Spatial Heterogeneity of Soil Bacterial Community Structure and Enzyme Activity along an Altitude Gradient in the Fanjingshan Area, Northeastern Guizhou Province, China
Abstract
1. Introduction
2. Research Area and Methods
2.1. Research Area
2.2. Soil Sample Collection
2.3. Determination Methods
2.3.1. PH and Soil Enzyme Activity Determination
2.3.2. DNA Extraction, NovaSeq Sequencing, and Bioinformatics Analysis
2.4. Data Analysis
3. Results
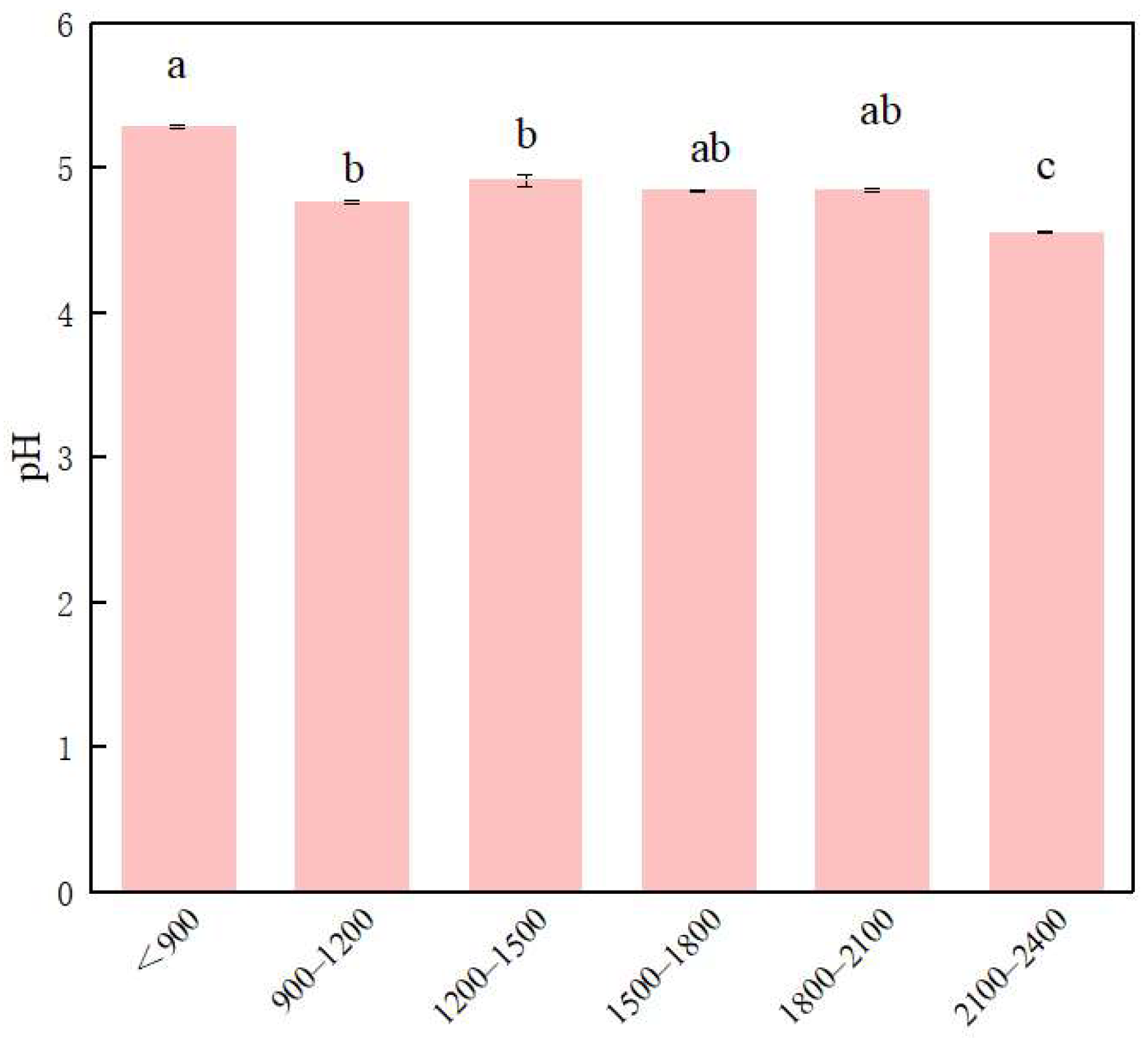
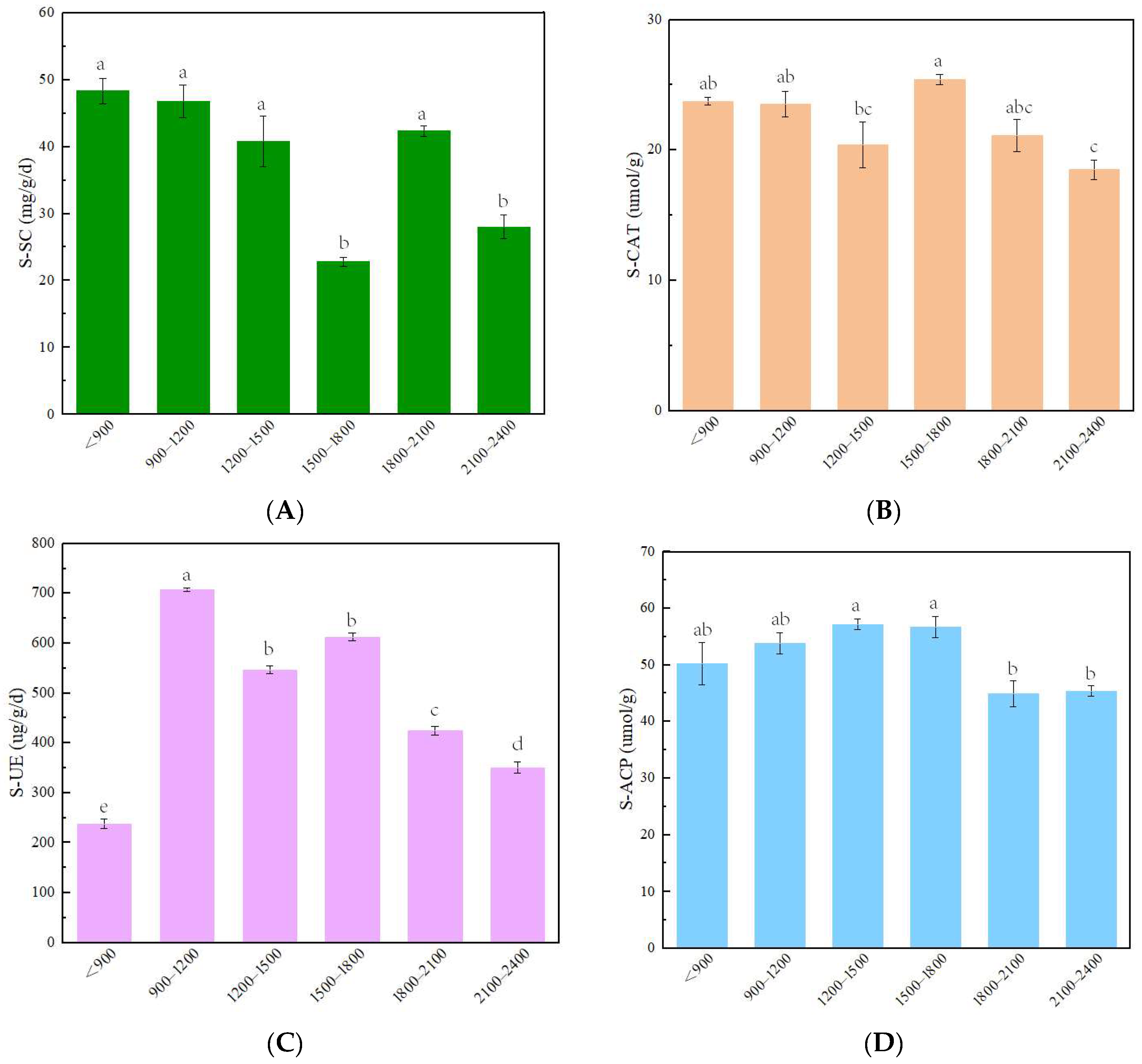
3.1. Changes in PH and Soil Enzymes along the Altitude Gradient
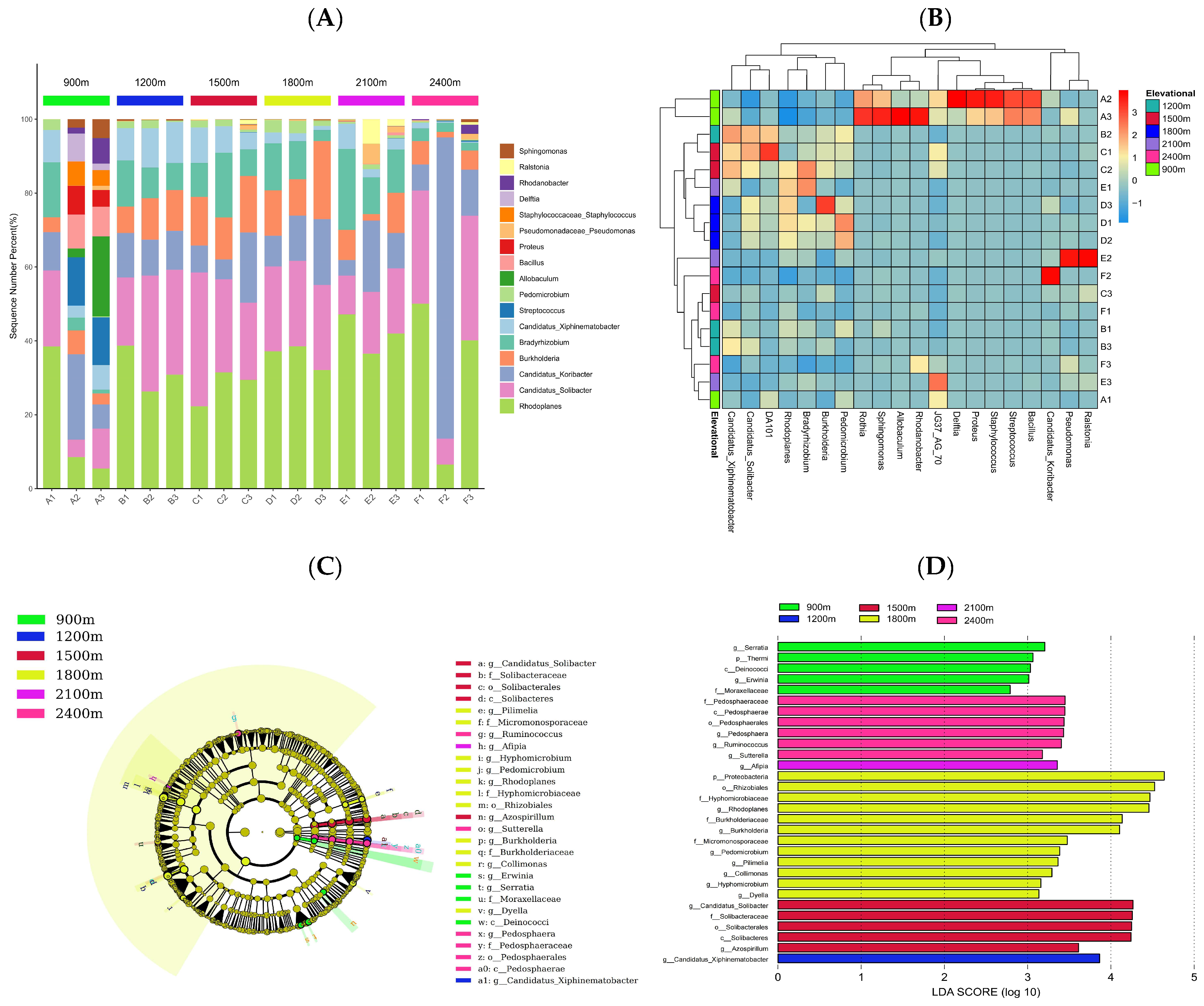
3.2. Changes in Soil Bacterial Community Composition and Species Differences among OTU Groups along the Altitude Gradient
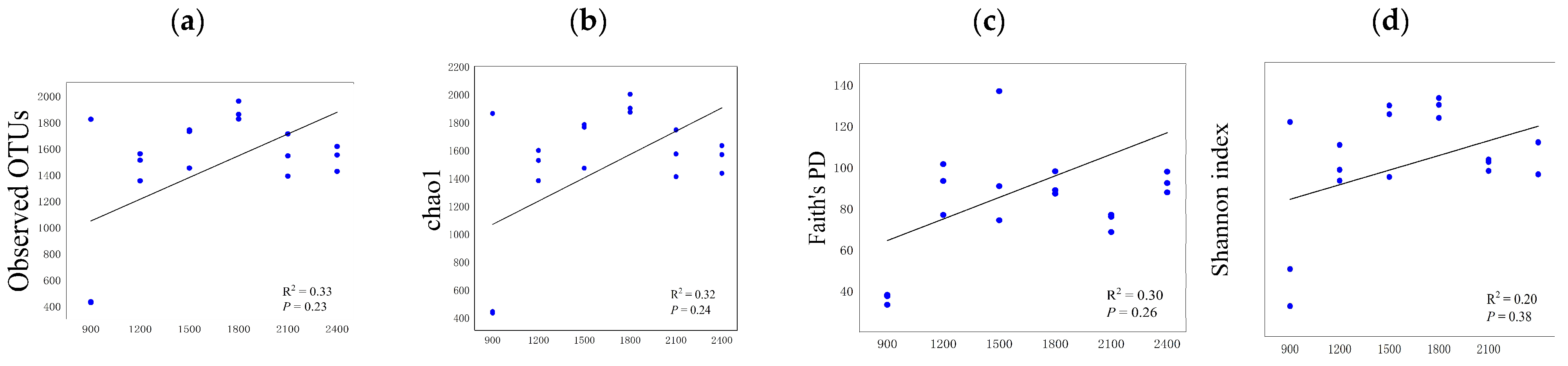
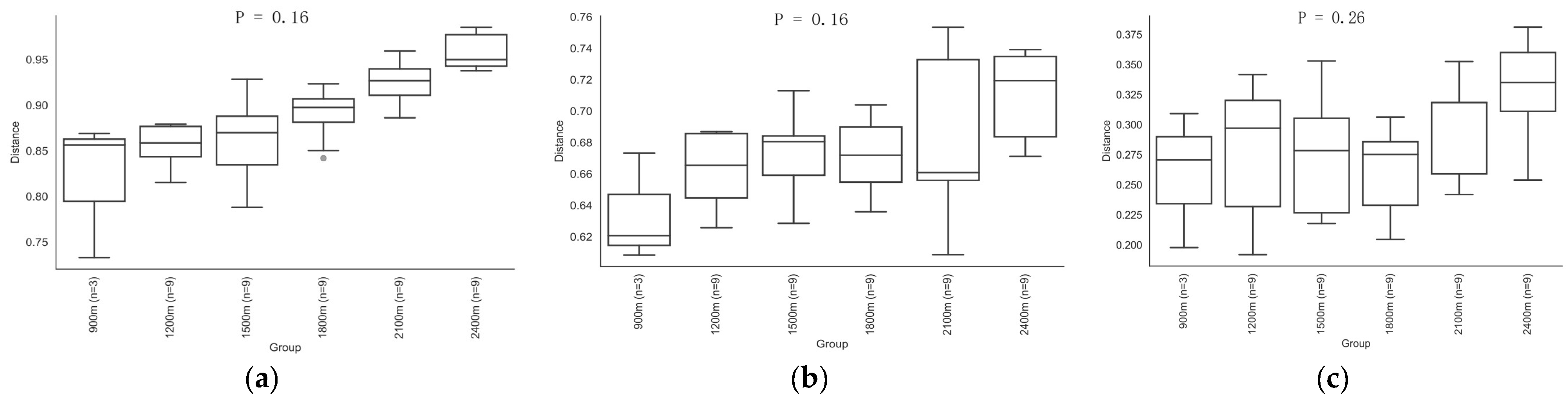
3.3. Differences in Soil Microbial Diversity and Community Structure along the Altitude Gradient
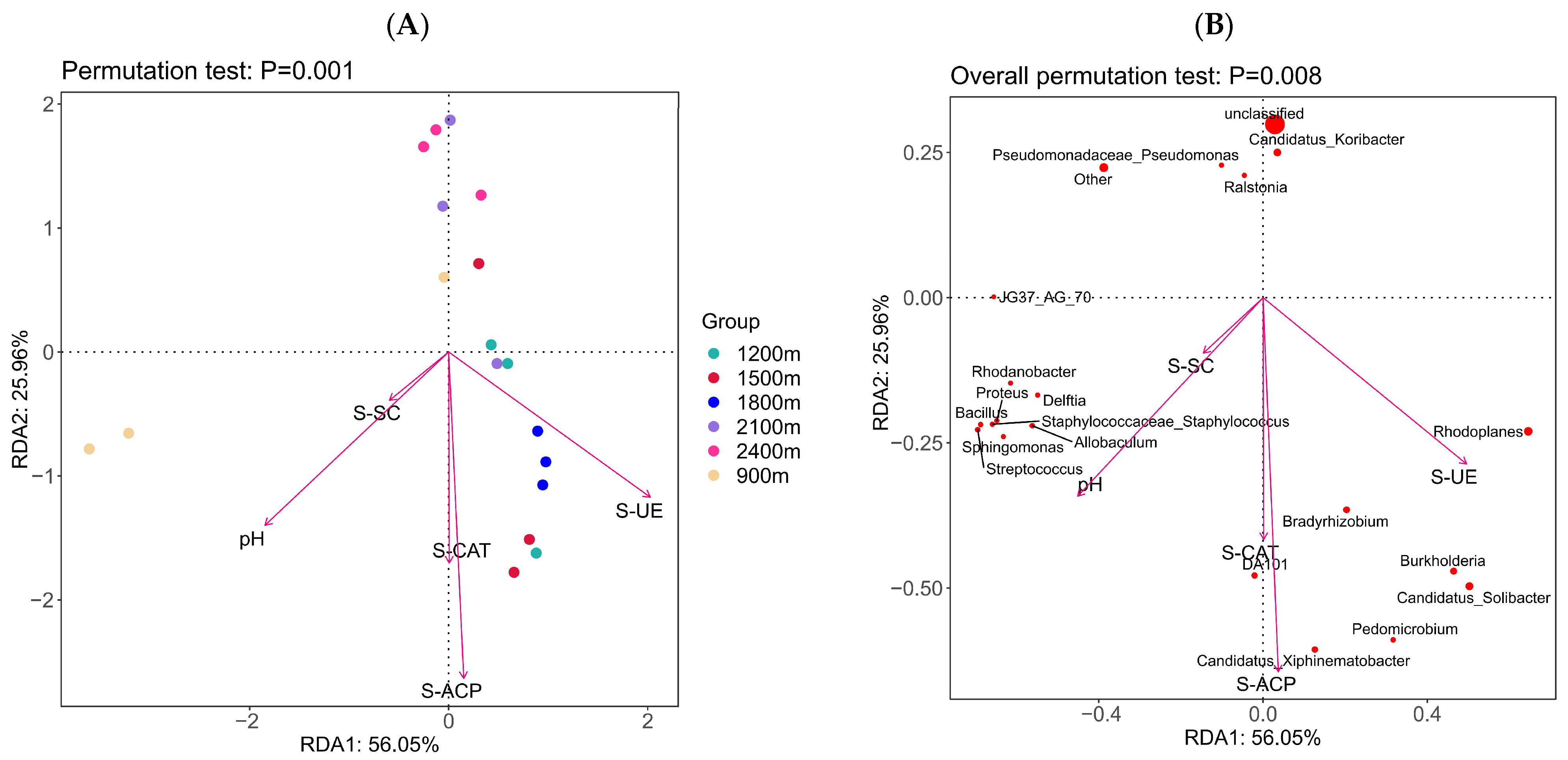
3.4. Effect of PH and Soil Enzyme on Microbial Community Structure
4. Discussion
4.1. Variation of Soil Enzyme Activity with Altitude in Fanjingshan
4.2. Vertical Variability in Soil Bacterial Community Structure and Phylogeny in Fanjingshan
4.3. Relationship between Soil Bacterial Community Structure and Soil pH or Enzyme Activity along the Altitude Gradient
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Caldwell, B.A. Enzyme activities as a component of soil biodiversity: A review. Pedobiologia 2005, 49, 637–644. [Google Scholar] [CrossRef]
- Li, F.; Liu, M.; Li, Z.; Jiang, C.; Han, F.; Che, Y. Changes in soil microbial biomass and functional diversity with a nitrogen gradient in soil columns. Appl. Soil Ecol. 2013, 64, 1–6. [Google Scholar] [CrossRef]
