Influence of Effective Microorganisms on Some Biological and Biochemical Aspects of Spodoptera littoralis (Boisduval) (Lepidoptera: Noctuidae)
Abstract
1. Introduction
2. Materials and Methods
2.1. Rearing Technique of S. littoralis
2.2. The Tested Agent
2.3. The EMs Preparation
2.4. Antifeedant Technique
2.5. Biological Aspects
2.6. Enzymes Assay
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martinez, S.S.; van Emden, H.F. Growth disruption, abnormalities and mortality of Spodoptera littoralis (Boisduval) (Lepidoptera: Noctuidae) caused by Azadirachtin. Neotrop. Entomol. 2001, 30, 113–125. [Google Scholar] [CrossRef]
- Dahi Hassan, F.; Abdel-rahman, A.R.G.; El-Bamby, M.M.; Gamil, W.E.; Rasheed, D.S. Insecticides application and the Egyptian cotton leafworm, Spodoptera littoralis (Boisd.) permanent larvae. Egypt. Acad. J. Biol. Sci. 2017, 10, 311–322. [Google Scholar]
- Tabashnik, B.E.; Mota-sanchez, D.; Whalon, M.E.; Hollingworth, R.M.; Carriere, Y. Defining terms for proactive management of resistance to Bt crops and pesticides. J. Econ. Entomol. 2014, 107, 496–507. [Google Scholar] [CrossRef]
- El-Naggar, J.B.; Jehan, B.A. Sublethal effect of certain insecticides on biological and physiological aspects of Spodoptera littoralis (Boisd.). Nat. Sci. 2013, 11, 19–25. [Google Scholar]
- Zayed, M.S.; Taha, E.-K.A.; Hassan, M.M.; Elnabawy, E.-S.M. Enhance systemic resistance significantly reduces the silverleaf whitefly population and increases the yield of sweet pepper, Capsicum annuum L. var. annuum. Sustainability 2022, 14, 6583. [Google Scholar] [CrossRef]
- Elnabawy, E.-S.M.; Hassan, S.; Taha, E.-K.A. Repellent and toxicant effects of eight essential oils against the red flour beetle, Tribolium castaneum Herbst (Coleoptera: Tenebrionidae). Biology 2022, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Nasr, G.M.; Taha, E.-K.A.; Hamza, A.M.; Negm, E.A.; Eryan, N.L.; Noureldeen, A.; Darwish, H.; Zayed, M.S.; Elnabawy, E.-S.M. Gamma radiation: An eco-friendly control method for the rice weevil, Sitophilus oryzae (L.) (Coleoptera: Curculionidae). Biology 2022, 11, 1295. [Google Scholar] [CrossRef] [PubMed]
- El-Nabawy, E.M.; Tsuda, K.; Sakamaki, Y. Attractiveness of spiders and insect predators and parasitoids to flowering plants. Egypt. J. Biol. Pest Control 2015, 25, 245–250. [Google Scholar]
- El-Nabawy, E.M.; Tsuda, K.; Sakamaki, Y.; Oda, A.; Ushijima, Y. The effect of organic fertilizers and flowering plants on sheet-web and wolf spider populations (Araneae: Lycosidae and Linyphiidae) and its importance for pest control. J. Insect Sci. 2016, 16, 18. [Google Scholar] [CrossRef] [PubMed]
