Associations between Deformation of the Thoracolumbar Fascia and Activation of the Erector Spinae and Multifidus Muscle in Patients with Acute Low Back Pain and Healthy Controls: A Matched Pair Case-Control Study
Abstract
1. Introduction
Aims
2. Materials and Methods
2.1. Study Design Overview
2.2. Setting and Participants
2.2.1. Inclusion Criteria for the aLBP Group
2.2.2. Exclusion Criteria
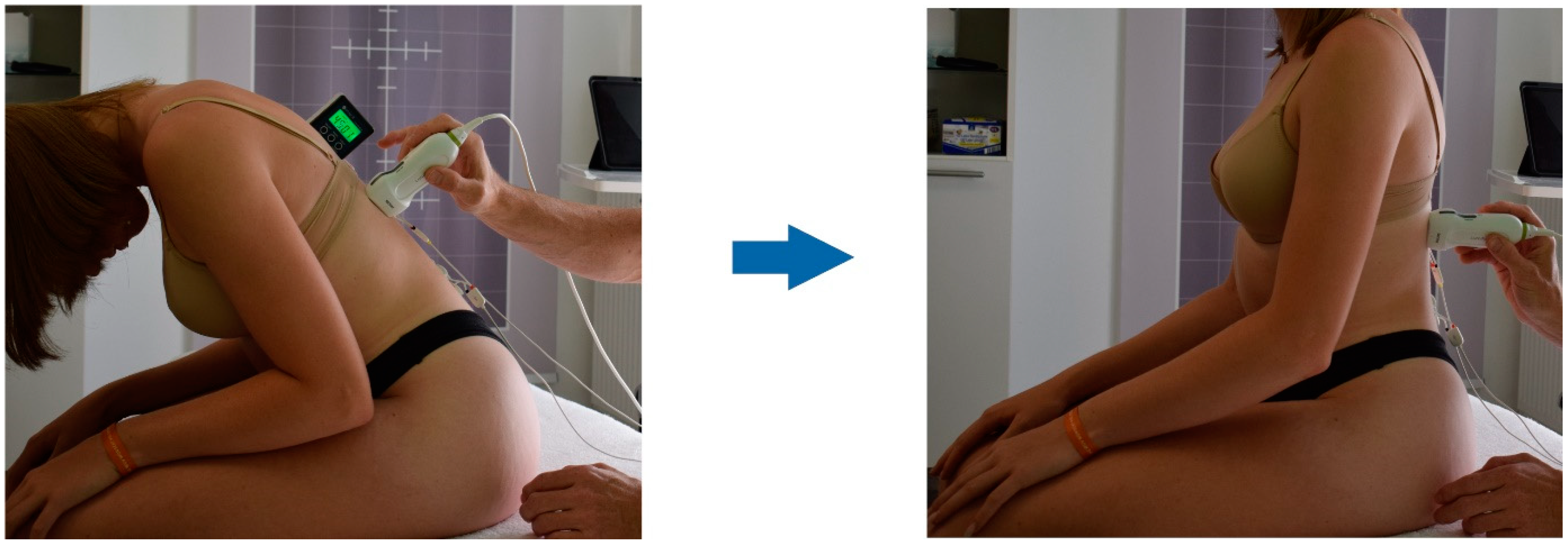
2.3. Procedure
2.4. Outcomes
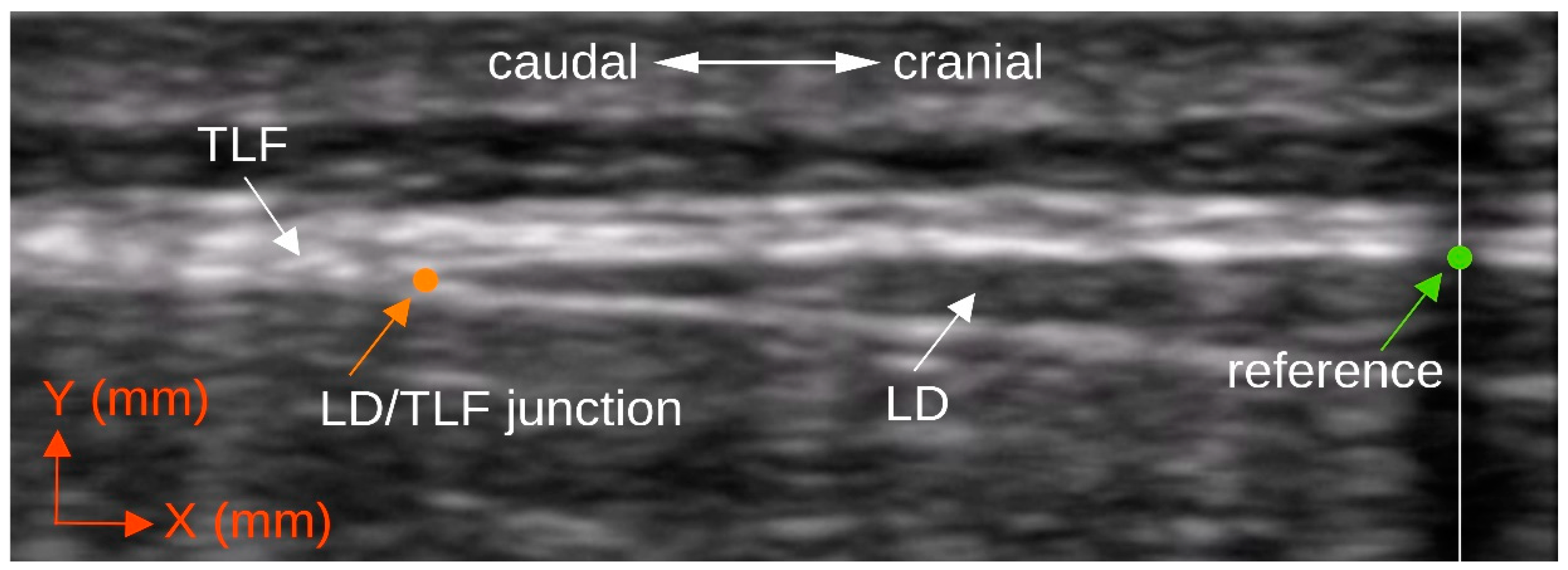
2.4.1. Ultrasound Measurement of the Deformation of the Thoracolumbar Fascia
2.4.2. Surface Electromyography of the Erector Spinae and Multifidus Muscle
2.4.3. Data Synchronization
2.4.4. Cross Correlation Analysis of the Measurement Series
2.5. Statistics
3. Results
4. Discussion
Limitations
- Mechanical forces to the muscle spindles during a dynamic task, triggering a stretch reflex.
- Muscle spindles are blocked by adhesions, and their function is disturbed, which is why they are unable to smooth muscle contraction.
- An epimysium incapable of shearing against the TLF cannot provide a proprioceptive function to unify muscle spindle tensions.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Levy, V.J.; Holt, C.T.; Haskins, A.E. Osteopathic Manipulative Medicine Consultations for Hospitalized Patients. J. Am. Osteopath Assoc. 2019, 119, 299–306. [Google Scholar] [CrossRef]
- van Tulder, M.; Becker, A.; Bekkering, T.; Breen, A.; Gil del Real, M.T.; Hutchinson, A.; Koes, B.; Laerum, E.; Malmivaara, A. European guidelines for the management of acute nonspecific low back pain in primary care. Eur. Spine J. 2006, 15, s169–s191. [Google Scholar] [CrossRef] [PubMed]
- Casser, H.-R.; Seddigh, S.; Rauschmann, M. Acute Lumbar Back Pain: Investigation, Differential Diagnosis, and Treatment. Dtsch. Arztebl. Int. 2016, 113, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Parreira, P.; Maher, C.G.; Steffens, D.; Hancock, M.J.; Ferreira, M.L. Risk factors for low back pain and sciatica: An umbrella review. Spine J. 2018, 18, 1715–1721. [Google Scholar] [CrossRef] [PubMed]
