Bacterial Communities Associated with Crude Oil Bioremediation through Composting Approaches with Indigenous Bacterial Isolate
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation of Crude Oil Degrading Bacteria
2.2. Screening of Bacterial Isolates Capable of Crude Oil Degradation
2.3. Identification of Selected Crude Oil Degrading Bacterial Isolates
2.4. Compost-Based Bioremediation of Crude Oil-Contaminated Soil
2.5. Chemical Analyses of Soil Samples
2.6. Microbiological Analyses of Soil Samples Subjected to Bioremediation Treatments
2.7. Statistical Analysis
3. Results
3.1. Isolation of Crude Oil Degrading Bacteria and Assessment of Crude Oil Degradation Capacity of Bacterial Isolates
3.2. Compost-Based Bioremediation Treatments of Crude Oil Contaminated Soil and Microbiological Analyses
3.3. Bacterial Community Analysis
3.4. Beta-Diversity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mallah, M.A.; Changxing, L.; Mallah, M.A.; Noreen, S.; Liu, Y.; Saeed, M.; Xi, H.; Ahmed, B.; Feng, F.; Mirjat, A.A.; et al. Polycyclic aromatic hydrocarbon and its effects on human health: An overeview. Chemosphere 2022, 296, 133948. [Google Scholar] [CrossRef] [PubMed]
- Hunt, L.J.; Duca, D.; Dan, T.; Knopper, L.D. Petroleum hydrocarbon (PHC) uptake in plants: A literature review. Environ. Pollut. 2019, 245, 472–484. [Google Scholar] [CrossRef] [PubMed]
- Honda, M.; Suzuki, N. Toxicities of polycyclic aromatic hydrocarbons for aquatic animals. Int. J. Environ. Res. Public Health 2020, 17, 1363. [Google Scholar] [CrossRef] [PubMed]
- Xuezhi, D. Remediation methods of crude oil contaminated soil. World J. Agric. Soil Sci. 2020, 4, 8. [Google Scholar] [CrossRef]
- Ali, N.; Dashti, N.; Khanafer, M.; Al-Awadhi, H.; Radwan, S. Bioremediation of soils saturated with spilled crude oil. Sci. Rep. 2020, 10, 1116. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liu, W.; Tian, S.; Wang, W.; Qi, Q.; Jiang, P.; Gao, X.; Li, F.; Li, H.; Yu, H. Petroleum hydrocarbon-degrading bacteria for the remediation of oil pollution under aerobic conditions: A perspective analysis. Front. Microbiol. 2018, 9, 2885. [Google Scholar] [CrossRef]