- Elnabawy, E.M.; Tsuda, K.; Sakamaki, Y.M. September. Enhancement of web-builder spider populations in eggplant fields by surrounding flowering plants. In 2016 International Congress of Entomology; ESA: Orlando, FL, USA, 2016. [Google Scholar]
- Shawer, M.B.; Sharshir, F.A.; Taha, E.-K.A.; Shenishen, E.Z.; Hassan, M.M.; Elnabawy, E.M. The impact of cold storage durations on Trichogramma evanescens (Westwood) (Hymenoptera: Trichogrammatidae) during their pupal stage. Saudi J. Biol. Sci. 2021, 28, 7202–7206. [Google Scholar] [CrossRef]
- Taha, E.-K.A.; Shawer, M.B.; Sharshir, F.A.; Shenishen, E.Z.; Hassan, M.M.; Elshazly, H.; Elnabawy, E.M. Effect of emergence time on some biological aspects of Trichogramma evanescens (Westwood) (Hymenoptera: Trichogrammatidae). J. King Saudi Univ. Sci. 2022, 34, 101981. [Google Scholar] [CrossRef]
- Copping, L.G. The Manual of Biocontrol Agents, 4th ed.; British Crop Production Council: Surrey, UK, 2009; p. 851. [Google Scholar]
- Sarwar, M. The inhibitory properties of organic pest control agents against aphid (Aphididae: Homoptera) on canola Brassica napus L. (Brassicaceae) under field environment. Int. J. Sci. Res. Environ. Sci. 2013, 1, 195–201. [Google Scholar] [CrossRef]
- Sarwar, M. Commonly available commercial insecticide formulations and their applications in the field. Int. J. Mater. Chem. Phys. 2015, 1, 116–123. [Google Scholar]
- Sarwar, M. The Dangers of pesticides associated with public health and preventing of the risks. Int. J. Bioinform. Biomed. Eng. 2015, 1, 130–136. [Google Scholar]
- Farag, A.M. Biochemical Studies on the Effect of Some Insect Growth Regulators on the Cotton Leafworm. Unpublished. Master’s Thesis, Faculty of Agriculture Cairo University, Giza, Egypt, 2001. [Google Scholar]
- Abdel-Aal, A.E. Effect of Some Insect Growth Regulators on Certain Biological, Biochemical and Histopathological Aspects of the Cotton Leafworm, Spodoptera littoralis (Boisd.). Unpublished. Ph.D. Thesis, Faculty of Agriculture Cairo University, Giza, Egypt, 2003; p. 119. [Google Scholar]
- Seth, R.K.; Kaur, J.J.; Rad, D.K.; Reynolds, S.E. Effect of larval exposure to sublethal concentrations of the ecdysteroid agonists RH-5849 and Tebufenozide (RH-5992) on male reproductive physiology in Spodoptera litura. J. Insect Physiol. 2004, 50, 505–517. [Google Scholar] [CrossRef] [PubMed]