- Lampert, T.; Prütz, F.; Seeling, S.; Starker, A.; Kroll, L.E.; Rommel, A.; Ryl, L.; Ziese, T. Gesundheit in Deutschland: Gesundheitsberichterstattung Des Bundes, Gemeinsam Getragen von RKI Und Destatis; Robert Koch-Institut: Berlin, Germany, 2015. [Google Scholar]
- Andersson, G.B. Epidemiological features of chronic low-back pain. Lancet 1999, 354, 581–585. [Google Scholar] [CrossRef]
- Dagenais, S.; Caro, J.; Haldeman, S. A systematic review of low back pain cost of illness studies in the United States and internationally. Spine J. 2008, 8, 8–20. [Google Scholar] [CrossRef]
- Wong, A.Y.; Karppinen, J.; Samartzis, D. Low back pain in older adults: Risk factors, management options and future directions. Scoliosis Spinal Disord. 2017, 12, 14. [Google Scholar] [CrossRef]
- Casato, G.; Stecco, C.; Busin, R. Role of fasciae in nonspecific low back pain. Eur. J. Transl. Myol. 2019, 29, 8330. [Google Scholar] [CrossRef]
- Steenstra, I.A.; Verbeek, J.H.; Heymans, M.; Bongers, P.M. Prognostic factors for duration of sick leave in patients sick listed with acute low back pain: A systematic review of the literature. Occup. Environ. Med. 2005, 62, 851–860. [Google Scholar] [CrossRef]
- Langevin, H.M.; Fox, J.R.; Koptiuch, C.; Badger, G.J.; Greenan-Naumann, A.C.; Bouffard, N.A.; Konofagou, E.E.; Lee, W.-N.; Triano, J.J.; Henry, S.M. Reduced thoracolumbar fascia shear strain in human chronic low back pain. BMC Musculoskelet. Disord. 2011, 12, 203. [Google Scholar] [CrossRef]
- Panjabi, M.M. A hypothesis of chronic back pain: Ligament subfailure injuries lead to muscle control dysfunction. Eur. Spine J. 2005, 15, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Schleip, R.; Vleeming, A.; Lehmann-Horn, F.; Klingler, W. Letter to the Editor concerning “A hypothesis of chronic back pain: Ligament subfailure injuries lead to muscle control dysfunction” (M. Panjabi). Eur. Spine J. 2007, 16, 1733–1735. [Google Scholar] [CrossRef] [PubMed]
