Wound Infections in Adult Patients after Berlin Heart® EXCOR Biventricular Assist Device Implantation
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
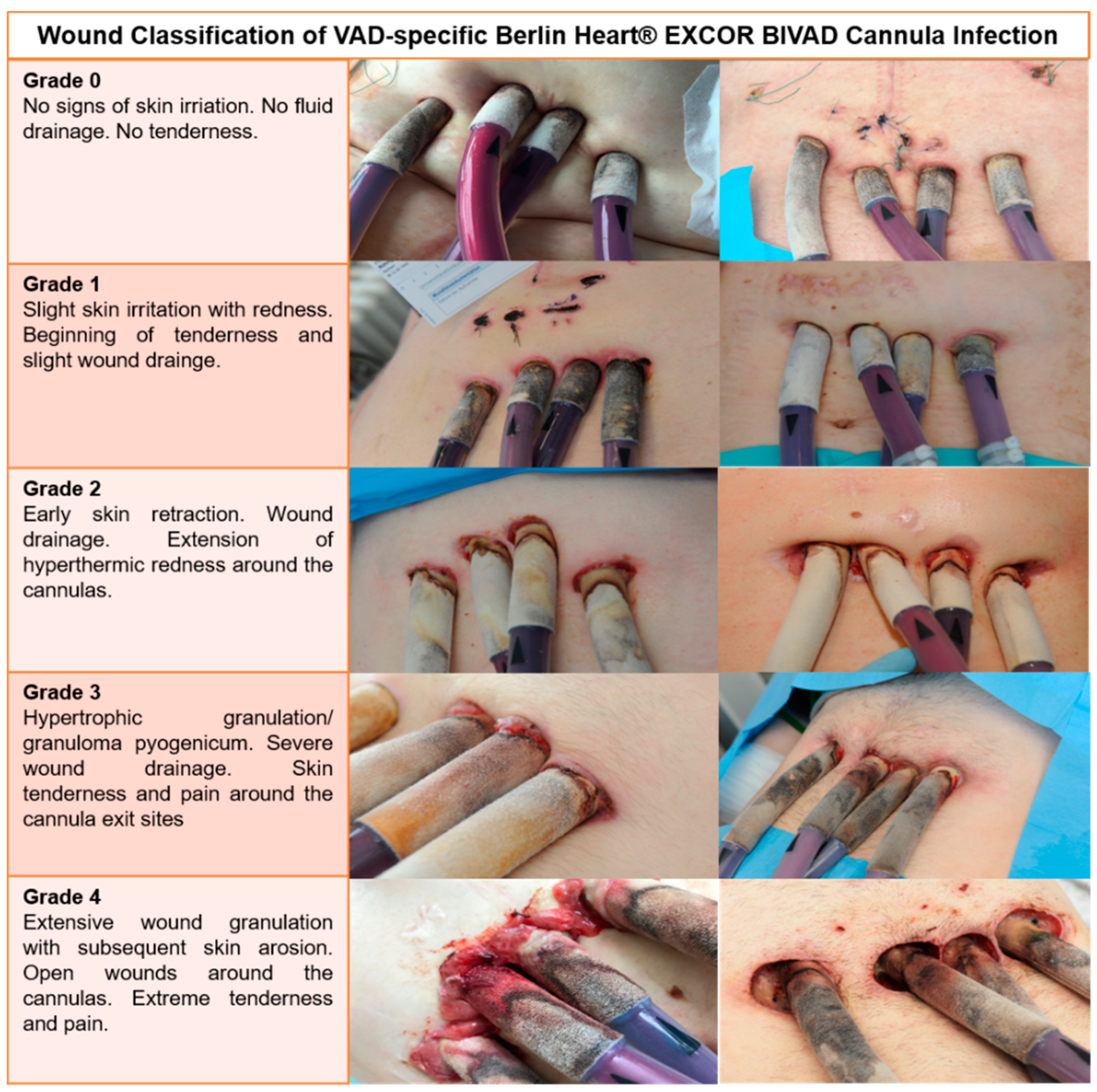
2.1. Definitions
2.2. Statistics
3. Results
3.1. Patient Characteristics
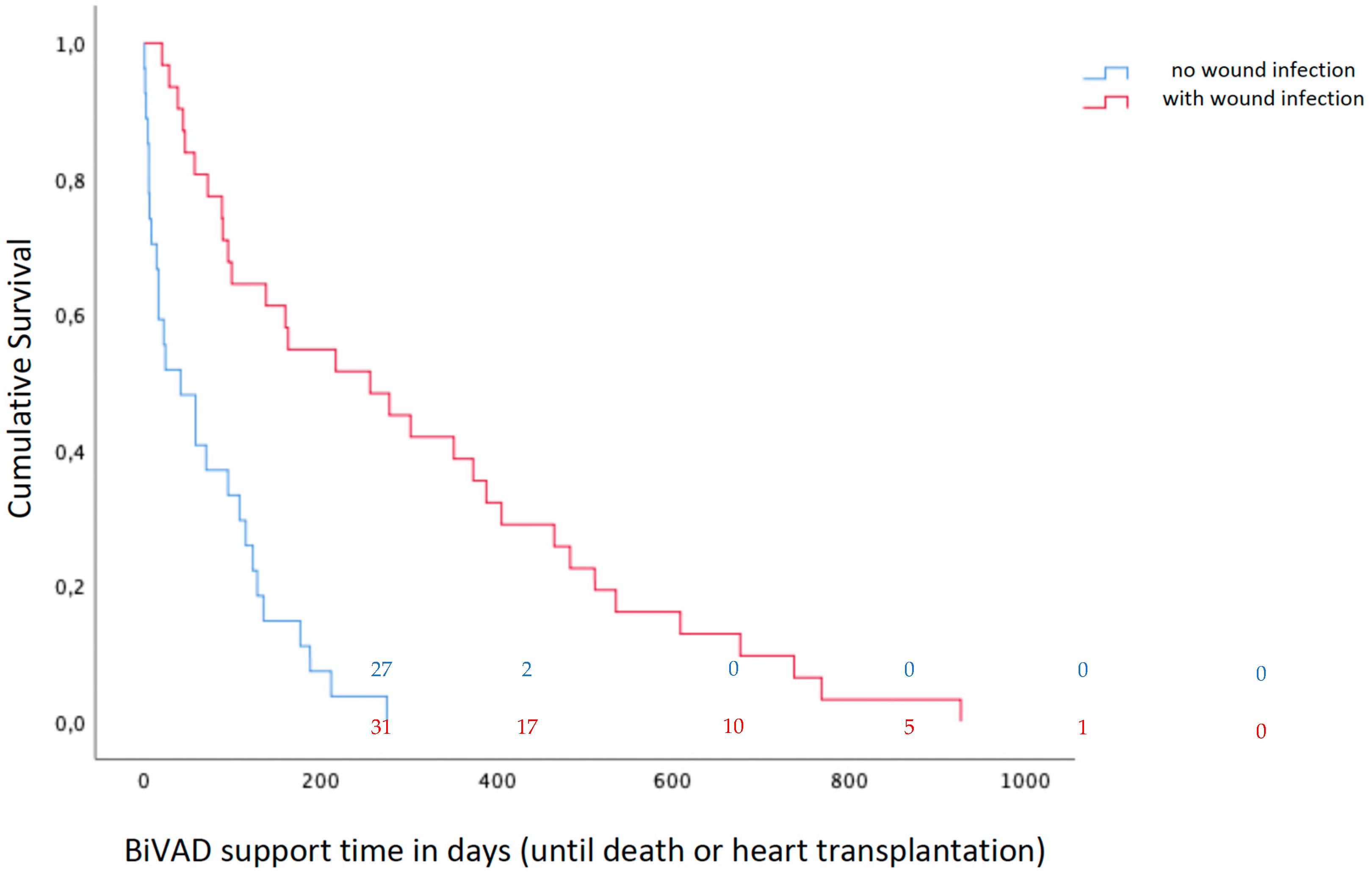
3.2. Overall Clinical Outcome after BiVAD Implantation
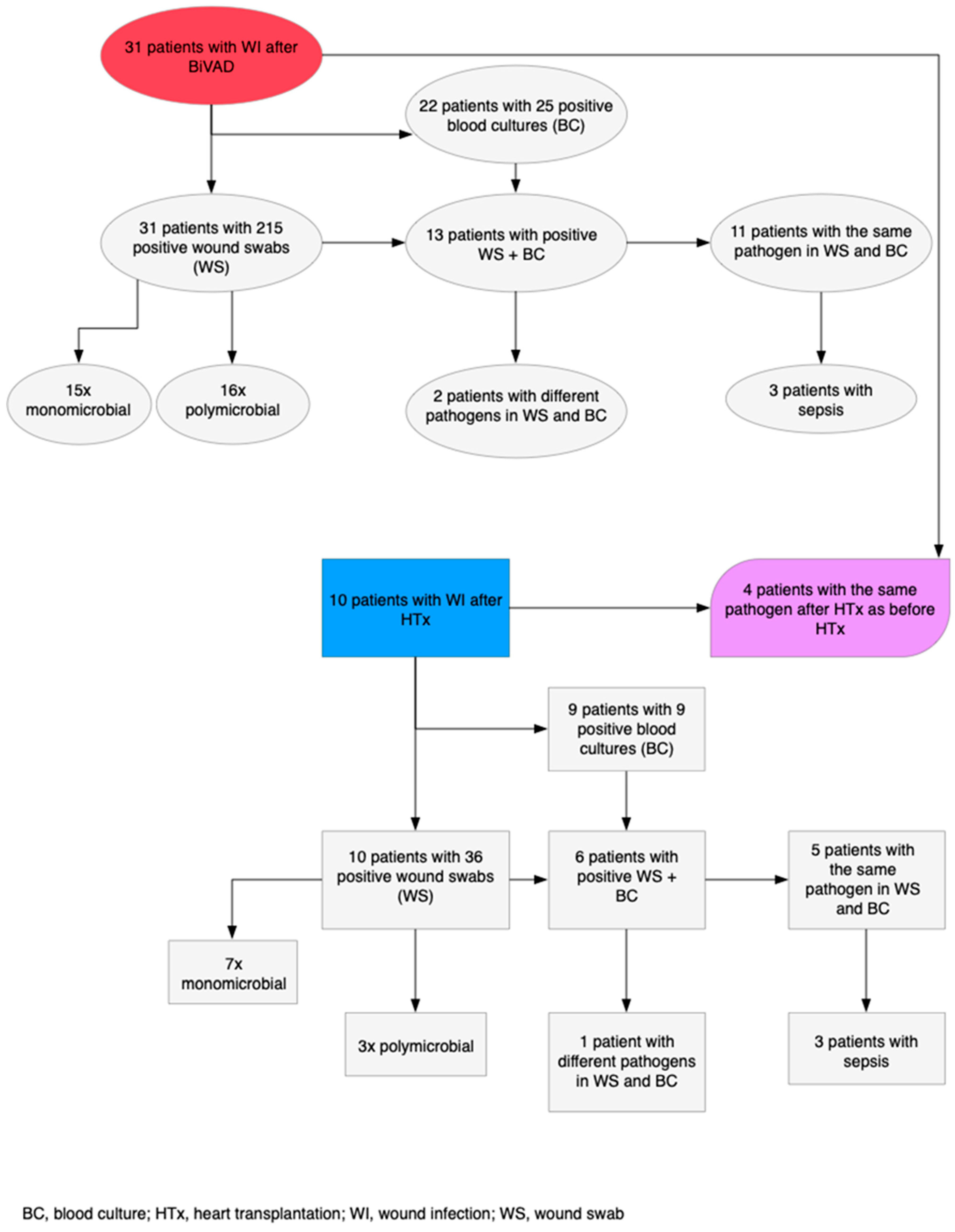
3.3. Infections Post-BiVAD
3.3.1. ISHLT Criteria
3.3.2. CDC Classification
3.4. Wound Infections Post-HTx
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Terzi, A. Mechanical circulatory support: 60 years of evolving knowledge. Int. J. Artif. Organs 2019, 42, 215–225. [Google Scholar] [CrossRef]
- Patel, S.R.; Saeed, O.; Naftel, D.; Myers, S.; Kirklin, J.; Jorde, U.P.; Goldstein, D.J. Outcomes of Restrictive and Hypertrophic Cardiomyopathies After LVAD: An INTERMACS Analysis. J. Card. Fail. 2017, 23, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Ozbaran, M.; Yagdi, T.; Engin, C.; Erkul, S.; Balcioglu, O.; Baysal, B.; Nalbantgil, S.; Ertugay, S. Long-term paracorporeal ventricular support systems: A single-center experience. Transplant. Proc. 2013, 45, 1013–1016. [Google Scholar] [CrossRef] [PubMed]
- Schmack, B.; Weymann, A.; Ruschitzka, F.; Autschbach, R.; Raake, P.W.; Jurrmann, N.; Menon, A.K.; Karck, M.; Wilhelm, M.J.; Ruhparwar, A. Successful support of biventricular heart failure patients by new EXCOR(®) Adult pumps with bileaflet valves: A prospective study. Clin. Res. Cardiol. 2018, 107, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Kremer, J.; El-Dor, A.; Sommer, W.; Tochtermann, U.; Warnecke, G.; Karck, M.; Ruhparwar, A.; Meyer, A.L. Long-term Paracorporeal Pulsatile Mechanical Circulatory Support in Adolescent and Adult Patients. Interact Cardiovasc. Thorac. Surg. 2022. [Google Scholar] [CrossRef] [PubMed]