- White, J.; Johnson, D. Vendors of Microbial and Botanical Insecticides and Insect Monitoring Devices; UK Cooperative Extention Service, University of Kentucky College of Agriculture: Lexington, KY, USA, 2012. [Google Scholar]
- Osman, H.H.; Abdle-Hafez, H.F.; Khidr, H.H. Comparison between the efficacy of two nano-particles and effective microorganisms on some biological and biochemical aspects of Spodoptera littorals. Inter. J. Agric. Innov. Res. 2015, 3, 1620–1626. [Google Scholar]
- Li, S.; Shen, Y.; Xie, A.; Yu, X.; Qui, L.; Zang, L.; Zang, Q. Green synthesis of silver nanoparticles using Capsicum annum L. extract. Green Chem. 2007, 9, 852–858. [Google Scholar] [CrossRef]
- Tang, J.; Niu, X.; Sun, Q.; Wang, R. Bioremediation of petroleum polluted soil by combination of rye grass with effective microorganisms. J. Environ. Technol. Eng. 2010, 3, 80–86. [Google Scholar]
- Bajwa, R. Effects of arbuscular mycorrhizae (AM) and effective microorganisms (EM) on various plants under allelopathic stress. Allelopath. J. 2005, 16, 261–271. [Google Scholar]
- Chrispaul, M.; David, M.M.; Joseph, A.O.; Samuel, V. Effective microorganisms and their influence on growth and yield of pigweed (Amaranthus dubians). ARPN J. Agric. Biol. Sci. 2010, 5, 17–22. [Google Scholar]
- Sekaran, V.; Rajagopal, K.; Karutha Pandian, S.T. Cost-effective activated sludge process using effective microorganisms (EM). Environ. Health 2007, 7, 71–83. [Google Scholar]
- Ndona, R. Einfluss von Behandlungen Mit EMs. Effektiven Mikroorganismen auf Tomato in Geschützten Anbau. Ph.D. Thesis, Universität für Bodenkultur, Vienna, Austria, 2008. [Google Scholar]
- Filipp, M.; Spornberger, A.; Keppel, H.; Brunmayer, R. Influence of effective microorganisms (EM) on yield and quality in organic apple production. Mitteilungen Klosterneuburg, Rebe und Wein, Obstbau und Früchteverwertung 2009, 59, 250–258. [Google Scholar]
- Perez-Montano, F.; Alias-Villegas, C.; Bellogin, R.A.; Del Cerro, P.; Espuny, M.R.; Jimenez-Guerrero, I.; Lopez-Baena, F.J.; Ollero, F.J.; CUBO, T. Plant growth promotion in cereal and leguminous agricultural important plants: From microorganism capacities to crop production. Microb. Res. 2014, 169, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Derbalah, A.S.; Khidr, A.A.; Moustafa, H.Z.; Taman, A. Laboratory evaluation of some non-conventional pest control agents against the pink bollworm Pectinophora gossypiella (Saunders). Egypt. J. Biol. Pest Control 2014, 24, 363. [Google Scholar]
- Keratum, A.Y.; Hamza, A.M.; El–Bassuoini, S.A.; Zayed, M.S. Evaluation of non-traditional agents against the spiny bollworm Earias insulana under laboratory conditions with safety concerns. Egypt. J. Plant Pro. Res. 2016, 4, 82–98. [Google Scholar]
