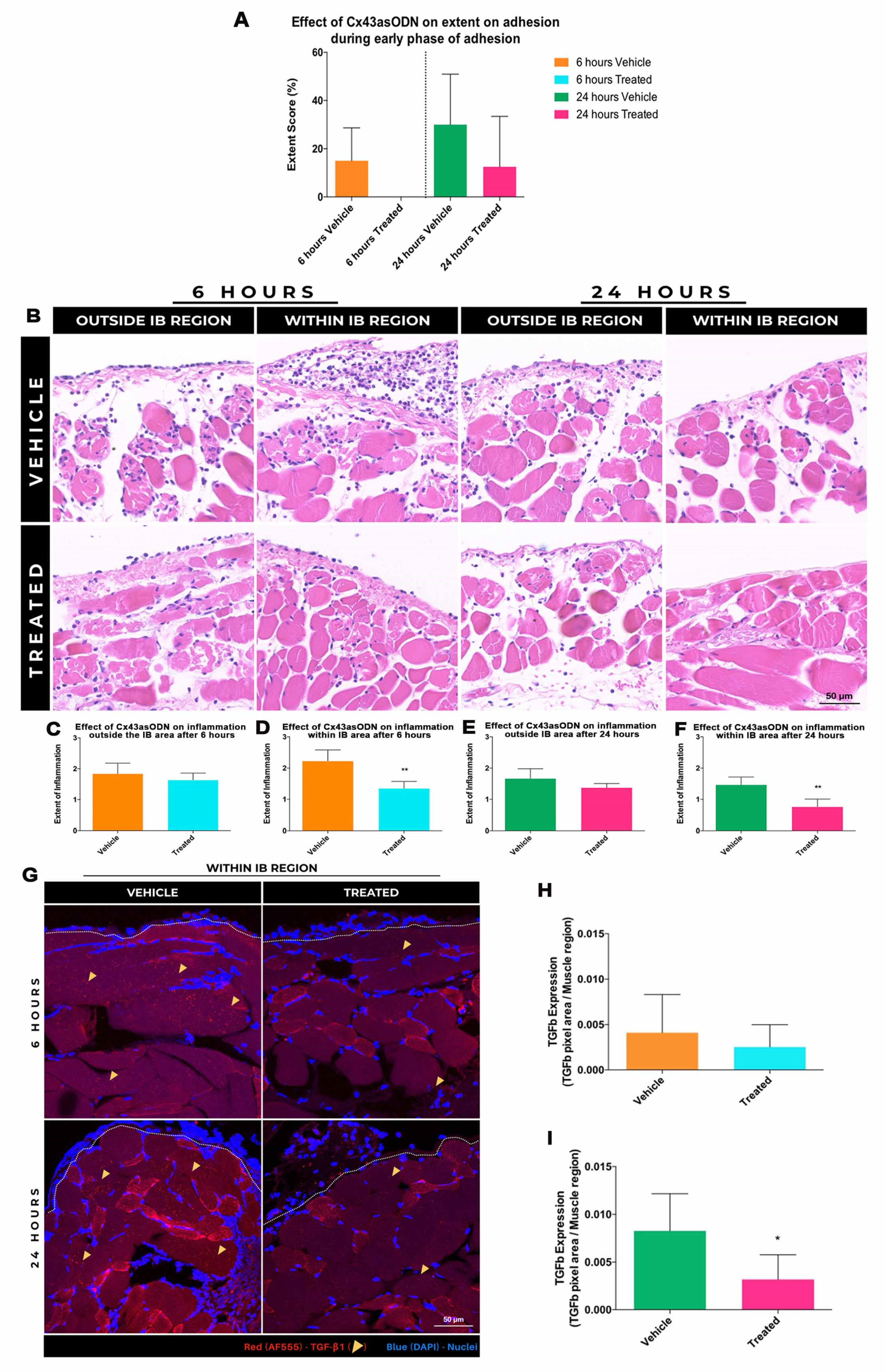
3.9. Treatment with Cx43asODN Reduced the Extent of Adhesion Formation
3.10. Effect of Cx43asODN Treatment on Inflammation during Early Adhesion Formation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martin, P. Wound Healing--Aiming for Perfect Skin Regeneration. Science 1997, 276, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Cogliati, B.; Mennecier, G.; Willebrords, J.; Da Silva, T.C.; Maes, M.; Pereira, I.V.A.; Yanguas, S.C.; Hernandez-Blazquez, F.J.; Dagli, M.L.Z.; Vinken, M. Connexins, Pannexins, and Their Channels in Fibroproliferative Diseases. J. Membr. Biol. 2016, 249, 199–213. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J. Clin. Investig. 2007, 117, 524–529. [Google Scholar] [CrossRef]
- Wynn, T.A.; Ramalingam, T.R. Mechanisms of fibrosis: Therapeutic translation for fibrotic disease. Nat. Med. 2012, 18, 1028–1040. [Google Scholar] [CrossRef]
- Naus, C.C.; Giaume, C. Bridging the gap to therapeutic strategies based on connexin/pannexin biology. J. Transl. Med. 2016, 14, 330. [Google Scholar] [CrossRef]
- Becker, D.L.; Thrasivoulou, C.; Phillips, A.R. Connexins in wound healing; perspectives in diabetic patients. Biochim. Biophys. Acta (BBA)—Biomembr. 2012, 1818, 2068–2075. [Google Scholar] [CrossRef]
- Becker, D.L.; Phillips, A.R.; Duft, B.J.; Kim, Y.; Green, C.R. Translating connexin biology into therapeutics. Semin. Cell Dev. Biol. 2016, 50, 49–58. [Google Scholar] [CrossRef]
- Wang, X.; Ma, A.; Zhu, W.; Zhu, L.; Zhao, Y.; Xi, J.; Becker, D.L. The role of connexin 43 and hemichannels correlated with the astrocytic death following ischemia/reperfusion insult. Cell. Mol. Neurobiol. 2013, 33, 401–410. [Google Scholar] [CrossRef]
- Mendoza-Naranjo, A.; Cormie, P.; Serrano, A.E.; Wang, C.M.; Thrasivoulou, C.; Sutcliffe, J.E.; Gilmartin, D.J.; Tsui, J.; Serena, T.E.; Phillips, A.R.; et al. Overexpression of the gap junction protein Cx43 as found in diabetic foot ulcers can retard fibroblast migration. Cell Biol. Int. 2012, 36, 661–667. [Google Scholar] [CrossRef]
- Lu, F.; Gao, J.; Ogawa, R.; Hyakusoku, H.; Ou, C. Biological Differences between Fibroblasts Derived from Peripheral and Central Areas of Keloid Tissues. Plast. Reconstr. Surg. 2007, 120, 625–630. [Google Scholar] [CrossRef]
- Tarzemany, R.; Jiang, G.; Jiang, J.X.; Larjava, H.; Häkkinen, L. Connexin 43 Hemichannels Regulate the Expression of Wound Healing-Associated Genes in Human Gingival Fibroblasts. Sci. Rep. 2017, 7, 14157. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Coutinho, P.; Frank, S.; Franke, S.; Law, L.-Y.; Martin, P.; Green, C.R.; Becker, D.L. Targeting Connexin43 Expression Accelerates the Rate of Wound Repair. Curr. Biol. 2003, 13, 1697–1703. [Google Scholar] [CrossRef] [PubMed]