- Mense, S. Innervation of the thoracolumbar fascia. Eur. J. Transl. Myol. 2019, 29, 8297. [Google Scholar] [CrossRef] [PubMed]
- Wilke, J.; Schleip, R.; Klingler, W.; Stecco, C. The Lumbodorsal Fascia as a Potential Source of Low Back Pain: A Narrative Review. BioMed Res. Int. 2017, 2017, 5349620. [Google Scholar] [CrossRef]
- Hodges, P.W.; Danneels, L. Changes in Structure and Function of the Back Muscles in Low Back Pain: Different Time Points, Observations, and Mechanisms. J. Orthop. Sports Phys. Ther. 2019, 49, 464–476. [Google Scholar] [CrossRef]
- Koppenhaver, S.L.; Gaffney, E.; Oates, A.; Eberle, L.; Young, B.; Hebert, J.; Proulx, L.; Shinohara, M. Lumbar muscle stiffness is different in individuals with low back pain than asymptomatic controls and is associated with pain and disability, but not common physical examination findings. Musculoskelet. Sci. Pract. 2019, 45, 102078. [Google Scholar] [CrossRef]
- Tong, M.H.; Mousavi, S.J.; Kiers, H.; Ferreira, P.; Refshauge, K.; van Dieën, J. Is There a Relationship between Lumbar Proprioception and Low Back Pain? A Systematic Review with Meta-Analysis. Arch. Phys. Med. Rehabil. 2017, 98, 120–136. [Google Scholar] [CrossRef]
- Strutton, P.H.; Theodorou, S.; Catley, M.; McGregor, A.H.; Davey, N.J. Corticospinal Excitability in Patients with Chronic Low Back Pain. J. Spinal Disord. Tech. 2005, 18, 420–424. [Google Scholar] [CrossRef]
- Arvanitidis, M.; Bikinis, N.; Petrakis, S.; Gkioka, A.; Tsimpolis, D.; Falla, D.; Martinez-Valdes, E. Spatial distribution of lumbar erector spinae muscle activity in individuals with and without chronic low back pain during a dynamic isokinetic fatiguing task. Clin. Biomech. 2020, 81, 105214. [Google Scholar] [CrossRef]
- Hao, Z.; Xie, L.; Wang, J.; Hou, Z. Spatial Distribution and Asymmetry of Surface Electromyography on Lumbar Muscles of Soldiers with Chronic Low Back Pain. Pain Res. Manag. 2020, 2020, 1–8. [Google Scholar] [CrossRef]