- Hetzer, R.; Kaufmann, F.; Walter, E.M.D. Mechanische Herzunterstützungssysteme. In Medizintechnik: Verfahren–Systeme–Informationsverarbeitung; Kramme, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1–23. [Google Scholar] [CrossRef]
- Bartfay, S.E.; Dellgren, G.; Hallhagen, S.; Wahlander, H.; Dahlberg, P.; Redfors, B.; Ekelund, J.; Karason, K. Durable circulatory support with a paracorporeal device as an option for pediatric and adult heart failure patients. J. Thorac. Cardiovasc. Surg. 2021, 161, 1453–1464.e1454. [Google Scholar] [CrossRef]
- McGiffin, D.; Kure, C.; McLean, J.; Marasco, S.; Bergin, P.; Hare, J.L.; Leet, A.; Patel, H.; Zimmet, A.; Rix, J.; et al. The results of a single-center experience with HeartMate 3 in a biventricular configuration. J. Heart Lung Transplant. 2021, 40, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Kirklin, J.K.; Pagani, F.D.; Kormos, R.L.; Stevenson, L.W.; Blume, E.D.; Myers, S.L.; Miller, M.A.; Baldwin, J.T.; Young, J.B.; Naftel, D.C. Eighth annual INTERMACS report: Special focus on framing the impact of adverse events. J. Heart Lung Transplant. 2017, 36, 1080–1086. [Google Scholar] [CrossRef]
- Gordon, R.J.; Quagliarello, B.; Lowy, F.D. Ventricular assist device-related infections. Lancet Infect. Dis. 2006, 6, 426–437. [Google Scholar] [CrossRef]
- Munoz, P.; Valerio, M.; Vasquez, V.; Velasquez-Rodriguez, J.; Sousa, I.; Zatarain, E.; Barrio, J.M.; Ruiz, M.; Cuerpo, G.; Rodriguez-Abella, H.; et al. Infections in patients after Berlin Heart((R)) EXCOR assist device implantation. Transpl. Infect. Dis. 2018, 20, e12936. [Google Scholar] [CrossRef]