- Mori, R.; Power, K.T.; Wang, C.M.; Martin, P.; Becker, D.L. Acute downregulation of connexin43 at wound sites leads to a reduced inflammatory response, enhanced keratinocyte proliferation and wound fibroblast migration. J. Cell Sci. 2006, 119, 5193–5203. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.S.; Berends, R.F.; Flint, D.J.; Martin, P.E. Cell motility in models of wounded human skin is improved by Gap27 despite raised glucose, insulin and IGFBP-5. Exp. Cell Res. 2013, 319, 390–401. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.A.; Richards, T.; Becker, D.L. Connexins and Diabetes. Cardiol. Res. Pract. 2012, 2012, 8. [Google Scholar] [CrossRef]
- Tamaoki, M.; Imanaka-Yoshida, K.; Yokoyama, K.; Nishioka, T.; Inada, H.; Hiroe, M.; Sakakura, T.; Yoshida, T. Tenascin-C Regulates Recruitment of Myofibroblasts during Tissue Repair after Myocardial Injury. Am. J. Pathol. 2005, 167, 71–80. [Google Scholar] [CrossRef]
- Serini, G.; Bochaton-Piallat, M.L.; Ropraz, P.; Geinoz, A.; Borsi, L.; Zardi, L.; Gabbiani, G. The fibronectin domain ED-A is crucial for myofibroblastic phenotype induction by transforming growth factor-beta1. J. Cell Biol. 1998, 142, 873–881. [Google Scholar] [CrossRef]
- Kaden, J.J.; Dempfle, C.-E.; Grobholz, R.; Fischer, C.S.; Vocke, D.C.; Kılıç, R.; Sarıkoç, A.; Piñol, R.; Hagl, S.; Lang, S.; et al. Inflammatory regulation of extracellular matrix remodeling in calcific aortic valve stenosis. Cardiovasc. Pathol. 2005, 14, 80–87. [Google Scholar] [CrossRef]
- Fu, X.; Khalil, H.; Kanisicak, O.; Boyer, J.G.; Vagnozzi, R.J.; Maliken, B.D.; Sargent, M.A.; Prasad, V.; Valiente-Alandi, I.; Blaxall, B.C.; et al. Specialized fibroblast differentiated states underlie scar formation in the infarcted mouse heart. J. Clin. Investig. 2018, 128, 2127–2143. [Google Scholar] [CrossRef]
- Andelova, K.; Szeiffova Bacova, B.; Sykora, M.; Pavelka, S.; Rauchova, H.; Tribulova, N. Cardiac Cx43 Signaling Is Enhanced and TGF-beta1/SMAD2/3 Suppressed in Response to Cold Acclimation and Modulated by Thyroid Status in Hairless SHR(M). Biomedicines 2022, 10, 1707. [Google Scholar] [CrossRef]
- Asazuma-Nakamura, Y.; Dai, P.; Harada, Y.; Jiang, Y.; Hamaoka, K.; Takamatsu, T. Cx43 contributes to TGF-β signaling to regulate differentiation of cardiac fibroblasts into myofibroblasts. Exp. Cell Res. 2009, 315, 1190–1199. [Google Scholar] [CrossRef] [PubMed]