- Ferreira, P.H.; Ferreira, M.L.; Nascimento, D.P.; Pinto, R.Z.; Franco, M.R.; Hodges, P.W. Discriminative and reliability analyses of ultrasound measurement of abdominal muscles recruitment. Man. Ther. 2011, 16, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.-Y.; Choi, J.-D.; Kim, S.-Y.; Oh, D.-W.; Kim, J.-K.; Park, J.-W. Comparison between muscle activation measured by electromyography and muscle thickness measured using ultrasonography for effective muscle assessment. J. Electromyogr. Kinesiol. 2014, 24, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Hodges, P.W.; Pengel, L.H.M.; Herbert, R.D.; Gandevia, S.C. Measurement of muscle contraction with ultrasound imaging. Muscle Nerve 2003, 27, 682–692. [Google Scholar] [CrossRef]
- Nakai, Y.; Kawada, M.; Miyazaki, T.; Kiyama, R. Trunk muscle activity during trunk stabilizing exercise with isometric hip rotation using electromyography and ultrasound. J. Electromyogr. Kinesiol. 2019, 49, 102357. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, J.L.; McLean, L.; Hodder, J.; Warner, M.B.; Stokes, M.J. Association Between Changes in Electromyographic Signal Amplitude and Abdominal Muscle Thickness in Individuals With and Without Lumbopelvic Pain. J. Orthop. Sports Phys. Ther. 2013, 43, 466–477. [Google Scholar] [CrossRef]
- Wilke, J.; Debelle, H.; Tenberg, S.; Dilley, A.; Maganaris, C. Ankle Motion Is Associated With Soft Tissue Displacement in the Dorsal Thigh: An in vivo Investigation Suggesting Myofascial Force Transmission Across the Knee Joint. Front. Physiol. 2020, 11, 180. [Google Scholar] [CrossRef]
- Thibault-Gagnon, S.; Auchincloss, C.; Graham, R.; McLean, L. The temporal relationship between activity of the pelvic floor muscles and motion of selected urogenital landmarks in healthy nulliparous women. J. Electromyogr. Kinesiol. 2018, 38, 126–135. [Google Scholar] [CrossRef]
- Dean, R.T.; Dunsmuir, W.T.M. Dangers and uses of Cross-Correlation in analyzing time series in perception, performance, movement, and neuroscience: The importance of constructing transfer function autoregressive models. Behav. Res. Methods 2015, 48, 783–802. [Google Scholar] [CrossRef]