- Zayed, M.S. Evaluation of novel nanoparticles against Tetranychus urticae and its predatory mite Amblyseius gossipi. Zagazig J. Agric. Res. 2020, 47, 951–961. [Google Scholar] [CrossRef]
- Golec, A.F.C.; Pérez, P.G.; Lokare, C. Effective microorganisms: Myth or reality? Rev. Peru. De Biol. 2007, 14, 315–319. [Google Scholar]
- Olle, M.; Williams, I.H. Effective microorganisms and their influence on vegetable production–a review. J. Hortic. Sci. Biotechnol. 2013, 88, 380–386. [Google Scholar] [CrossRef]
- Siegel, M.R.; Latch, G.C.M.; Bush, L.P.; Fanin, F.F.; Rowan, D.D.; Tapper, B.A.; Bacon, C.W.; Johnson, M.C. Fungal endophyte-infected grasses: Alkaloid accumulation and aphid response. J. Chem. Ecol. 1990, 16, 3301–3316. [Google Scholar] [CrossRef]
- Abdullah, M.M.A.B.; Ma’Radzi, A.H.; Saleh, N.A.M.; Kamal, A.Z.; Yaacob, N.D. Production of effective microorganism using halalbased sources: A review. Afr. J. Biotechnol. 2011, 10, 18649–18652. [Google Scholar]
- Fincheira, P.; Quiroz, A. Microbial volatiles as plant growth inducers. Microb. Res. 2018, 2, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Moon, Y.H.; Lee, K.B.; Kim, Y.J.; Koo, Y.M. Current status of EM (effective microorganisms) utilization. KSBB J. 2011, 26, 365–373. [Google Scholar] [CrossRef][Green Version]
- El-Defrawi, M.E.; Toppozada, A.; Mansoue, N.; Zeid, M. Toxicological studies on Egyptian cotton leafworm Prodenia litura (F.). Susceptibility of different larval instars to insecticides. J. Econ. Entomol. 1964, 57, 591–593. [Google Scholar] [CrossRef]
- Pushpa, T.B.; Sekaran, V.; Bash, S.J.; Jegan, J. Investigation on preparation, characterization, and application of effective microorganisms (em) based composts—An eco-friendly solution. Nat. Environ. Pollut. Technol. 2016, 15, 153–158. [Google Scholar]
- El-Malla, M.A.; Radwan, E.M.M. Residual toxicity of abamectin and spinosad insecticides on the cotton leafworm, Spodoptera littoralis (Boisd.). Bull. Ent. Soc. Egypt. Econ. Ser. 2008, 34, 119–129. [Google Scholar]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Abivardi, C.; Benz, G. Tests with the extracts of 21 medicinal plants for antifeedant activity against larvae of Pieris brassicae L. (Lep., Pieridae). Bull. Soc. Entomol. Swisse 1984, 57, 383–392. [Google Scholar]
- Waldbauer, G.P. The consumption and utilization of food in insects. Insect Physiol. 1968, 5, 229–288. [Google Scholar]