- Dai, P.; Nakagami, T.; Tanaka, H.; Hitomi, T.; Takamatsu, T. Cx43 mediates TGF-beta signaling through competitive Smads binding to microtubules. Mol. Biol. Cell 2007, 18, 2264–2273. [Google Scholar] [CrossRef]
- Hills, C.E.; Squires, P.E. TGF-beta1-induced epithelial-to-mesenchymal transition and therapeutic intervention in diabetic nephropathy. Am. J. Nephrol. 2010, 31, 68–74. [Google Scholar] [CrossRef]
- Zeisberg, M.; Kalluri, R. The role of epithelial-to-mesenchymal transition in renal fibrosis. Klin. Wochenschr. 2004, 82, 175–181. [Google Scholar] [CrossRef]
- Zheng, G.; Lyons, J.G.; Tan, T.K.; Wang, Y.; Hsu, T.T.; Min, D.; Harris, D.C. Disruption of E-cadherin by matrix metalloproteinase directly mediates epithelial-mesenchymal transition downstream of transforming growth factor-beta1 in renal tubular epithelial cells. Am. J. Pathol. 2009, 175, 580–591. [Google Scholar] [CrossRef]
- Abed, A.; Toubas, J.; Kavvadas, P.; Authier, F.; Cathelin, D.; Alfieri, C.; Boffa, J.-J.; Dussaule, J.-C.; Chatziantoniou, C.; Chadjichristos, C.E. Targeting connexin 43 protects against the progression of experimental chronic kidney disease in mice. Kidney Int. 2014, 86, 768–779. [Google Scholar] [CrossRef]
- Lu, F.; Gao, J.; Ogawa, R.; Hyakusoku, H. Variations in Gap Junctional Intercellular Communication and Connexin Expression in Fibroblasts Derived from Keloid and Hypertrophic Scars. Plast. Reconstr. Surg. 2007, 119, 844–851. [Google Scholar] [CrossRef]
- Arung, W.; Meurisse, M.; Detry, O. Pathophysiology and prevention of postoperative peritoneal adhesions. World J. Gastroenterol. 2011, 17, 4545–4553. [Google Scholar] [CrossRef]
- Braun, K.M.; Diamond, M.P. The biology of adhesion formation in the peritoneal cavity. Semin. Pediatr. Surg. 2014, 23, 336–343. [Google Scholar] [CrossRef]
- Sandoval, P.; A Jiménez-Heffernan, J.; Guerra-Azcona, G.; Pérez-Lozano, M.L.; Rynne-Vidal, Á.; Albar-Vizcaíno, P.; Gil-Vera, F.; Martín, P.; Coronado, M.J.; Barcena, C.; et al. Mesothelial-to-mesenchymal transition in the pathogenesis of post-surgical peritoneal adhesions. J. Pathol. 2016, 239, 48–59. [Google Scholar] [CrossRef]
- Adhesion Scoring Group. Improvement of interobserver reproducibility of adhesion scoring systems. Fertil. Steril. 1994, 62, 984–988. [Google Scholar] [CrossRef]
- Cassidy, M.R.; Sherburne, A.C.; Heydrick, S.J.; Stucchi, A.F. Combined intraoperative administration of a histone deacetylase inhibitor and a neurokinin-1 receptor antagonist synergistically reduces intra-abdominal adhesion formation in a rat model. Surgery 2015, 157, 581–589. [Google Scholar] [CrossRef]