- Gryta, A.; Frc, M.; Oszust, K. The application of the bio-log ecoplate approach in ecotoxicological evaluation of dairy sewage sludge. Appl. Biochem. Biotechnol. 2014, 174, 1434–1443. [Google Scholar] [CrossRef]
- Djukic, I.; Zehetner, F.; Mentler, A.; Gerzabek, M.H. Microbial community composition and activity in different Alpine vegetation zones. Soil Biol. Biochem. 2010, 42, 155–161. [Google Scholar] [CrossRef]
- Deng, Y.F.; Tian, S.Y.; Cheng, Y.H.; Hu, Z.K.; Liu, M.Q.; Hu, F.; Chen, X.Y. Effects of liming on rhizosphere soil microbial communities of dominant plants in fallowed red soil under simulated nitrogen deposition. Acta Pedol. Sin. 2019, 56, 1449–1458. [Google Scholar]
- Rani, A.; Porwal, S.; Sharma, R.; Kapley, A.; Purohit, H.J.; Kalia, V.C. Assessment of microbial diversity in effluent treatment plants by culture dependent and culture independent approaches. Bioresour. Technol. 2008, 99, 7098–7107. [Google Scholar] [CrossRef]
- Chu, H.; Feng, M.; Liu, X.; Shi, Y.; Yang, T.; Gao, G. Soil Microbial Biogeography: Recent Advances in China and Research Frontiers in the World. Acta Pedol. Sin. 2020, 57, 515–529. [Google Scholar]
- Fierer, N.; McCain, C.M.; Meir, P.; Zimmermann, M.; Rapp, J.M.; Silman, M.R.; Knight, R. Microbes do not follow the elevational diversity patterns of plants and animals. Ecology 2011, 92, 797–804. [Google Scholar] [CrossRef]
- Gaston, K.J. Global patterns in biodiversity. Nature 2000, 405, 220–227. [Google Scholar] [CrossRef]
- Ma, L.; Liu, L.; Lu, Y.; Chen, L.; Zhang, Z.; Zhang, H.; Wang, X.; Shu, L.; Yang, Q.; Song, Q.; et al. When microclimates meet soil microbes: Temperature controls soil microbial diversity along an elevational gradient in subtropical forests. Soil Biol. Biochem. 2022, 166, 108566. [Google Scholar] [CrossRef]