- Tao, J.; Qin, C.; Feng, X.; Ma, L.; Liu, X.; Yin, H.; Liang, Y.; Liu, H.; Huang, C.; Zhang, Z.; et al. Traits of exogenous species and indigenous community contribute to the species colonization and community succession. Front. Microbiol. 2018, 9, 3087. [Google Scholar] [CrossRef]
- Ejaz, M.; Zhao, B.; Wang, X.; Bashir, S.; Haider, F.U.; Aslam, Z.; Khan, M.I.; Shabaan, M.; Naveed, M.; Mustafa, A. Isolation and characterization of oil-degrading Enterobacter sp. From naturally hydrocarbon-contaminated soils and their potential use against the bioremediation of crude oil. Appl. Sci. 2021, 11, 3504. [Google Scholar] [CrossRef]
- Kästner, M.; Miltner, A. Application of compost for effective bioremediation of organic contaminants and pollutants in soil. Appl. Microbiol. Biotechnol. 2016, 100, 3433–3449. [Google Scholar] [CrossRef]
- Tran, H.-T.; Lin, C.; Bui, X.-T.; Ngo, H.-H.; Cheruiyot, N.K.; Hoang, H.-G.; Vu, C.-T. Aerobic composting remediation of petroleum hydrocarbon-contaminated soil. Current and future perspectives. Sci. Total Environ. 2021, 753, 142250. [Google Scholar] [CrossRef]
- Ubani, O.; Atagana, H.I.; Selvarajan, R.; Ogola, H.J. Unravelling the genetic and functional diversity of dominant bacterial communities involved in manure co-composting bioremediation of complex crude oil waste sludge. Heliyon 2022, 8, e08945. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, Q.; Li, D.; Du, J.; Wang, C.; Qin, J. Rapid degradation of long-chain crude oil in soil by indigenous bacteria using fermented food waste supernatant. Waste Manag. 2019, 85, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Phetcharat, T.; Dawkrajai, P.; Chitov, T.; Wongpornchai, P.; Saenton, S.; Mhuantong, W.; Kanokratana, P.; Champreda, V.; Bovonsombut, S. Effect of inorganic nutrients on bacterial community composition in oil-bearing sandstones from the subsurface strata of an onshore oil reservoir and its potential use in microbial enhanced oil recovery. PLoS ONE 2018, 13, e0198050. [Google Scholar] [CrossRef] [PubMed]