- Hoffmann, N.E.; Siddiqui, S.A.; Agarwal, S.; McKellar, S.H.; Kurtz, H.J.; Gettman, M.T.; Ereth, M.H. Choice of Hemostatic Agent Influences Adhesion Formation in a Rat Cecal Adhesion Model. J. Surg. Res. 2009, 155, 77–81. [Google Scholar] [CrossRef]
- Nair, S.K.; Bhat, I.K.; Aurora, A.L. Role of Proteolytic Enzyme in the Prevention of Postoperative Intraperitoneal Adhesions. Arch. Surg. 1974, 108, 849–853. [Google Scholar] [CrossRef] [PubMed]
- Chua, J.W.; Madden, L.; Lim, S.B.H.; Philips, A.R.J.; Becker, D.L. Development of a refined ex vivo model of peritoneal adhesion formation, and a role for connexin 43 in their development. Mol. Cell. Biochem. 2021, 477, 295–305. [Google Scholar] [CrossRef]
- Schipke, J.; Brandenberger, C.; Rajces, A.; Manninger, M.; Alogna, A.; Post, H.; Mühlfeld, C. Assessment of cardiac fibrosis: A morphometric method comparison for collagen quantification. J. Appl. Physiol. 2017, 122, 1019–1030. [Google Scholar] [CrossRef]
- Diamond, M.P.; Nezhat, F. Adhesions after resection of ovarian endometriomas. Fertil. Steril. 1993, 59, 934–935. [Google Scholar] [CrossRef]
- Tsai, J.M.; Sinha, R.; Seita, J.; Fernhoff, N.; Christ, S.; Koopmans, T.; Krampitz, G.W.; McKenna, K.M.; Xing, L.; Sandholzer, M.; et al. Surgical adhesions in mice are derived from mesothelial cells and can be targeted by antibodies against mesothelial markers. Sci. Transl. Med. 2018, 10, eaan6735. [Google Scholar] [CrossRef]
- diZerega, G.S.; Campeau, J.D. Peritoneal repair and post-surgical adhesion formation. Hum. Reprod. Update 2001, 7, 547–555. [Google Scholar] [CrossRef]
- Mutsaers, S.E.; birnie, K.; lansley, S.; Herrick, S.; Elim, C.B.; PrãªLe, C.M. Mesothelial cells in tissue repair and fibrosis. Front. Pharmacol. 2015, 6, 113. [Google Scholar] [CrossRef]
- Ozel, H.; Avsar, F.M.; Topaloglu, S.; Sahin, M. Induction and assessment methods used in experimental adhesion studies. Wound Repair Regen. 2005, 13, 358–364. [Google Scholar] [CrossRef]
- Okabayashi, K.; Ashrafian, H.; Zacharakis, E.; Hasegawa, H.; Kitagawa, Y.; Athanasiou, T.; Darzi, A. Adhesions after abdominal surgery: A systematic review of the incidence, distribution and severity. Surg. Today 2013, 44, 405–420. [Google Scholar] [CrossRef]
- Merlo, G.; Fausone, G.; Castagna, B. Fibrinolytic Activity of Mesothelial Lining of the Displaced Peritoneum. Am. J. Med. Sci. 1983, 286, 12–14. [Google Scholar] [CrossRef]