- Bryant, J.A.; Lamanna, C.; Morlon, H.; Kerkhoff, A.J.; Enquist, B.J.; Green, J.L. Microbes on mountainsides: Contrasting elevational patterns of bacterial and plant diversity. Proc. Natl. Acad. Sci. USA 2008, 105 (Suppl. 1), 11505–11511. [Google Scholar] [CrossRef]
- Wu, Z.Y.; Lin, W.X.; Chen, Z.F.; Fang, C.X.; Zhang, Z.X.; Wu, L.K.; Zhou, M.M.; Chen, T. Variations of soil microbial community diversity along an elevational gradient in mid-subtropical forest. Chin. J. Plant Ecol. 2013, 37, 397–406. [Google Scholar] [CrossRef]
- Tang, M.; Li, L.; Wang, X.; You, J.; Li, J.; Chen, X. Elevational is the main factor controlling the soil microbial community structure in alpine tundra of the Changbai Mountain. Sci. Rep. 2020, 10, 12442. [Google Scholar] [CrossRef]
- Cao, R.; Wu, F.Z.; Yang, W.Q.; Xu, Z.F.; Tani, B.; Wang, B.; Li, J.; Chang, C.H. Effects of altitudes on soil microbial biomass and enzyme activity in alpine-gorge regions. J. Appl. Ecol. 2016, 27, 1257–1264. [Google Scholar]
- Siles, J.A.; Cajthaml, T.; Minerbi, S.; Margesin, R. Effect of altitude and season on microbial activity, abundance and community structure in Alpine forest soils. FEMS Microbiol. Ecol. 2016, 92, fiw008. [Google Scholar] [CrossRef]
- Xue, H.M.; Ma, F.; Song, Y.Q. Mafic-ultramafic rocks from the Fanjingshan region, southwestern margin of the Jiangnan orogenic belt: Ages, geochemical characteristics and tectonic setting. Acta Petrol. Sin. 2012, 28, 3015–3030. [Google Scholar]
- Gao, L.Z.; Chen, J.S.; Dai, C.G.; Ding, X.Z.; Wang, X.H.; Liu, Y.X.; Wang, M.; Zhang, H. SHRIMP zircon U-Pb dating of tuff in Fan-jingshan Group and Xiajiang Group from Guizhou and Hunan Provinces and its stratigraphic implications. Geol. Bull. China 2014, 33, 949–959. [Google Scholar]