- Auerbach, S.R.; Richmond, M.E.; Schumacher, K.R.; Lopez-Colon, D.; Mitchell, M.B.; Turrentine, M.W.; Cantor, R.S.; Niebler, R.A.; Eghtesady, P. Infectious complications of ventricular assist device use in children in the United States: Data from the Pediatric Interagency Registry for Mechanical Circulatory Support (Pedimacs). J. Heart Lung Transplant. 2018, 37, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Hannan, M.M.; Xie, R.; Cowger, J.; Schueler, S.; de By, T.; Dipchand, A.I.; Chu, V.H.; Cantor, R.S.; Koval, C.E.; Krabatsch, T.; et al. Epidemiology of infection in mechanical circulatory support: A global analysis from the ISHLT Mechanically Assisted Circulatory Support Registry. J. Heart Lung Transplant. 2019, 38, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, A.G.; Khan, M.S.; Morales, D.L.; Chen, D.W.; Moffett, B.S.; Price, J.F.; Dreyer, W.J.; Denfield, S.W.; Jeewa, A.; Fraser, C.D., Jr.; et al. Infectious complications and outcomes in children supported with left ventricular assist devices. J. Heart Lung Transplant. 2013, 32, 518–524. [Google Scholar] [CrossRef]
- Hannan, M.M.; Husain, S.; Mattner, F.; Danziger-Isakov, L.; Drew, R.J.; Corey, G.R.; Schueler, S.; Holman, W.L.; Lawler, L.P.; Gordon, S.M.; et al. Working formulation for the standardization of definitions of infections in patients using ventricular assist devices. J. Heart Lung Transplant. 2011, 30, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Garner, J.S.; Jarvis, W.R.; Emori, T.G.; Horan, T.C.; Hughes, J.M. CDC definitions for nosocomial infections, 1988. Am. J. Infect. Control 1988, 16, 128–140. [Google Scholar] [CrossRef]
- Stehlik, J.; Kobashigawa, J.; Hunt, S.A.; Reichenspurner, H.; Kirklin, J.K. Honoring 50 Years of Clinical Heart Transplantation in Circulation: In-Depth State-of-the-Art Review. Circulation 2018, 137, 71–87. [Google Scholar] [CrossRef]
- Hetzer, R.; Kaufmann, F.; Delmo Walter, E.M. Paediatric mechanical circulatory support with Berlin Heart EXCOR: Development and outcome of a 23-year experience. Eur. J. Cardiothorac. Surg. 2016, 50, 203–210. [Google Scholar] [CrossRef]