- Senthil-Nathan, S.; Kalaivani, K. Efficacy of nucleopolyhedrovirus (NPV) and azadirachtin on Spodoptera litura Fabricius (Lepidoptera: Noctuidae). Biol. Control 2005, 43, 93–98. [Google Scholar] [CrossRef]
- Mohamed, H.A. Integrated Mite Management. Master’s Thesis, Faculty of Agriculture Kafrelsheikh University, Kafr el-Sheikh, Egypt, 2006. [Google Scholar]
- El-doksh, R.A. Toxicological Studies on Some Agricultural Pests. Ph.D. Thesis, Faculty of Agriculture Kafrelsheikh University, Tanta, Egypt, 2001. [Google Scholar]
- Ishaaya, I.; Swirski, E. Trehalase, Invertase and amylase activities in the black scale, Saissetia oleae and their relation to host adaptability. J. Insect Physiol. 1976, 22, 1025–1029. [Google Scholar] [CrossRef]
- Henry, R.J. Colorimetric Method of Total Proteins; Clinical Chemistry Harper Row Pub: New York, NY, USA, 1964; p. 181. [Google Scholar]
- Schmit, J.M. Kits for Determination of Serum Total Lipids Concentration. Ph.D. Thesis, Lyon University, Lyon, France, 1964. [Google Scholar]
- Costat. Costat Statistical Software: Micro Computer Program Analysis Version 4.20; Cohort Software: Berkeley, CA, USA, 2006. [Google Scholar]
- Ebeid, A.R.; Geseaha, M.A. Impact of three commercial insecticides on some biological aspects of the cotton leafworm, Spodoptera littoralis (Lepidoptera: Noctuidae). J. Appl. Sci. Res. 2012, 8, 2620–2625. [Google Scholar]
- Isman, M.B. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annual. Rev. Entomol. 2006, 51, 45–66. [Google Scholar] [CrossRef]
- Monnerat, R.G.; Soares, C.M.; Capdeville, G.; Jones, G.; Martins, E.S.; Praca, L.; Cordeiro, B.A.; Braz, S.V.; Dos Santos, R.C.; Berry, C. Translocation and insecticidal activity of Bacillus thuringiensis living inside of plants. Micro Biotechnol. 2009, 2, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Sidorova, D.E.; Plyuta, V.A.; Padiy, D.A.; Kupriyanova, E.V.; Roshina, N.V.; Koksharova, O.A.; Khmel, I.A. The effect of volatile organic compounds on different organisms: Agrobacteria, plants, and insects. Microorganisms 2022, 10, 69. [Google Scholar] [CrossRef] [PubMed]
- Yasui, H.; Kato, A.; Yazawa, M. Antifeedants to armyworm, Spodoptera litura and Pseudaletia separate, from bitter gourd leaves, Momordica charantia. J. Chem. Ecol. 1998, 24, 803–813. [Google Scholar] [CrossRef]
- Tolba, H.I. Biochemical studies on Serratia marcescens for controlling the black cutworm, Agrotis ipsilon (Huf.). Ph.D. Thesis, Faculty of Agriculture, Cairo University, Giza, Egypt, 2006. [Google Scholar]