- Shi, L.; Li, H.B. Fanjingshan: The best protected virgin forest at the same latitude. For. Hum. 2015, 9, 71–73. [Google Scholar]
- Gao, Y.R. Study on Species Diversity of Leafhopper and Its Relationship with Host Plants in Fanjing Mountain; Guizhou Normal University: Guiyang, China, 2019. [Google Scholar]
- Shang, K.; Shi, L.; Li, H.B.; Yao, L.M.; Zhou, G.Y.; Jiang, L. Diversity of Arbuscular Mycorrhizal Fungi in Different Heights of Fanjingshan Mountain. J. Northeast. For. Univ. 2020, 48, 76–80. [Google Scholar]
- The Group of the Scientific Survey of the Fanjingshan Mountain. Scientific Survey of the Fanjingshan Mountain Preserve; Guizhou Environmental Protection Bureau: Guiyang, China, 1982. [Google Scholar]
- Xiao, Z.; Sommar, J.; Lindqvist, O.; Tan, H.; He, J. Atmospheric mercury deposition on Fanjing mountain nature reserve. Guizhou, China. Chemosphere 1998, 36, 2191–2200. [Google Scholar] [CrossRef]
- Kandeler, E.; Gerber, H. Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol. Fertil. Soils 1988, 6, 68–72. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [PubMed]
- Gao, M.; Song, W.; Zhou, Q.; Ma, X.; Chen, X. Interactive effect of oxytetracycline and lead on soil enzymatic activity and microbial biomass. Environ. Toxicol. Pharmacol. 2013, 36, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Guan, S.Y. Soil Enzymes and Their Research Methods; Agricultural Press: Beijing, China, 1986; pp. 294–302. [Google Scholar]
- Xie, Y.G.; Liao, X.F.; Liu, J.M.; Chen, J.Z. A Case Study Demonstrates That the Littler of the Rare Species Cinnamomum migao Composed of Different Tissues Can Affect the Chemical Properties and Microbial Community Diversity in Topsoil. Microorganisms 2022, 10, 1125. [Google Scholar] [CrossRef] [PubMed]