- Sandica, E.; Blanz, U.; Mime, L.B.; Schultz-Kaizler, U.; Kececioglu, D.; Haas, N.; Kirchner, G.; zu Knyphausen, E.; Lauenroth, V.; Morshuis, M. Long-Term Mechanical Circulatory Support in Pediatric Patients. Artif. Organs 2016, 40, 225–232. [Google Scholar] [CrossRef]
- Michel, S.; Buchholz, S.; Buech, J.; Veit, T.; Fabry, T.; Abicht, J.; Thierfelder, N.; Mueller, C.; Rosenthal, L.L.; Pabst von Ohain, J.; et al. Bridging patients in cardiogenic shock with a paracorporeal pulsatile biventricular assist device to heart transplantation-a single-centre experience. Eur. J. Cardiothorac. Surg. 2022. [Google Scholar] [CrossRef]
- Tan, Z.; Zeng, L.A. Post-operative infection in mechanical circulatory support patients. Ann. Transl. Med. 2020, 8, 831. [Google Scholar] [CrossRef] [PubMed]
- Simon, D.; Fischer, S.; Grossman, A.; Downer, C.; Hota, B.; Heroux, A.; Trenholme, G. Left ventricular assist device-related infection: Treatment and outcome. Clin. Infect. Dis. 2005, 40, 1108–1115. [Google Scholar] [CrossRef]
- Engin, C.; Ayik, F.; Oguz, E.; Eygi, B.; Yagdi, T.; Karakula, S.; Ozbaran, M. Ventricular assist device as a bridge to heart transplantation in adults. Transplant. Proc. 2011, 43, 927–930. [Google Scholar] [CrossRef]
| Characteristics | With Wound Infection (n = 31) | Without Wound Infection (n = 27) | p-Value |
|---|---|---|---|
| Age years | 47 (34–55) | 49 (34–53) | 0.668 |
| Male sex | 28 (90.3%) | 20 (74.1%) | 0.105 |
| Body mass index, kg/m2 | 26.9 (21.3–30.9) | 27.0 (22.4–33.2) | 0.725 |
| Body surface area, m2 | 2.02 (1.85–2.2) | 1.97 (1.77–2.08) | 0.171 |
| Time of BiVAD | 0.027 | ||
| 2006–2014 | 14 (45.2%) | 20 (74.1%) | |
| 2015–2020 | 17 (54.8%) | 7 (25.9%) | |
| INTERMACS Profile | 0.585 | ||
| 1 | 20 (64.5%) | 20 (74.1%) | |
| 2 | 8 (25.8%) | 3 (11.1%) | |
| 3 | 3 (9.7%) | 4 (14.8%) | |
| CMP aetiology | 0.928 | ||
| Non-ischemic | 21 (67.7%) | 15 (55.6%) | |
| Ischemic | 6 (19.4%) | 7 (25.9%) | |