- Zhang, P.; Zhu, X.; Huang, F.; Liu, Y.; Zhang, J.; Lu, Y.; Ruan, Y. Suppression of jasmonic acid-dependent defense in the cotton plant by the mealybug Phenacoccus solenopsis. PLoS ONE 2011, 6, e22378. [Google Scholar] [CrossRef]
- Rawi, S.M.; El-Gindy, H.; Haggag, A.M.; Abou-El Hassan, A.; Abdel Kader, A. Few possible molluscicides from calendula Micrantha Officinalis and Ammi majus plants. I. Physiological effect on B. alexandrina and B. truncates. J. Egypt. Ger. Soc. Zool. 1995, 16, 69–75. [Google Scholar]
- Rasheed, D.M.S.; Abdel-Rahman, A.G.; Dahi, H.F.; El-Bamby, M.M.; Gamil, W.E. Spinosyn resistance mechanism in Egyptian cotton leafworm Spodoptera littoralis (Boisd.). Al-Azhar J. Agric. Res. 2015, 24, 99–121. [Google Scholar]
- Abdel-Aal, A.E.; Abdel-Khalek, A.A. Effect of three insect growth regulators on some biological and physiological aspects of Spodoptera littoralis (Boisd.). Bull. Ent. Soc. Egypt. Econ. Ser. 2006, 32, 101–112. [Google Scholar]
- El-Shershaby, M.; Farag, N.A.; Ahmed, A.I. Impact of B. thuringiensis on protein content and enzymes activity of Spodoptera littoralis. Res. J. Agric. Biol. Sci. 2008, 4, 861–865. [Google Scholar]
- Kamel, S.A.; Abd-El Aziz, F.A.; El-Bakry, M.N. Biochemical effects of three commercial formulations of B. thuringiensis (Agerrin, Dipel 2X and Dipel DF) on S. littoralis larvae. Egypt. Acad. J. Biol. Sci. 2010, 3, 21–29. [Google Scholar]
- Assar, A.A.; Abo El-Mahasen, M.M.; Khalil, M.E.; Mahmoud, S.H. Biochemical effects of some insect growth regulators on the house fly, Musca domestica (Diptera: Muscidae). Egypt. Acad. J. Biol. Sci. 2010, 2, 33–44. [Google Scholar]
- Gunnindg, R.V.; Moores, G.D.; Devonshire, A.L. Esterases and fenvalerate resistance in a field population of Helicoverpa punctigera (Lepidoptera: Noctuidae) in Australia. Pest Biochem. Physiol. 1997, 58, 155–162. [Google Scholar] [CrossRef]
- Mosleh, Y.Y.; Ismail, S.M.; Ahmed, M.T.; Ahmed, Y.M. Comparative toxicity and biochemical responses of certain pesticides to the mature earthworm Aporrectodea caliginosa under laboratory conditions. Environ. Toxicol. 2003, 18, 338–346. [Google Scholar] [CrossRef] [PubMed]

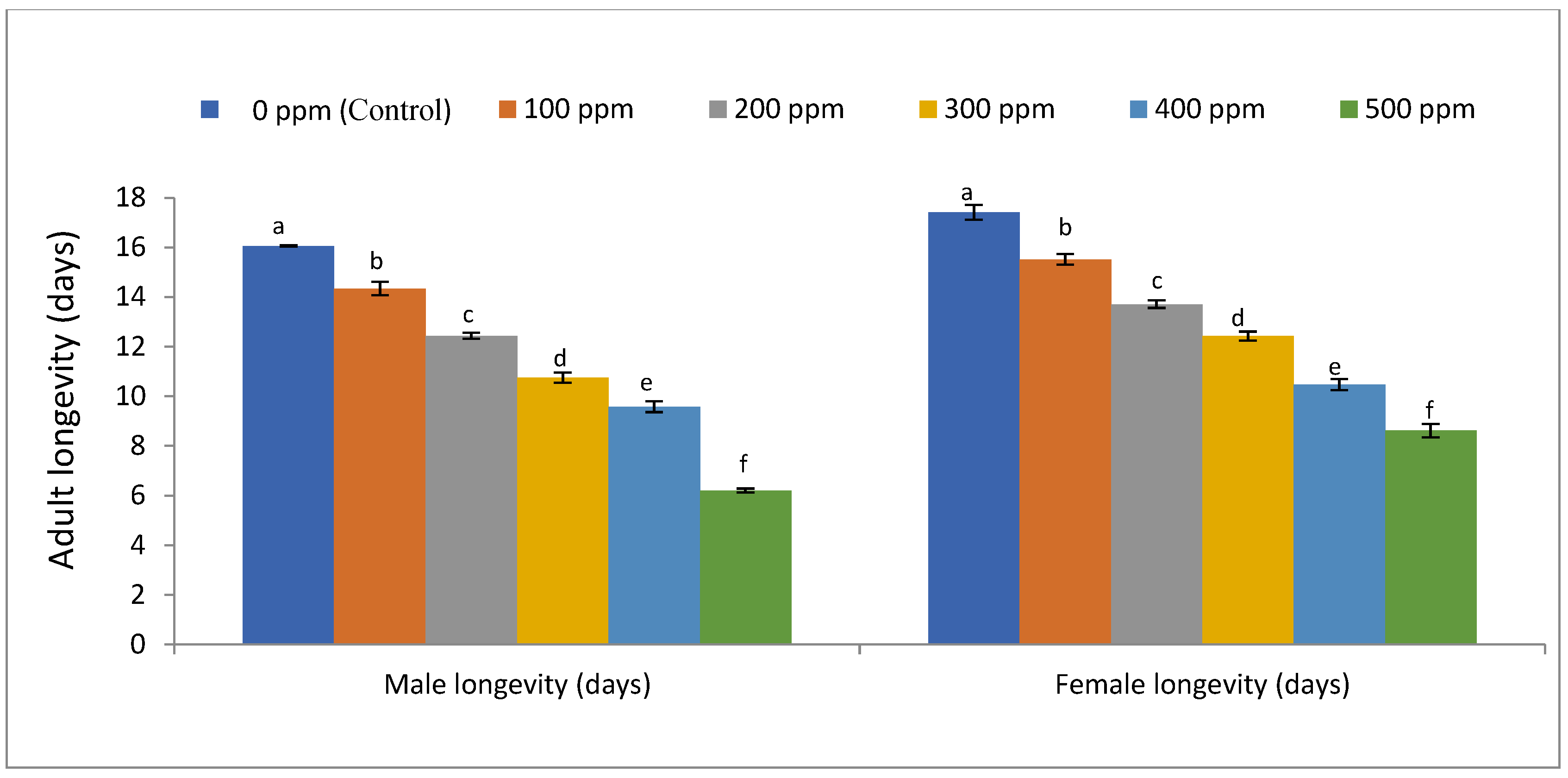
| EMs (ppm) | % Antifeedant Activity | Mean | ||||
|---|---|---|---|---|---|---|
| 1st Day | 2nd Day | 3rd Day | 4th Day | 5th Day | ||
| 100.00 | 9.85 ± 0.30 e | 15.79 ± 0.04 e | 22.03 ± 0.21 e | 33.01 ± 0.37 e | 41.71 ± 0.86 e | 22.03 ± 0.38 e |
| 200.00 | 13.80 ± 0.33 d | 19.62 ± 0.30 d | 28.18 ± 0.36 d | 38.29± 0.86 d | 49.87 ± 0.43 d | 28.18 ±0.21 d |