- Youssef, N.H.; Duncan, K.E.; Nagle, D.P.; Savage, K.N.; Knapp, R.M.; McInerney, M.J. Comparison of methods to detect biosurfactant production by diverse microorganisms. J. Microbiol. Methods 2004, 56, 339–347. [Google Scholar] [CrossRef]
- Zargar, A.N.; Mishra, S.; Kumar, M.; Srivastava, P. Isolation and chemical characterization of the biosurfactant produced by Gordonia sp. IITR100. PLoS ONE 2022, 17, e0264202. [Google Scholar] [CrossRef]
- Rani, M.; Weadge, J.T.; Jabaji, S. Isolation and characterization of biosurfactant-producing bacteria from oil well batteries with antimicrobial activities against food-borne and plant pathogens. Front. Microbiol. 2020, 11, 64. [Google Scholar] [CrossRef]
- Kalaimurugan, D.; Balamuralikrishnan, B.; Govindarajan, R.K.; Al-Dhabi, N.A.; Valan Arasu, M.; Vadivalagan, C.; Venkatesan, S.; Kamyab, H.; Chelliapan, S.; Khanongnuch, C. Production and characterization of a novel biosurfactant molecule from Bacillus safensis YKS2 and assessment of its efficiencies in wastewater treatment by a directed metagenomic approach. Sustainability 2022, 14, 2142. [Google Scholar] [CrossRef]
- Panda, S.K.; Kar, R.N.; Panda, C.R. Isolation and identification of petroleum hydrocarbon degrading microorganisms from oil contaminated environment. Int. J. Environ. Sci. 2013, 3, 1314–1321. [Google Scholar] [CrossRef]
- Hendrickx, B.; Dejonghe, W.; Boënne, W.; Brennerova, M.; Cernik, M.; Lederer, T.; Bucheli-Witschel, M.; Bastiaens, L.; Verstraete, W.; Top, E.M.; et al. Dynamics of an oligotrophic bacterial aquifer community during contact with a groundwater plume contaminated with benzene, toluene, ethylbenzene, and xylenes: An in situ mesocosm study. Appl. Environ. Microbiol. 2005, 71, 3815–3825. [Google Scholar] [CrossRef]