- Pelin, K.; Hirvonen, A.; Linnainmaa, K. Expression of cell adhesion molecules and connexins in gap junctional intercellular communication deficient human mesothelioma tumour cell lines and communication competent primary mesothelial cells. Carcinogenesis 1994, 15, 2673–2675. [Google Scholar] [CrossRef]
- Mikuła-Pietrasik, J.; Uruski, P.; Szubert, S.; Szpurek, D.; Sajdak, S.; Tykarski, A.; Książek, K. Malignant ascites determine the transmesothelial invasion of ovarian cancer cells. Int. J. Biochem. Cell Biol. 2017, 92, 6–13. [Google Scholar] [CrossRef]
- Ogawa, T.; Hayashi, T.; Tokunou, M.; Nakachi, K.; Trosko, J.E.; Chang, C.-C.; Yorioka, N. Suberoylanilide Hydroxamic Acid Enhances Gap Junctional Intercellular Communication via Acetylation of Histone Containing Connexin 43 Gene Locus. Cancer Res. 2005, 65, 9771–9778. [Google Scholar] [CrossRef]
- Tang, B.; Peng, Z.-H.; Yu, P.-W.; Yu, G.; Qian, F.; Zeng, D.-Z.; Zhao, Y.-L.; Shi, Y.; Hao, Y.-X.; Luo, H.-X. Aberrant Expression of Cx43 Is Associated with the Peritoneal Metastasis of Gastric Cancer and Cx43-Mediated Gap Junction Enhances Gastric Cancer Cell Diapedesis from Peritoneal Mesothelium. PLoS ONE 2013, 8, e74527. [Google Scholar] [CrossRef]
- Al-Ghadban, S.; Kaissi, S.; Homaidan, F.R.; Naim, H.Y.; El-Sabban, M.E. Cross-Talk between intestinal epithelial cells and immune cells in inflammatory bowel disease. Sci. Rep. 2016, 6, 29783. [Google Scholar] [CrossRef]
- Eugenín, E.A.; Brañes, M.C.; Berman, J.W.; Sáez, J.C. TNF-alpha plus IFN-gamma induce connexin43 expression and formation of gap junctions between human monocytes/macrophages that enhance physiological responses. J. Immunol. 2003, 170, 1320–1328. [Google Scholar] [CrossRef]
- Eugenín, E.A.; González, H.E.; Sánchez, H.A.; Brañes, M.C.; Sáez, J.C. Inflammatory conditions induce gap junctional communication between rat Kupffer cells both in vivo and in vitro. Cell. Immunol. 2007, 247, 103–110. [Google Scholar] [CrossRef]
- Branes, M.C.; E Contreras, J.; Sáez, J.C. Activation of human polymorphonuclear cells induces formation of functional gap junctions and expression of connexins. Med. Sci. Monit. 2002, 8, BR313–BR323. [Google Scholar]
- Calder, B.W.; Rhett, J.M.; Bainbridge, H.; Fann, S.A.; Gourdie, R.G.; Yost, M.J. Inhibition of Connexin 43 Hemichannel-Mediated ATP Release Attenuates Early Inflammation During the Foreign Body Response. Tissue Eng. Part A 2015, 21, 1752–1762. [Google Scholar] [CrossRef]
- Zernecke, A.; Bidzhekov, K.; Ozuyaman, B.; Fraemohs, L.; Liehn, E.A.; Luscher-Firzlaff, J.M.; Weber, C. CD73/ecto-5′-nucleotidase protects against vascular inflammation and neointima formation. Circulation 2006, 113, 2120–2127. [Google Scholar] [CrossRef]