| 300.00 | 19.46 ± 0.12 c | 23.16 ±0.16 c | 34.01 ± 0.40 c | 45.26 ± 0.32 c | 55.09 ±0.36 c | 34.01± 0.22 c |
| 400.00 | 21.41 ± 0.20 b | 37.56 ± 0.26 b | 48.30 ± 0.60 b | 60.51 ± 0.18 b | 68.22 ± 0.36 b | 48.29 ± 0.40 b |
| 500.00 | 34.29 ± 0.02 a | 45.01 ± 0.46 a | 56.52 ± 0.74 a | 66.39 ± 0.05 a | 78.52 ± 0.23 a | 56.52 ±0.16 a |
| EMs (ppm) | CI | RGR% | AD% | ECI% | CD% |
|---|---|---|---|---|---|
| 0.00 (Control) | 6.37 ± 0.15 a | 24.19 ± 0.11 a | 84.70 ± 0.09 f | 8.53 ± 0.06 a | 10.37 ± 0.04 a |
| 100.00 | 5.61 ± 0.16 b | 19.63 ± 0.57 b | 86.33 ± 0.40 e | 7.24 ± 0.11 b | 8.09 ± 0.15 b |
| 200.00 | 4.52 ± 0.10 c | 18.44 ± 0.06 c | 89.02 ± 0.14 c | 6.51 ± 0.15 c | 7.15 ± 0.09 c |
| 300.00 | 3.76 ± 0.09 d | 16.51 ± 0.12 d | 87.68 ± 0.28 d | 5.62 ± 0.15 d | 6.27 ± 0.13 d |
| 400.00 | 3.09 ± 0.04 e | 12.59 ± 0.13 e | 94.81 ± 0.38 b | 4.11 ± 0.06 e | 4.77± 0.06 e |
| 500.00 | 2.87 ± 0.02 f | 9.60 ± 0.16 f | 97.38 ± 0.03 a | 3.44 ± 0.16 f | 3.90 ± 0.06 f |
| EMs (ppm) | Reduction% | |||||
|---|---|---|---|---|---|---|
| 1st Day | 2nd Day | 3rd Day | 4th Day | 5th Day | Mean | |
| 100.00 | 1.40 ±0.94 e | 18.77 ± 2.17 e | 34.44 ± 0.74 e | 50.63 ± 0.24 d | 62.91 ± 0.36 c | 33.06 ± 0.59 e |
| 200.00 | 19.68 ±1.26 d | 33.67 ± 0.83 d | 42.36 ± 0.95 d | 59.65 ± 0.51 c | 69.62 ±0.94 b | 44.07 ± 0.44 d |
| 300.00 | 33.29 ±1.35 c | 43.25± 0.63 c | 52.36 ± 0.26 c | 65.44 ±1.81 b | 71.94 ± 1.96 b | 52.49 ± 0.96 c |
| 400.00 | 46.42 ±1.07 b | 53.45 ± 1.09 b | 57.19 ± 1.19 b | 67.39 ±1.31 b | 76.64 ±1.40 a | 59.66 ±0.63 b |
| 500.00 | 67.40 ±0.97 a | 71.07 ± 0.28 a | 72.32 ± 0.79 a | 75.64± 0.56 a | 78.53 ±0.50 a | 72.78 ± 0.11 a |
| EMs (ppm) | Larvae | |||
|---|---|---|---|---|
| Weight (mg) | % Normal | % Malformed | Duration | |
| 0.00 (Control) | 60.58 ± 0.77 a | 100.00 ± 0.00 a | 00.00 ± 0.00 f | 14.37± 0.10 f |
| 100.00 | 60.50 ± 3.68 a | 55.73± 0.45 b | 44.27 ± 0.45 e | 22.07 ± 0.08 a |
| 200.00 | 51.91 ± 1.10 b | 42.85± 0.37 c | 57.14 ± 0.37 d | 20.33 ± 0.07 b |
| 300.00 | 44.48± 0.72 c | 33.27 ± 0.63 d | 66.73 ±0.63 c | 18.16 ± 0.20 c |
| 400.00 | 37.65 ± 0.59 d | 23.45± 0.38 e | 76.55 ±0.38 b | 16.81 ± 0.04 d |
| 500.00 | 30.16 ± 0.39 e | 18.15± 0.22 f | 81.85 ± 0.22 a | 15.62 ± 0.15 e |
| Pupae | ||||
| EMs (ppm) | Weight (mg) | Normal % | Malformed % | Duration |
| 0.00 (Control) | 375.22 ±0.48 a | 93.33 ±1.66 a | 6.67 ±1.67 f | 13.7 ± 0.12 a |
| 100.00 | 354.99 ±0.30 b | 64.18 ±0.93 b | 35.81 ±0.93 e | 8.82 ± 0.06 f |
| 200.00 | 344.75 ± 0.88 c | 47.71 ± 1.40 c | 52.29 ±1.4 d | 9.19 ± 0.06 e |
| 300.00 | 335.70 ±1.48 d | 34.45 ± 0.77 d | 65.5 ±0.77 c | 10.42 ±0.06 d |