- Sriwichai, M.; Malem, F.; Pholchan, M.K.; Bovonsombut, S. Detection of bacterial communities in volatile-organic-compound (VOC)-contaminated soil in an industrial estate in eastern Thailand by PCR-DGGE analysis. Chiang Mai J. Sci. 2017, 44, 742–750. [Google Scholar]
- Wan, C.Y.; De Wever, H.; Diels, L.; Thoeye, C.; Liang, J.B.; Huang, L.N. Biodiversity and population dynamics of microorganisms in a full-scale membrane bioreactor for municipal wastewater treatment. Water Res. 2011, 45, 1129–1138. [Google Scholar] [CrossRef] [PubMed]
- Lasudee, K.; Tokuyama, S.; Lumyong, S.; Pathom-Aree, W. Actinobacteria associated with arbuscular mycorrhizal funneliformis mosseae spores, taxonomic characterization and their beneficial traits to plants: Evidence obtained from mung bean (Vigna radiata) and Thai jasmine rice (Oryza sativa). Front. Microbiol. 2018, 9, 1247. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, A.; Ishiwata, H.; Kitamura, K.; Sunamura, M.; Fujita, T.; Matsuo, M.; Higashihara, T. Dynamics of microbial populations and strong selection for Cycloclasticus pugetii following the Nakhodka oil spill. Microb. Ecol. 2003, 46, 442–453. [Google Scholar] [CrossRef]
- Meyer, M.; Stenzel, U.; Hofreiter, M. Parallel tagged sequencing on the 454 platform. Nat. Protoc. 2008, 3, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Romanus, A.A.; Ikechukwu, E.F.; Patrick, A.S.; Goddey, U.; Helen, O. Efficiency of plantain peels and guinea corn shaft for bioremediation of crude oil polluted soil. J. Microbiol. Res. 2015, 5, 31–40. [Google Scholar] [CrossRef]
- Sansupa, C.; Purahong, W.; Wubet, T.; Tiansawat, P.; Pathom-Aree, W.; Teaumroong, N.; Chantawannakul, P.; Buscot, F.; Elliott, S.; Disayathanoowat, T. Soil bacterial communities and their associated functions for forest restoration on a limestone mine in northern Thailand. PLoS ONE 2021, 16, e0248806. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wen, X.; Zhao, L.; Shi, Y.; Jin, H. Crude oil treatment leads to shift of bacterial communities in soils from the deep active layer and upper permafrost along the China-Russia crude oil pipeline route. PLoS ONE 2014, 9, e96552. [Google Scholar] [CrossRef] [PubMed]