- Wren, T.A.L.; Do, K.P.; Rethlefsen, S.A.; Healy, B. Cross-correlation as a method for comparing dynamic electromyography signals during gait. J. Biomech. 2006, 39, 2714–2718. [Google Scholar] [CrossRef]
- Brandl, A.; Egner, C.; Schleip, R. Immediate Effects of Myofascial Release on the Thoracolumbar Fascia and Osteopathic Treatment for Acute Low Back Pain on Spine Shape Parameters: A Randomized, Placebo-Controlled Trial. Life 2021, 11, 845. [Google Scholar] [CrossRef]
- Stecco, A.; Gesi, M.; Stecco, C.; Stern, R. Fascial Components of the Myofascial Pain Syndrome. Curr. Pain Headache Rep. 2013, 17, 352. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. Ann. Intern. Med. 2007, 147, 573–577. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Gaul, C.; Mette, E.; Schmidt, T.; Grond, S. ODQ—Oswestry Low Back Pain Disability Questionnaire-deutsche Fassung. BioMed Res. Int. 2008, 2008, 51–58. [Google Scholar] [CrossRef]
- Norkin, C.C.; White, D.J. Measurement of Joint Motion: A Guide to Goniometry; FA Davis: Philadelphia, PA, USA, 2016. [Google Scholar]
- Watanabe, K.; Miyamoto, K.; Masuda, T.; Shimizu, K. Use of Ultrasonography to Evaluate Thickness of the Erector Spinae Muscle in Maximum Flexion and Extension of the Lumbar Spine. Spine 2004, 29, 1472–1477. [Google Scholar] [CrossRef]
- Wong, K.-K.; Chai, H.-M.; Chen, Y.-J.; Wang, C.-L.; Shau, Y.-W.; Wang, S.-F. Mechanical deformation of posterior thoracolumbar fascia after myofascial release in healthy men: A study of dynamic ultrasound imaging. Musculoskelet. Sci. Pract. 2017, 27, 124–130. [Google Scholar] [CrossRef]
- Jhu, J.-L.; Chai, H.-M.; Jan, M.-H.; Wang, C.-L.; Shau, Y.-W.; Wang, S.-F. Reliability and Relationship Between 2 Measurements of Transversus Abdominis Dimension Taken During an Abdominal Drawing-in Maneuver Using a Novel Approach of Ultrasound Imaging. J. Orthop. Sports Phys. Ther. 2010, 40, 826–832. [Google Scholar] [CrossRef]
- Mohr, L.; Vogt, L.; Wilke, J. Use of Reflective Tape to Detect Ultrasound Transducer Movement: A Validation Study. Life 2021, 11, 104. [Google Scholar] [CrossRef]