- Bokulich, N.A.; Maldonado, J.; Kang, D.W.; Krajmalnik-Brown, R.; Caporaso, J.G. Rapidly processed stool swabs approximate stool microbiota profiles. Msphere 2019, 4, e00208-19. [Google Scholar] [CrossRef] [PubMed]
- Brockett, B.F.; Prescott, C.E.; Grayston, S.J. Soil moisture is the major factor influencing microbial community structure and enzyme activities across seven biogeoclimatic zones in western Canada. Soil Biol. Biochem. 2012, 44, 9–20. [Google Scholar] [CrossRef]
- Garcıa-Gil, J.C.; Plaza, C.; Soler-Rovira, P.; Polo, A. Long-term effects of municipal solid waste compost application on soil enzyme activities and microbial biomass. Soil Biol. Biochem. 2000, 32, 1907–1913. [Google Scholar] [CrossRef]
- Jang, I.Y.; Kang, H.J. Controlling environmental factors of soil enzyme activities at three altitudes on Mt. Jumbong. J. Ecol. Field Biol. 2010, 33, 223–228. [Google Scholar] [CrossRef]
- Gomez, E.J.; Delgado, J.A.; Gonzalez, J.M. Environmental factors affect the response of microbial extracellular enzyme activity in soils when determined as a function of water availability and temperature. Ecol. Evol. 2020, 10, 10105–10115. [Google Scholar] [CrossRef]
- Smith, A.P.; Marín-Spiotta, E.; Balser, T. Successional and seasonal variations in soil and litter microbial community structure and function during tropical postagricultural forest regeneration: A multiyear study. Glob. Change Biol. 2015, 21, 3532–3547. [Google Scholar] [CrossRef]
- Peay, K.G.; von Sperber, C.; Cardarelli, E.; Toju, H.; Francis, C.A.; Chadwick, O.A.; Vitousek, P.M. Convergence and contrast in the community structure of Bacteria, Fungi and Archaea along a tropical elevation–climate gradient. FEMS Microbiol. Ecol. 2017, 93, fix045. [Google Scholar] [CrossRef]