- Medić, A.; Lješević, M.; Inui, H.; Beškoski, V.; Kojić, I.; Stojanović, K.; Karadžić, I. Efficient biodegradation of petroleum: N-alkanes and polycyclic aromatic hydrocarbons by polyextremophilic Pseudomonas aeruginosa san ai with multidegradative capacity. RSC Adv. 2020, 10, 14060–14070. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yang, G.; Jia, H.; Sun, B. Crude oil degradation by a novel strain Pseudomonas aeruginosa AQNU-1 isolated from an oil-contaminated lake wetland. Processes 2022, 10, 307. [Google Scholar] [CrossRef]
- Ramavandi, B.; Ghafarizadeh, F.; Alavi, N.; Babaei, A.A.; Ahmadi, M. Biotreatment of total petroleum hydrocarbons from an oily sludge using co-composting approach. Soil Sediment Contam. 2018, 27, 524–537. [Google Scholar] [CrossRef]
- Lin, C.; Sheu, D.S.; Lin, T.C.; Kao, C.M.; Grasso, D. Thermophilic biodegradation of diesel oil in food waste composting processes without bioaugmentation. Environ. Eng. Sci. 2012, 29, 117–123. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, D.; Ren, H. Bioremediation of oil contaminated soil using agricultural wastes via microbial consortium. Sci. Rep. 2020, 10, 9188. [Google Scholar] [CrossRef] [PubMed]
- Koolivand, A.; Abtahi, H.; Parhamfar, M.; Saeedi, R.; Coulon, F.; Kumar, V.; Villaseñor, J.; Sartaj, M.; Najarian, N.; Shahsavari, M.; et al. The effect of petroleum hydrocarbons concentration on competition between oil-degrading bacteria and indigenous compost microorganisms in petroleum sludge bioremediation. Environ. Technol. Innov. 2022, 26, 102319. [Google Scholar] [CrossRef]
- Klein, S.; Lorenzo, C.; Hoffmann, S.; Walther, J.M.; Storbeck, S.; Piekarski, T.; Tindall, B.J.; Wray, V.; Nimtz, M.; Moser, J. Adaptation of Pseudomonas aeruginosa to various conditions includes tRNA-dependent formation of alanyl-phosphatidylglycerol. Mol. Microbiol. 2009, 71, 551–565. [Google Scholar] [CrossRef]