- Maciver, A.H.; McCall, M.; Shapiro, A.J. Intra-Abdominal adhesions: Cellular mechanisms and strategies for prevention. Int. J. Surg. 2011, 9, 589–594. [Google Scholar] [CrossRef]
- Beyene, R.T.; Kavalukas, S.L.; Barbul, A. Intra-Abdominal adhesions: Anatomy, physiology, pathophysiology, and treatment. Curr. Probl. Surg. 2015, 52, 271–319. [Google Scholar] [CrossRef]
- Vural, B.; Cantürk, N.Z.; Esen, N.; Solakoglu, S.; Cantürk, Z.; Kirkali, G.; Sökmensüer, C. The role of neutrophils in the formation of peritoneal adhesions. Hum. Reprod. 1999, 14, 49–54. [Google Scholar] [CrossRef]
- Hoshino, A.; Kawamura, Y.I.; Yasuhara, M.; Toyama-Sorimachi, N.; Yamamoto, K.; Matsukawa, A.; Lira, S.A.; Dohi, T. Inhibition of CCL1-CCR8 Interaction Prevents Aggregation of Macrophages and Development of Peritoneal Adhesions. J. Immunol. 2007, 178, 5296–5304. [Google Scholar] [CrossRef]
- Sarieddine, M.Z.R.; Scheckenbach, K.E.L.; Foglia, B.; Maass, K.; Garcia, I.; Kwak, B.; Chanson, M. Connexin43 modulates neutrophil recruitment to the lung. J. Cell. Mol. Med. 2009, 13, 4560–4570. [Google Scholar] [CrossRef]
- Oviedo-Orta, E.; Gasque, P.; Evans, W.H. Immunoglobulin and cytokine expression in mixed lymphocyte cultures is reduced by disruption of gap junction intercellular communication. FASEB J. 2001, 15, 768–774. [Google Scholar] [CrossRef]
- Desmoulière, A.; Geinoz, A.; Gabbiani, F. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J. Cell Biol. 1993, 122, 103–111. [Google Scholar] [CrossRef]
- Chegini, N. TGF-beta system: The principal profibrotic mediator of peritoneal adhesion formation. Semin. Reprod. Med. 2008, 26, 298–312. [Google Scholar] [CrossRef]
- Rosenkranz, S. TGF-beta1 and angiotensin networking in cardiac remodeling. Cardiovasc. Res. 2004, 63, 423–432. [Google Scholar] [CrossRef]
- Tomasek, J.J.; McRae, J.; Owens, G.K.; Haaksma, C.J. Regulation of α-Smooth Muscle Actin Expression in Granulation Tissue Myofibroblasts Is Dependent on the Intronic CArG Element and the Transforming Growth Factor-β1 Control Element. Am. J. Pathol. 2005, 166, 1343–1351. [Google Scholar] [CrossRef]
- Goetsch, K.; Niesler, C. The extracellular matrix regulates the effect of decorin and transforming growth factor beta-2 (TGF-β2) on myoblast migration. Biochem. Biophys. Res. Commun. 2016, 479, 351–357. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J. Role of transforming growth factor-beta in muscle damage and regeneration: Focused on eccentric muscle contraction. J. Exerc. Rehabil. 2017, 13, 621–626. [Google Scholar] [CrossRef]