- Shen, C.; Gunina, A.; Luo, Y.; Wang, J.; He, J.Z.; Kuzyakov, Y.; Hemp, A.; Classen, A.T.; Ge, Y. Contrasting patterns and drivers of soil bacterial and fungal diversity across a mountain gradient. Environ. Microbiol. 2020, 22, 3287–3301. [Google Scholar] [CrossRef]
- Bahram, M.; Hildebrand, F.; Forslund, S.K.; Anderson, J.L.; Soudzilovskaia, N.A.; Bodegom, P.M.; Bengtsson-Palme, J.; Anslan, S.; Coelho, L.P.; Harend, H.; et al. Structure and function of the global topsoil microbiome. Nature 2018, 560, 233–237. [Google Scholar] [CrossRef]
- Kalam, S.; Anirban, B.A.S.U.; Podile, A.R. Difficult-to-culture bacteria in the rhizosphere: The underexplored signature microbial groups. Pedosphere 2022, 32, 75–89. [Google Scholar] [CrossRef]
- del Barrio-Duque, A.; Samad, A.; Nybroe, O.; Antonielli, L.; Sessitsch, A.; Compant, S. Interaction between endophytic Proteobacteria strains and Serendipita indica enhances biocontrol activity against fungal pathogens. Plant Soil 2020, 451, 277–305. [Google Scholar] [CrossRef]
- Liu, L.; Zhu, K.; Krause, S.M.; Li, S.; Wang, X.; Zhang, Z.; Shen, M.; Yang, Q.; Lian, J.; Wang, X.; et al. Changes in assembly processes of soil microbial communities during secondary succession in two subtropical forests. Soil Biol. Biochem. 2021, 154, 108144. [Google Scholar] [CrossRef]
- Lavergne, S.; Molina, J.; Debussche, M. Fingerprints of environmental change on the rare mediterranean flora: A 115-year study. Glob. Change Biol. 2010, 12, 1466–1478. [Google Scholar] [CrossRef]
- Hou, Q.; Wang, W.; Yang, Y.; Hu, J.; Bian, C.; Jin, L.; Li, G.; Xiong, X. Rhizosphere microbial diversity and community dynamics during potato cultivation. Eur. J. Soil Biol. 2020, 98, 103176. [Google Scholar] [CrossRef]