| Myocarditis | 4 (12.9%) | 5 (18.5%) | |
| Comorbidities | |||
| Dialysis pre-BiVAD | 9 (29.0%) | 9 (33.3%) | 0.726 |
| Diabetes mellitus | 10 (32.3%) | 7 (25.9%) | 0.668 |
| Hyperlipidaemia | 8 (25.8) | 7 (25.9%) | 0.992 |
| Arterial hypertension | 12 (38.7%) | 11 (40.7%) | 0.876 |
| Pulmonary hypertension | 17 (54.8%) | 14 (51.9%) | 0.242 |
| Previous cardiac surgery | 4 (12.9%) | 7 (25.9%) | 0.211 |
| Pre-operative support | |||
| IABP | 7 (22.6%) | 8 (29.6%) | 0.544 |
| ECLS | 14 (45.2%) | 15 (55.6%) | 0.434 |
| Urgency of BiVAD | 0.387 | ||
| Elective | 4 (12.9%) | 2 (7.4%) | |
| Urgent | 16 (51.6%) | 12 (44.4%) | |
| Emergency | 7 (22.6%) | 10 (37.0%) | |
| Ultima ratio/reanimation | 4 (12.9%) | 3 (11.1%) |
| With Wound Infection (n = 31) | Without Wound Infection (n = 27) | p-Value | |
|---|---|---|---|
| Cardiopulmonary bypass time minutes | 217.70 (138–336) | 221.23 (98–494) | 0.532 |
| Aortic cross-clamp time minutes | 33.97 (0–291) | 27.23 (0–215) | 0.869 |
| Concomitant cardiac surgery after BiVAD implantation | 2 (6.5%) | 2 (7.4%) | 0.887 |
| Gortex membrane | 23 (74.2%) | 15 (55.6%) | 0.140 |
| Minimal body temperature °C | 35.23 (32–37) | 34.41 (28–36.8) | 0.084 |
| Packed red blood cells units | 9.29 ± 6.43 | 10.93 ± 7.85 | 0.462 |
| Fresh frozen plasma units | 5.24 ± 6.11 | 5.47 ± 5.93 | 0.842 |
| Surgery time minutes | 421.32 (273–640) | 411.00 (145–980) | 0.322 |
| B | SE | Wald | df | Sig. | Exp(B) | 95.0% CI for Exp(B) | ||
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| Time of BiVAD | 0.881 | 0.405 | 4.741 | 1 | 0.029 | 2.413 | 1.092 | 5.334 |
| Gortex membrane | 1.003 | 0.469 | 4.572 | 1 | 0.033 | 2.726 | 1.087 | 6.833 |
| Superficial Wound Infection (n = 13) | Deep Wound Infection (n = 18) | p-Value | |
|---|---|---|---|
| Polymicrobial | 4 | 12 | 0.052 |
| Monomicrobial | 9 | 6 | 0.052 |