- Smith, C.A.; Stauber, F.; Waters, C.; Alway, S.E.; Stauber, W.T. Transforming growth factor-beta following skeletal muscle strain injury in rats. J. Appl. Physiol. 1985, 102, 755–761. [Google Scholar] [CrossRef]
- Delaney, K.; Kasprzycka, P.; Ciemerych, M.A.; Zimowska, M. The role of TGF-beta1 during skeletal muscle regeneration. Cell Biol. Int. 2017, 41, 706–715. [Google Scholar] [CrossRef]
- Zimowska, M.; Duchesnay, A.; Dragun, P.; Oberbek, A.; Moraczewski, J.; Maretlly, I. Immunoneutralization of TGFbeta1 Improves Skeletal Muscle Regeneration: Effects on Myoblast Differentiation and Glycosaminoglycan Content. Int. J. Cell Biol. 2009, 2009, 659372. [Google Scholar] [CrossRef]
- Holmdahl, L.; Eriksson, E.; Al-Jabreen, M.; Risberg, B. Fibrinolysis in human peritoneum during operation. Surgery 1996, 119, 701–705. [Google Scholar] [CrossRef]
- Lang, N.N.; Myles, R.C.; Burton, F.L.; Hall, D.P.; Chin, Y.Z.; Boon, N.A.; Newby, D.E. The vascular effects of rotigaptide in vivo in man. Biochem. Pharmacol. 2008, 76, 1194–1200. [Google Scholar] [CrossRef]
- Dubuis, C.; May, L.; Alonso, F.; Luca, L.; Mylonaki, I.; Meda, P.; Delie, F.; Jordan, O.; Déglise, S.; Corpataux, J.-M.; et al. Atorvastatin-Loaded Hydrogel Affects the Smooth Muscle Cells of Human Veins. J. Pharmacol. Exp. Ther. 2013, 347, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Soleymani, S.; Madakshire, R.; Insel, P.A. ATP released from cardiac fibroblasts via connexin hemichannels activates profibrotic P2Y 2 receptors. FASEB J. 2012, 26, 2580–2591. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chua, J.W.; Thangaveloo, M.; Lim, D.X.E.; Madden, L.E.; Phillips, A.R.J.; Becker, D.L. Connexin43 in Post-Surgical Peritoneal Adhesion Formation. Life 2022, 12, 1734. https://doi.org/10.3390/life12111734
Chua JW, Thangaveloo M, Lim DXE, Madden LE, Phillips ARJ, Becker DL. Connexin43 in Post-Surgical Peritoneal Adhesion Formation. Life. 2022; 12(11):1734. https://doi.org/10.3390/life12111734
Chicago/Turabian StyleChua, Jia Wang, Moogaambikai Thangaveloo, Debbie Xiu En Lim, Leigh E. Madden, Anthony R. J. Phillips, and David L. Becker. 2022. "Connexin43 in Post-Surgical Peritoneal Adhesion Formation" Life 12, no. 11: 1734. https://doi.org/10.3390/life12111734
APA StyleChua, J. W., Thangaveloo, M., Lim, D. X. E., Madden, L. E., Phillips, A. R. J., & Becker, D. L. (2022). Connexin43 in Post-Surgical Peritoneal Adhesion Formation. Life, 12(11), 1734. https://doi.org/10.3390/life12111734