| 400.00 | 320.51± 0.35 e | 19.77 ± 2.84 e | 76.89 ±0.58 b | 11.13 ±0.04 c |
| 500.00 | 303.88 ± 0.53 f | 14.90 ± 0.41 e | 85.09 ±0.40 a | 12.56 ±0.05 b |
| Adult | ||||
| EMs (ppm) | Emergence % | Malformed % | ||
| 0.00 (Control) | 90.67 ± 0.67 a | 9.33 ±0.66 f | ||
| 100.00 | 51.32 ±0.44 f | 48.68 ±0.40 a | ||
| 200.00 | 56.6 ± 0.40 e | 43.4 ± 0.42 b | ||
| 300.00 | 61.72 ±0.42 d | 38.28 ±0.42 c | ||
| 400.00 | 75.63 ± 0.59 c | 24.37 ± 0.59 d | ||
| 500.00 | 80.89 ± 0.44 b | 19.11 ±0.44 e | ||
| EMs (ppm) | Carbohydrates Enzymes | Total Protein (g/L) | Total Lipids (g/L) | ||
|---|---|---|---|---|---|
| Trehalose (µ/L) | Amylase (µ/L) | Invertase (µ/L) | |||
| 0.00 (Control) | 180.01 ± 0.32 c | 18.86 ± 0.26 b | 112.09 ± 0.62 e | 5.01 ± 0.10 a | 7.04 ± 0.03 a |
| 100.00 | 189.35 ± 0.78 a | 19.51 ± 0.23 a | 111.49 ± 0.00 e | 4.77 ±0.02 b | 6.65 ± 0.02 a |
| 200.00 | 185.72 ± 0.33 b | 17.48 ± 0.26 c | 115.84 ± 0.33 d | 4.25 ± 0.03 c | 6.36 ± 0.29 b |
| 300.00 | 170.37 ± 0.27 d | 16.38 ± 0.04 d | 119.73 ± 0.49 c | 4.14 ±0.26 cd | 6.30 ± 0.22 b |
| 400.00 | 152.33 ± 0.47 e | 15.16 ± 0.16 e | 124.83 ± 0.22 b | 4.01 ± 0.26 de | 5.52 ± 0.00 c |
| 500.00 | 145.94 ± 0.01 f | 14.51 ± 0.00 f | 127.67 ± 0.32 a | 3.95 ± 0.00 e | 4.09 ± 0.00 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zayed, M.S.; Taha, E.-K.A.; Hegazy, F.H.; Albogami, B.; Noureldeen, A.; Elnabawy, E.-S.M. Influence of Effective Microorganisms on Some Biological and Biochemical Aspects of Spodoptera littoralis (Boisduval) (Lepidoptera: Noctuidae). Life 2022, 12, 1726. https://doi.org/10.3390/life12111726
Zayed MS, Taha E-KA, Hegazy FH, Albogami B, Noureldeen A, Elnabawy E-SM. Influence of Effective Microorganisms on Some Biological and Biochemical Aspects of Spodoptera littoralis (Boisduval) (Lepidoptera: Noctuidae). Life. 2022; 12(11):1726. https://doi.org/10.3390/life12111726
Chicago/Turabian StyleZayed, Mohamed S., El-Kazafy A. Taha, Fatma H. Hegazy, Bander Albogami, Ahmed Noureldeen, and El-Said M. Elnabawy. 2022. "Influence of Effective Microorganisms on Some Biological and Biochemical Aspects of Spodoptera littoralis (Boisduval) (Lepidoptera: Noctuidae)" Life 12, no. 11: 1726. https://doi.org/10.3390/life12111726
APA StyleZayed, M. S., Taha, E.-K. A., Hegazy, F. H., Albogami, B., Noureldeen, A., & Elnabawy, E.-S. M. (2022). Influence of Effective Microorganisms on Some Biological and Biochemical Aspects of Spodoptera littoralis (Boisduval) (Lepidoptera: Noctuidae). Life, 12(11), 1726. https://doi.org/10.3390/life12111726