- Shen, C.; Xiong, J.; Zhang, H.; Feng, Y.; Lin, X.; Li, X.; Liang, W.; Chu, H. Soil pH drives the spatial distribution of bacterial communities along elevation on Changbai Mountain. Soil Biol. Biochem. 2013, 57, 204–211. [Google Scholar] [CrossRef]
- Luo, S.P.; He, B.H.; Zeng, Q.P.; Li, N.J.; Yang, L. Effects of seasonal variation on soil microbial community structure and enzyme activity in a Masson pine forest in Southwest China. J. Mt. Sci. 2020, 17, 1398–1409. [Google Scholar] [CrossRef]
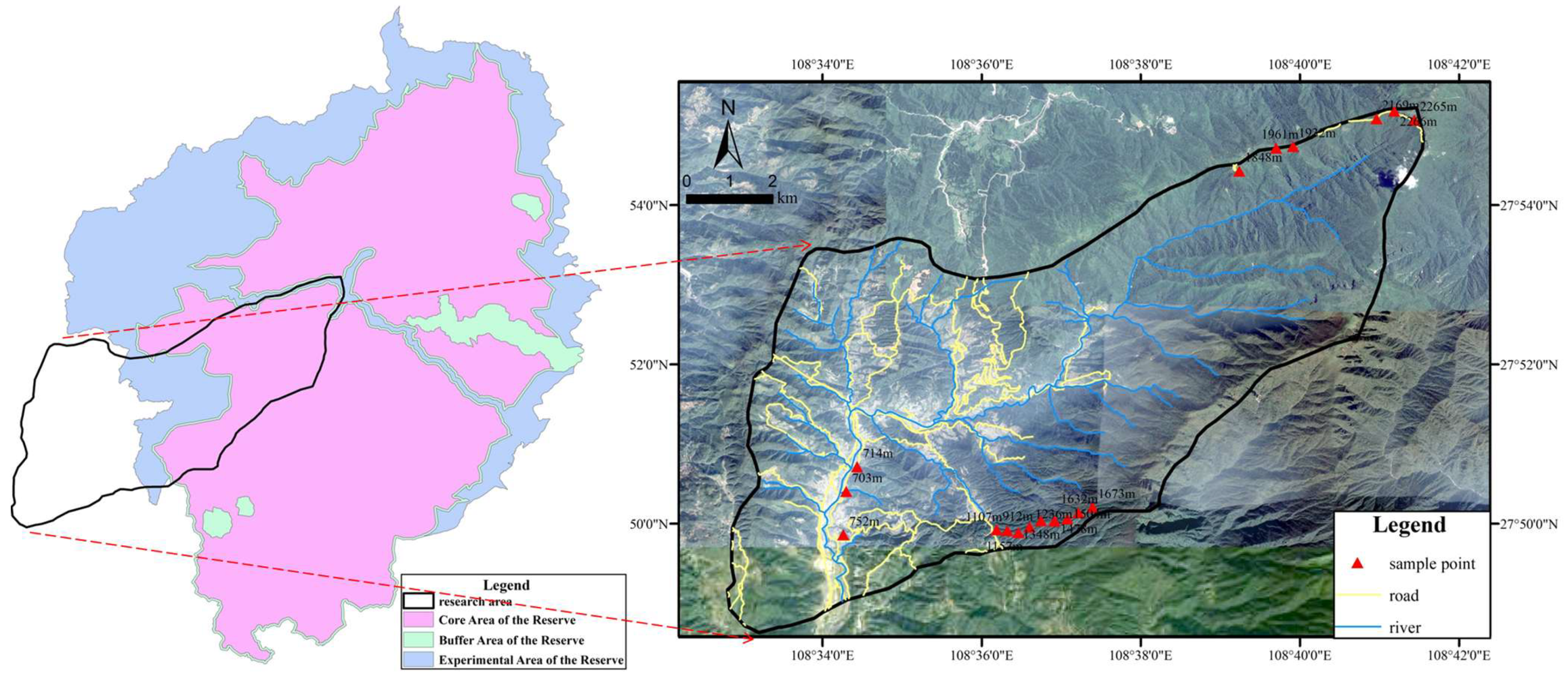
| No. | Latitude | Longitude | Altitude (m) | Slope (°) | Slope Position | Soil Type |
|---|---|---|---|---|---|---|
| A900-① | 27°50′24.1″ | 108°34′17.9″ | 703 | 50 | downhill | yellow soil |
| A900-② | 27°49′51.5″ | 108°34′15.7″ | 752 | 40 | downhill | yellow soil |
| A900-③ | 27°50′42.8″ | 108°34′26″ | 714 | 40 | downhill | yellow soil |
| B1200-① | 27°49′55.3″ | 108°36′11.4″ | 1107 | 30 | downhill | yellow soil |
| B1200-② | 27°51′42.1″ | 108°37′3.3″ | 912 | 30 | downhill | yellow soil |
| B1200-③ | 27°49′53.2″ | 108°36′27.8″ | 1157 | 20 | mid-slope | yellow soil |
| C1500-① | 27°49′57.4″ | 108°36′36.2″ | 1236 | 35 | mid-slope | yellow soil |
| C1500-② | 27°50′2.1″ | 108°36′44.7″ | 1348 | 30 | uphill | yellow soil |
| C1500-③ | 27°50′2.1″ | 108°36′55.5″ | 1478 | 35 | top of the slope | yellow soil |
| D1800-① | 27°50′35″ | 108°37′4.7″ | 1504 | 35 | mid-slope | dark yellow-brown soil |
| D1800-② | 27°50′8.1″ | 108°37′13.0″ | 1632 | 40 | mid-slope | dark yellow-brown soil |
| D1800-③ | 27°50′12.3″ | 108°37′23.6″ | 1673 | 40 | base of the slope | dark yellow-brown soil |
| E2100-① | 27°54′25.6″ | 108°39′14.4″ | 1848 | 50 | uphill | dark yellow-brown soil |
| E2100-② | 27°54′43.0″ | 108°39′42.1″ | 1961 | 75 | uphill | dark mountain dwarf forest soil |