- Wu, M.; Li, X. Chapter 87-Klebsiella pneumoniae and Pseudomonas aeruginosa. In Molecular Medical Microbiology, 2nd ed.; Tang, Y.-W., Sussman, M., Liu, D., Poxton, I., Schwartzman, J.B.T.-M.M.M., Eds.; Academic Press: Boston, MA, USA, 2015; pp. 1547–1564. ISBN 978-0-12-397169-2. [Google Scholar]
- Hussain, H.; Mamadalieva, N.Z.; Hussain, A.; Hassan, U.; Rabnawaz, A.; Ahmed, I.; Green, I.R. Fruit peels: Food waste as a valuable source of bioactive natural products for drug discovery. Curr. Issues Mol. Biol. 2022, 44, 1960–1994. [Google Scholar] [CrossRef]
- Du, W.X.; Olsen, C.W.; Avena-Bustillos, R.J.; Friedman, M.; McHugh, T.H. Physical and antibacterial properties of edible films formulated with apple skin polyphenols. J. Food Sci. 2011, 76, M149–M155. [Google Scholar] [CrossRef]
- Abdelmalek, S.; Mohsen, E.; Awwad, A.; Issa, R. Peels of Psidium guajava fruit possess antimicrobial properties. Int. Arab. J. Antimicrob. Agents 2016, 6, 3. [Google Scholar] [CrossRef]
- Neglo, D.; Tettey, C.O.; Essuman, E.K.; Kortei, N.K.; Boakye, A.A.; Hunkpe, G.; Amarh, F.; Kwashie, P.; Devi, W.S. Comparative antioxidant and antimicrobial activities of the peels, rind, pulp and seeds of watermelon (Citrullus lanatus) fruit. Sci. African 2021, 11, e00582. [Google Scholar] [CrossRef]
- Könönen, E.; Gursoy, U.K. Oral Prevotella species and their connection to events of clinical relevance in gastrointestinal and respiratory tracts. Front. Microbiol. 2022, 12, 798763. [Google Scholar] [CrossRef] [PubMed]
- Ueki, A.; Akasaka, H.; Satoh, A.; Suzuki, D.; Ueki, K. Prevotella paludivivens sp. nov., a novel strictly anaerobic, Gram-negative, hemicellulose-decomposing bacterium isolated from plant residue and rice roots in irrigated rice-field soil. Int. J. Syst. Evol. Microbiol. 2007, 57, 1803–1809. [Google Scholar] [CrossRef] [PubMed]
- Salam, L.B.; Obayori, O.S.; Ilori, M.O.; Amund, O.O. Effects of cadmium perturbation on the microbial community structure and heavy metal resistome of a tropical agricultural soil. Bioresour. Bioprocess. 2020, 7, 1–19. [Google Scholar] [CrossRef]