- Willard, F.H.; Vleeming, A.; Schuenke, M.D.; Danneels, L.; Schleip, R. The thoracolumbar fascia: Anatomy, function and clinical considerations. J. Anat. 2012, 221, 507–536. [Google Scholar] [CrossRef]
- Stecco, C. Functional Atlas of the Human Fascial System, 1st ed.; Churchill Livingstone: Edinburgh, Scotland, 2014; ISBN 978-0-7020-4430-4. [Google Scholar]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of recommendations for SEMG sensors and sensor placement procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef]
- Hofste, A.; Soer, R.; Salomons, E.; Peuscher, J.; Wolff, A.; Van Der Hoeven, H.; Oosterveld, F.; Groen, G.; Hermens, H. Intramuscular EMG Versus Surface EMG of Lumbar Multifidus and Erector Spinae in Healthy Participants. Spine 2020, 45, E1319–E1325. [Google Scholar] [CrossRef]
- Cohen, J. A Power Primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef]
- Ho, J.; Tumkaya, T.; Aryal, S.; Choi, H.; Claridge-Chang, A. Moving beyond P values: Data analysis with estimation graphics. Nat. Methods 2019, 16, 565–566. [Google Scholar] [CrossRef]
- Giuriati, W.; Ravara, B.; Porzionato, A.; Albertin, G.; Stecco, C.; Macchi, V.; De Caro, R.; Martinello, T.; Gomiero, C.; Patruno, M.; et al. Muscle spindles of the rat sternomastoid muscle. Eur. J. Transl. Myol. 2018, 28, 7904. [Google Scholar] [CrossRef]
- Fede, C.; Petrelli, L.; Guidolin, D.; Porzionato, A.; Pirri, C.; Fan, C.; De Caro, R.; Stecco, C. Evidence of a new hidden neural network into deep fasciae. Sci. Rep. 2021, 11, 12623. [Google Scholar] [CrossRef]
- Liu, L.; Huang, Q.-M.; Liu, Q.-G.; Nguyen, T.-T.; Yan, J.-Q.; Bo, C.-Z. Relationship between muscle spindles and myofascial trigger spots according to Hoffmann reflex pathway and tissue morphology characteristics in a rat model. Acupunct. Med. 2020, 38, 109–116. [Google Scholar] [CrossRef]
- Amonoo-Kuofi, H.S. The density of muscle spindles in the medial, intermediate and lateral columns of human intrinsic postvertebral muscles. J. Anat. 1983, 136, 509–519. [Google Scholar]