| E2100-③ | 27°54′44.2″ | 108°39′54.9″ | 1932 | 60 | uphill | dark mountain dwarf forest soil |
| F2400-① | 27°55′5.0″ | 108°40′57.9″ | 2169 | 45 | uphill | dark mountain dwarf forest soil |
| F2400-② | 27°55′10.9″ | 108°41′11.1″ | 2266 | 35 | top of the slope | mountainous scrub meadow soil |
| F2400-③ | 27°55′4.0″ | 108°41′26.3″ | 2265 | 40 | top of the slope | mountainous scrub meadow soil |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Y.; Zhang, L.; Wang, J.; Chen, M.; Liu, J.; Xiao, S.; Tian, X.; Wu, T. Spatial Heterogeneity of Soil Bacterial Community Structure and Enzyme Activity along an Altitude Gradient in the Fanjingshan Area, Northeastern Guizhou Province, China. Life 2022, 12, 1862. https://doi.org/10.3390/life12111862
Xie Y, Zhang L, Wang J, Chen M, Liu J, Xiao S, Tian X, Wu T. Spatial Heterogeneity of Soil Bacterial Community Structure and Enzyme Activity along an Altitude Gradient in the Fanjingshan Area, Northeastern Guizhou Province, China. Life. 2022; 12(11):1862. https://doi.org/10.3390/life12111862
Chicago/Turabian StyleXie, Yuangui, Lanyue Zhang, Juncai Wang, Meng Chen, Jiming Liu, Shengyang Xiao, Xiu Tian, and Tingting Wu. 2022. "Spatial Heterogeneity of Soil Bacterial Community Structure and Enzyme Activity along an Altitude Gradient in the Fanjingshan Area, Northeastern Guizhou Province, China" Life 12, no. 11: 1862. https://doi.org/10.3390/life12111862
APA StyleXie, Y., Zhang, L., Wang, J., Chen, M., Liu, J., Xiao, S., Tian, X., & Wu, T. (2022). Spatial Heterogeneity of Soil Bacterial Community Structure and Enzyme Activity along an Altitude Gradient in the Fanjingshan Area, Northeastern Guizhou Province, China. Life, 12(11), 1862. https://doi.org/10.3390/life12111862