- Pampillón-González, L.; Ortiz-Cornejo, N.L.; Luna-Guido, M.; Dendooven, L.; Navarro-Noya, Y.E. Archaeal and bacterial community structure in an anaerobic digestion reactor (Lagoon Type) used for biogas production at a pig farm. J. Mol. Microbiol. Biotechnol. 2017, 27, 306–317. [Google Scholar] [CrossRef]
- Lin, Y.; Lay, J.J.; Shieh, W.K. Diesel degradation in soil catalyzed by the addition of a bioagent. Int. J. Environ. Sci. Technol. 2016, 13, 551–560. [Google Scholar] [CrossRef][Green Version]
- Tudela, J.; Martínez, M.; Valdivia, R.; Romo, J.; Portillo, M.; Rangel, R. Bioremediation of PAH-contaminated soils: Process enhancement through composting/compost. Appl. Sci. 2020, 10, 3684. [Google Scholar]
- Chikere, C.B.; Chikere, B.O.; Okpokwasili, G.C. Bioreactor-based bioremediation of hydrocarbon-polluted Niger Delta marine sediment, Nigeria. Biotech 2012, 2, 53–66. [Google Scholar] [CrossRef]
- Khalid, F.E.; Lim, Z.S.; Sabri, S.; Gomez-Fuentes, C.; Zulkharnain, A.; Ahmad, S.A. Bioremediation of diesel contaminated marine water by bacteria: A review and bibliometric analysis. J. Mar. Sci. Eng. 2021, 9, 155. [Google Scholar] [CrossRef]
- Parnian, A.; Parnian, A.; Pirasteh-Anosheh, H.; Furze, J.N.; Prasad, M.N.V.; Race, M.; Hulisz, P.; Ferraro, A. Full-scale bioremediation of petroleum-contaminated soils via integration of co-composting. J. Soils Sediments 2022, 22, 2209–2218. [Google Scholar] [CrossRef]
- Sayara, T.; Sánchez, A. Gaseous emissions from the composting process: Controlling parameters and strategies of mitigation. Processes 2021, 9, 1844. [Google Scholar] [CrossRef]
- Ba, S.; Qu, Q.; Zhang, K.; Groot, J.C.J. Meta-analysis of greenhouse gas and ammonia emissions from dairy manure composting. Biosyst. Eng. 2020, 193, 126–137. [Google Scholar] [CrossRef]
- Al-Jabi, L.F.; Halalsheh, M.M.; Badarneh, D.M. Conservation of ammonia during food waste composting. Environ. Technol. 2008, 29, 1067–1107. [Google Scholar] [CrossRef] [PubMed]