- Shirzadfar, H. The Structure and Function of Nervous System and Skeletal Muscle: A Review. Curr. Neuropsychiatry Clin. Neurosci. Rep. 2021, 3, 1–25. [Google Scholar]
- Stokes, I.A.F.; Henry, S.M.; Single, R.M. Surface EMG electrodes do not accurately record from lumbar multifidus muscles. Clin. Biomech. 2003, 18, 9–13. [Google Scholar] [CrossRef]
- Bandpei, M.A.M.; Rahmani, N.; Majdoleslam, B.; Abdollahi, I.; Ali, S.S.; Ahmad, A. Reliability of Surface Electromyography in the Assessment of Paraspinal Muscle Fatigue: An Updated Systematic Review. J. Manip. Physiol. Ther. 2014, 37, 510–521. [Google Scholar] [CrossRef]
- Partanen, J.V.; Vanhanen, J.; Liljander, S.K. Electromyography of the muscle spindle. Sci. Rep. 2022, 12, 4220. [Google Scholar] [CrossRef]





| Baseline Characteristics | LBP Group (n = 10) Mean ± SD | Controls (n = 10) Mean ± SD | p Value |
|---|---|---|---|
| Sex (men/women) | 4/6 | 4/6 | |
| Age (years) | 43.6 ± 15.9 | 39.0 ± 15.0 | 0.38 a |
| 95% CI | 32.2–54.9 | 28.2–49.7 | |
| Min–Max | 18.5–59.6 | 18.–58.0 | |
| Height (m) | 1.72 ± 0.08 | 1.67 ± 0.12 | 0.30 |
| 95% CI | 1.67–1.78 | 1.59–1.76 | |
| Min–Max | 1.61–1.83 | 1.51–1.83 | |
| Weight (kg) | 74.5 ± 12.9 | 66.0 ± 15.6 | 0.16 |
| 95% CI | 66.3–84.7 | 54.9–77.2 | |
| Min–Max | 57.0–98.0 | 48.0–99.0 | |
| BMI (kg/m2) | 25.6 ± 4.5 | 23.5 ± 4.6 | 0.24 a |
| 95% CI | 22.3–28.8 | 20.3–26.7 | |
| Min–Max | 19.6–33.5 | 19.5–34.3 | |
| ODQ-D (0–100) | 49.4 ± 16.8 | ||
| 95% CI | 37.4–61.4 | ||
| Min–Max | 32.0–78.0 | ||
| VAS (0–10) | 5.3 ± 2.5 | ||
| 95% CI | 3.5–7.0 | ||
| Min–Max | 2.6–10.0 | ||
| Pain duration (days) | 9.1 ± 3.7 | ||
| 95% CI | 6.4–11.8 | ||
| Min–Max | 3.0–14.0 |
| Student’s t-Test | |||||
|---|---|---|---|---|---|
| Outcome | Mean ± SD | 95% CI | t | p | d |
| TLF deformation (%) | 27.5 ± 31.1 | 5.3–49.8 | 2.80 | 0.01 * | 0.88 |
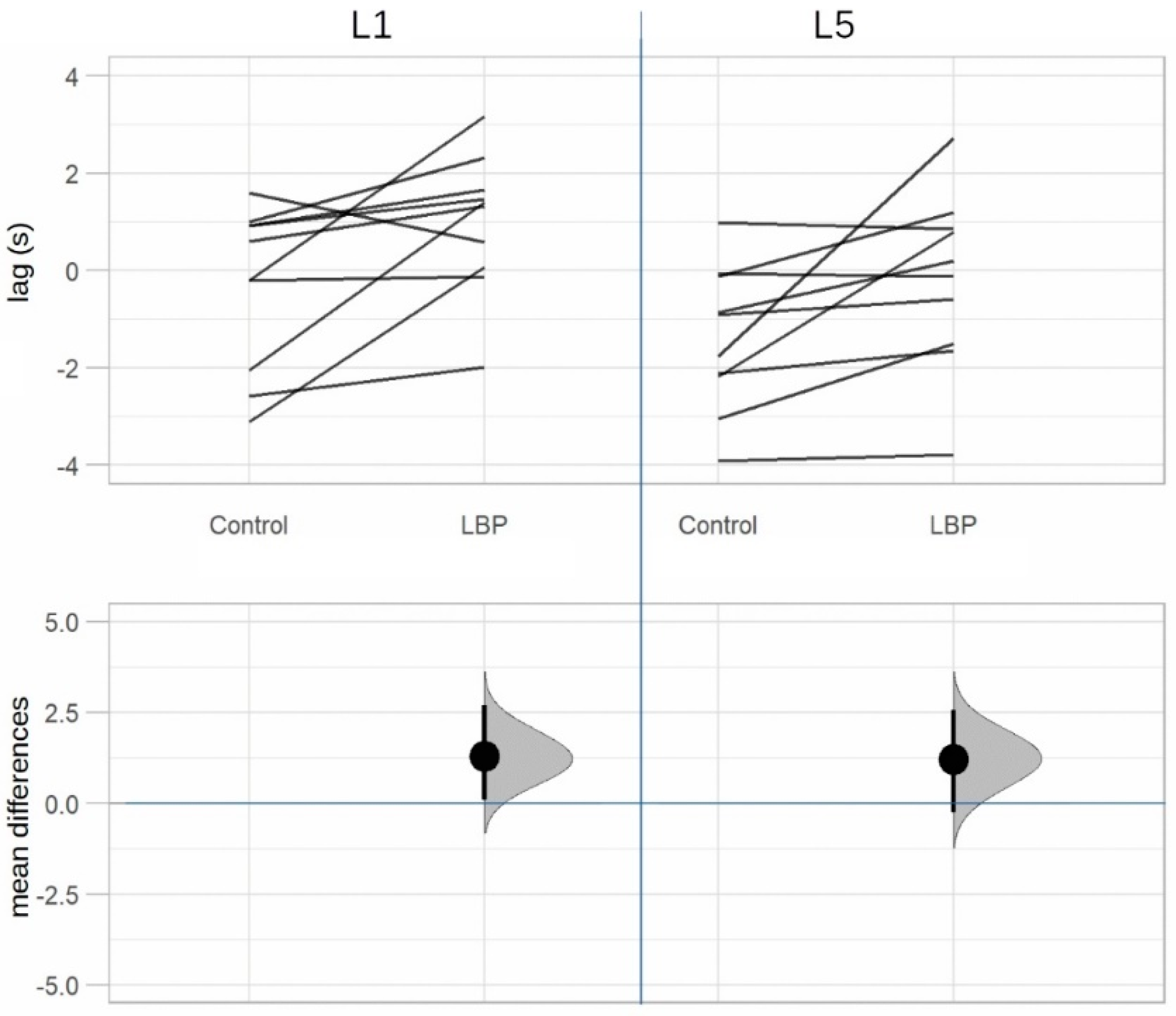
| L1 time lag (s) | 1.30 ± 1.53 | 0.21–2.39 | 2.69 | 0.02 * (0.04 **) | 0.85 |
| L5 time lag (s) | 1.21 ± 1.49 | 0.15–2.28 | 2.57 | 0.03 * (0.04 **) | 0.81 |