| Gram-positive | 5 | 13 | 0.064 |
| S. aureus | 4 | 5 | 0.859 |
| Enterococcus spec. | 1 | 8 | 0.029 |
| Gram-negative | 2 | 10 | 0.026 |
| P. aeruginosa | 0 | 4 | 0.073 |
| Candida spec. | 4 | 5 | 0.859 |
| Others * | 7 | 15 | 0.079 |
| Pathogen (VAD-R) | Number of Patients | Pathogen (N-VAD) | Number of Patients |
|---|---|---|---|
| Gram-positive | 5 | Gram-positive | 4 |
| Enterococcus spec. | 1 | Enterococcus spec. | 4 |
| Coagulase-negative Staphylococcus | 4 | ||
| Gram-negative | 4 | Gram-negative | 14 |
| P. aeruginosa | 3 | P. aeruginosa | 4 |
| Candida spec. | 4 | Candida spec. | 6 |
| Clostridium difficile | 7 | ||
| Herpes simplex virus | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kremer, J.; El-Dor, A.; Rivinius, R.; Schlegel, P.; Sommer, W.; Warnecke, G.; Karck, M.; Ruhparwar, A.; Meyer, A.L. Wound Infections in Adult Patients after Berlin Heart® EXCOR Biventricular Assist Device Implantation. Life 2022, 12, 1550. https://doi.org/10.3390/life12101550
Kremer J, El-Dor A, Rivinius R, Schlegel P, Sommer W, Warnecke G, Karck M, Ruhparwar A, Meyer AL. Wound Infections in Adult Patients after Berlin Heart® EXCOR Biventricular Assist Device Implantation. Life. 2022; 12(10):1550. https://doi.org/10.3390/life12101550
Chicago/Turabian StyleKremer, Jamila, Abbas El-Dor, Rasmus Rivinius, Philipp Schlegel, Wiebke Sommer, Gregor Warnecke, Matthias Karck, Arjang Ruhparwar, and Anna L. Meyer. 2022. "Wound Infections in Adult Patients after Berlin Heart® EXCOR Biventricular Assist Device Implantation" Life 12, no. 10: 1550. https://doi.org/10.3390/life12101550
APA StyleKremer, J., El-Dor, A., Rivinius, R., Schlegel, P., Sommer, W., Warnecke, G., Karck, M., Ruhparwar, A., & Meyer, A. L. (2022). Wound Infections in Adult Patients after Berlin Heart® EXCOR Biventricular Assist Device Implantation. Life, 12(10), 1550. https://doi.org/10.3390/life12101550