- Park, K.J.; Choi, M.H.; Hong, J.H. Control of composting odor using biofiltration. Compost Sci. Util. 2002, 10, 356–362. [Google Scholar] [CrossRef]
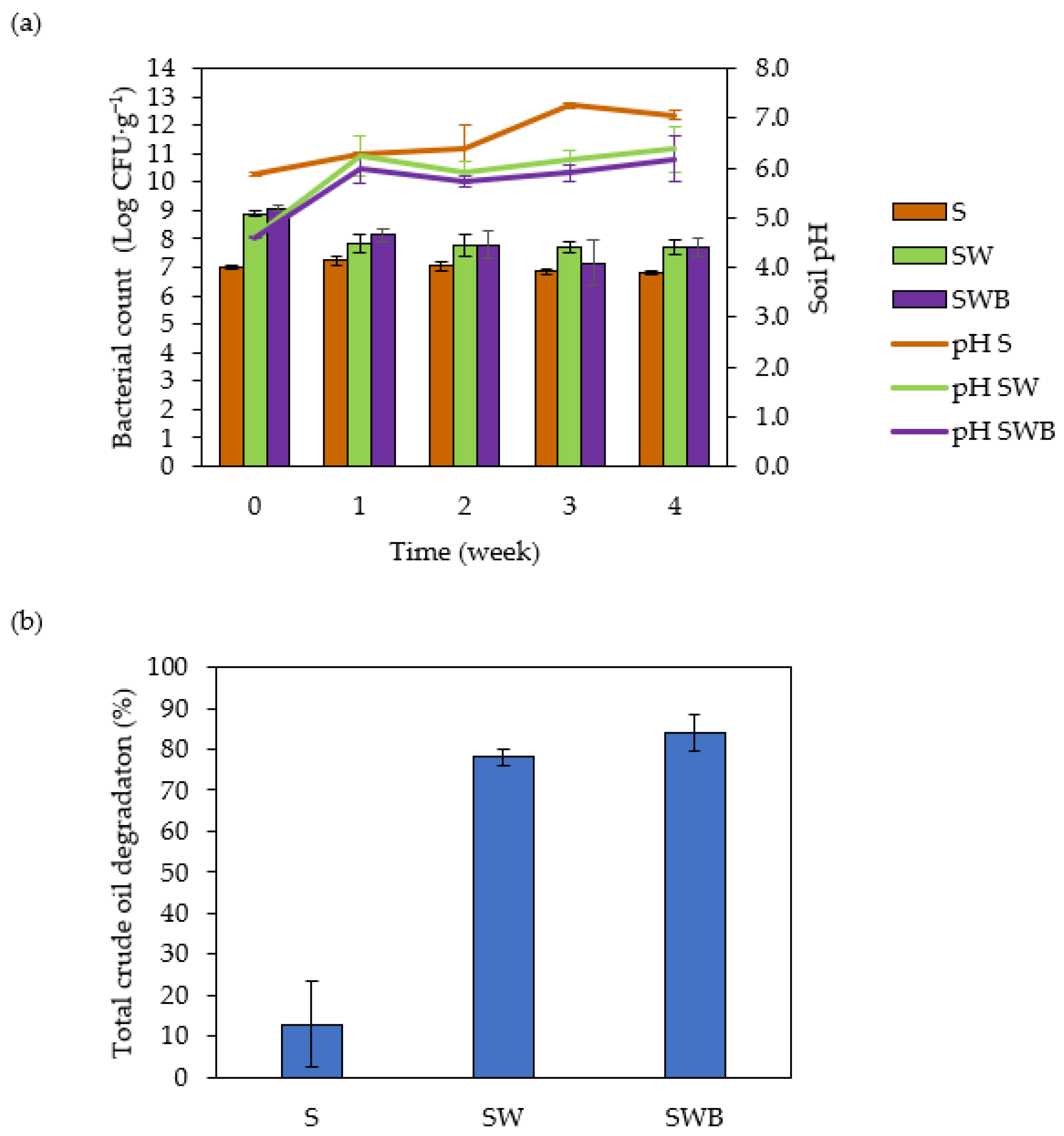
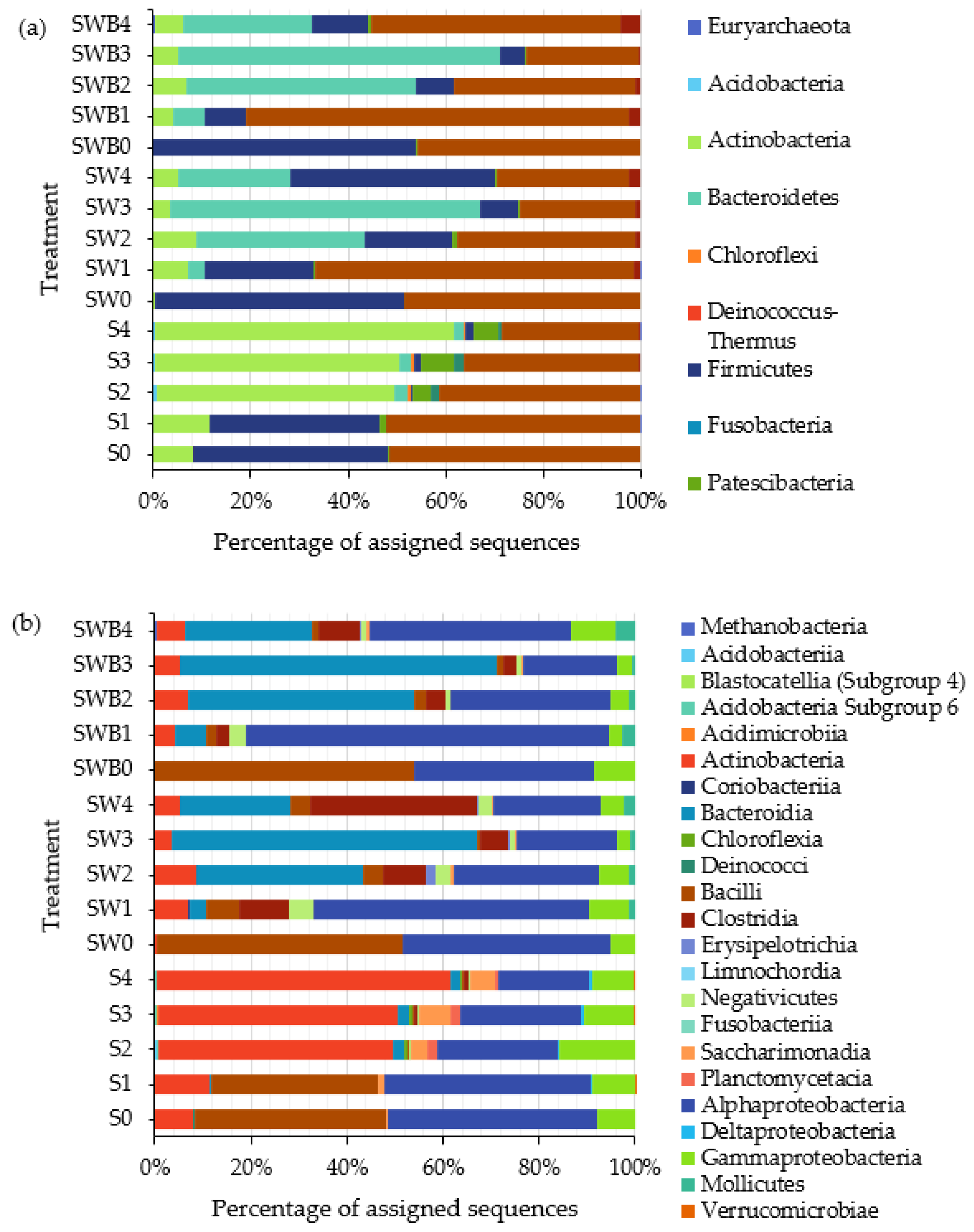
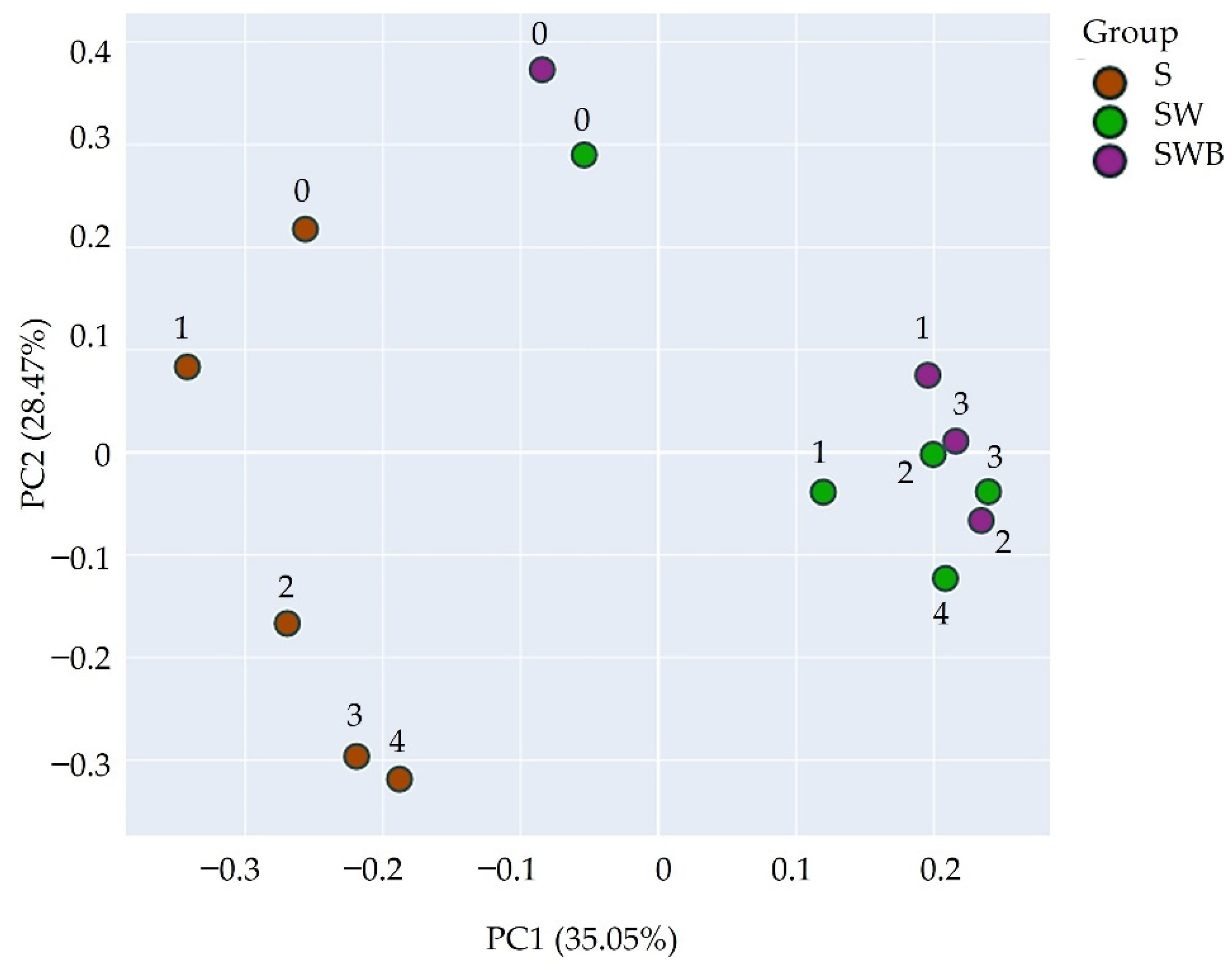
| Sample/ Isolate | Cell Morphology | Drop Collapse Score | Crude Oil Degradation (%) 1 |
|---|---|---|---|
| Control 2 | - | 0 | 4.67 ± 2.69 |
| M02 | Gram-positive, rod-shaped | 2 | 11.11 ± 6.42 |
| M07 3 | Gram-negative, rod-shaped | 2 | 21.56 ± 1.57 |
| M10 | Gram-positive, rod-shaped | 2 | 11.30 ± 4.02 |
| M16 | Gram-negative, rod-shaped | 2 | 7.44 ± 4.30 |
| S104 | Gram-positive, rod-shaped | 3 | 12.71 ± 7.34 |
| S120 | Gram-negative, rod-shaped | 2 | 10.37 ± 4.90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mukjang, N.; Chitov, T.; Mhuantong, W.; Champreda, V.; Pathom-aree, W.; Sattayawat, P.; Bovonsombut, S. Bacterial Communities Associated with Crude Oil Bioremediation through Composting Approaches with Indigenous Bacterial Isolate. Life 2022, 12, 1712. https://doi.org/10.3390/life12111712
Mukjang N, Chitov T, Mhuantong W, Champreda V, Pathom-aree W, Sattayawat P, Bovonsombut S. Bacterial Communities Associated with Crude Oil Bioremediation through Composting Approaches with Indigenous Bacterial Isolate. Life. 2022; 12(11):1712. https://doi.org/10.3390/life12111712
Chicago/Turabian StyleMukjang, Nilita, Thararat Chitov, Wuttichai Mhuantong, Verawat Champreda, Wasu Pathom-aree, Pachara Sattayawat, and Sakunnee Bovonsombut. 2022. "Bacterial Communities Associated with Crude Oil Bioremediation through Composting Approaches with Indigenous Bacterial Isolate" Life 12, no. 11: 1712. https://doi.org/10.3390/life12111712
APA StyleMukjang, N., Chitov, T., Mhuantong, W., Champreda, V., Pathom-aree, W., Sattayawat, P., & Bovonsombut, S. (2022). Bacterial Communities Associated with Crude Oil Bioremediation through Composting Approaches with Indigenous Bacterial Isolate. Life, 12(11), 1712. https://doi.org/10.3390/life12111712