| L1 TLF/sEMG (%) | 30.6 ± 44.6 | −1.2–62.4 | 2.17 | 0.03 * (0.03 **) | 0.69 |
| L5 TLF/sEMG (%) | 34.6 ± 40.6 | 5.5–63.6 | 2.69 | 0.01 * (0.02 **) | 0.85 |
| L1 sEMG/TLF (%) | 2.8 ± 45.8 | −30.0–35.5 | 0.19 | 0.85 | 0.06 |
| L5 sEMG/TLF (%) | 5.6 ± 43.3 | −25.3–36.6 | 0.41 | 0.69 | 0.13 |
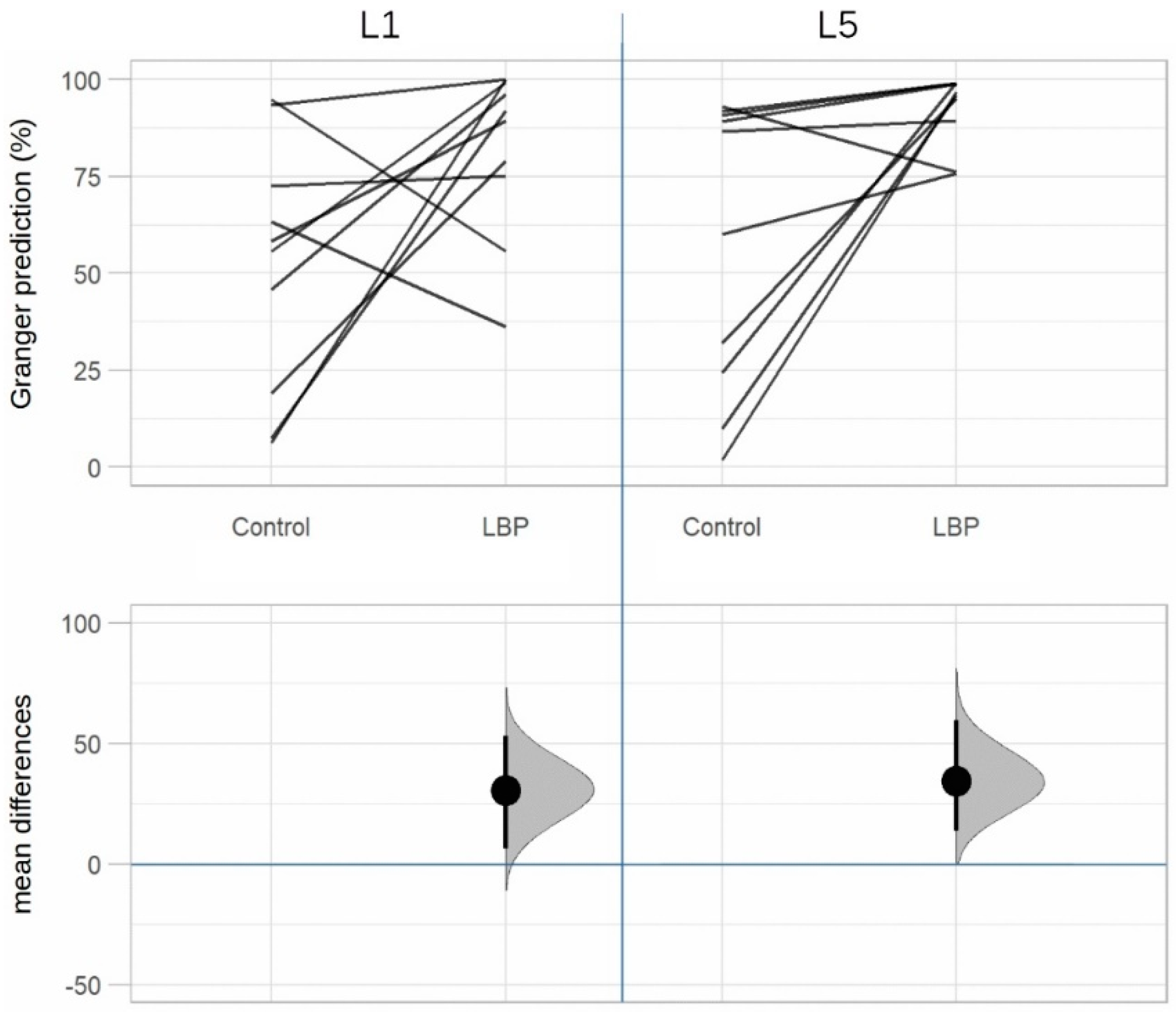
| Granger Prediction | |||||
|---|---|---|---|---|---|
| L1 | L5 | ||||
| Direction | Group | True | False | True | False |
| TLF/sEMG | LBP | 82.3% | 17.7% | 92.5% | 7.5% |
| Control | 51.7% | 48.3% | 58.0% | 42.0% | |
| sEMG/TLF | LBP | 61.0% | 39.0% | 52.3% | 47.7% |
| Control | 58.2% | 41.8% | 46.7% | 53.3% | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brandl, A.; Egner, C.; Reer, R.; Schmidt, T.; Schleip, R. Associations between Deformation of the Thoracolumbar Fascia and Activation of the Erector Spinae and Multifidus Muscle in Patients with Acute Low Back Pain and Healthy Controls: A Matched Pair Case-Control Study. Life 2022, 12, 1735. https://doi.org/10.3390/life12111735
Brandl A, Egner C, Reer R, Schmidt T, Schleip R. Associations between Deformation of the Thoracolumbar Fascia and Activation of the Erector Spinae and Multifidus Muscle in Patients with Acute Low Back Pain and Healthy Controls: A Matched Pair Case-Control Study. Life. 2022; 12(11):1735. https://doi.org/10.3390/life12111735
Chicago/Turabian StyleBrandl, Andreas, Christoph Egner, Rüdiger Reer, Tobias Schmidt, and Robert Schleip. 2022. "Associations between Deformation of the Thoracolumbar Fascia and Activation of the Erector Spinae and Multifidus Muscle in Patients with Acute Low Back Pain and Healthy Controls: A Matched Pair Case-Control Study" Life 12, no. 11: 1735. https://doi.org/10.3390/life12111735
APA StyleBrandl, A., Egner, C., Reer, R., Schmidt, T., & Schleip, R. (2022). Associations between Deformation of the Thoracolumbar Fascia and Activation of the Erector Spinae and Multifidus Muscle in Patients with Acute Low Back Pain and Healthy Controls: A Matched Pair Case-Control Study. Life, 12(11), 1735. https://doi.org/10.3390/life12111735







