Why Iron Deficiency in Acute Heart Failure Should Be Treated: A Real-World Clinical Practice Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study and Patient Cohort
2.2. Statistical Analysis
3. Results
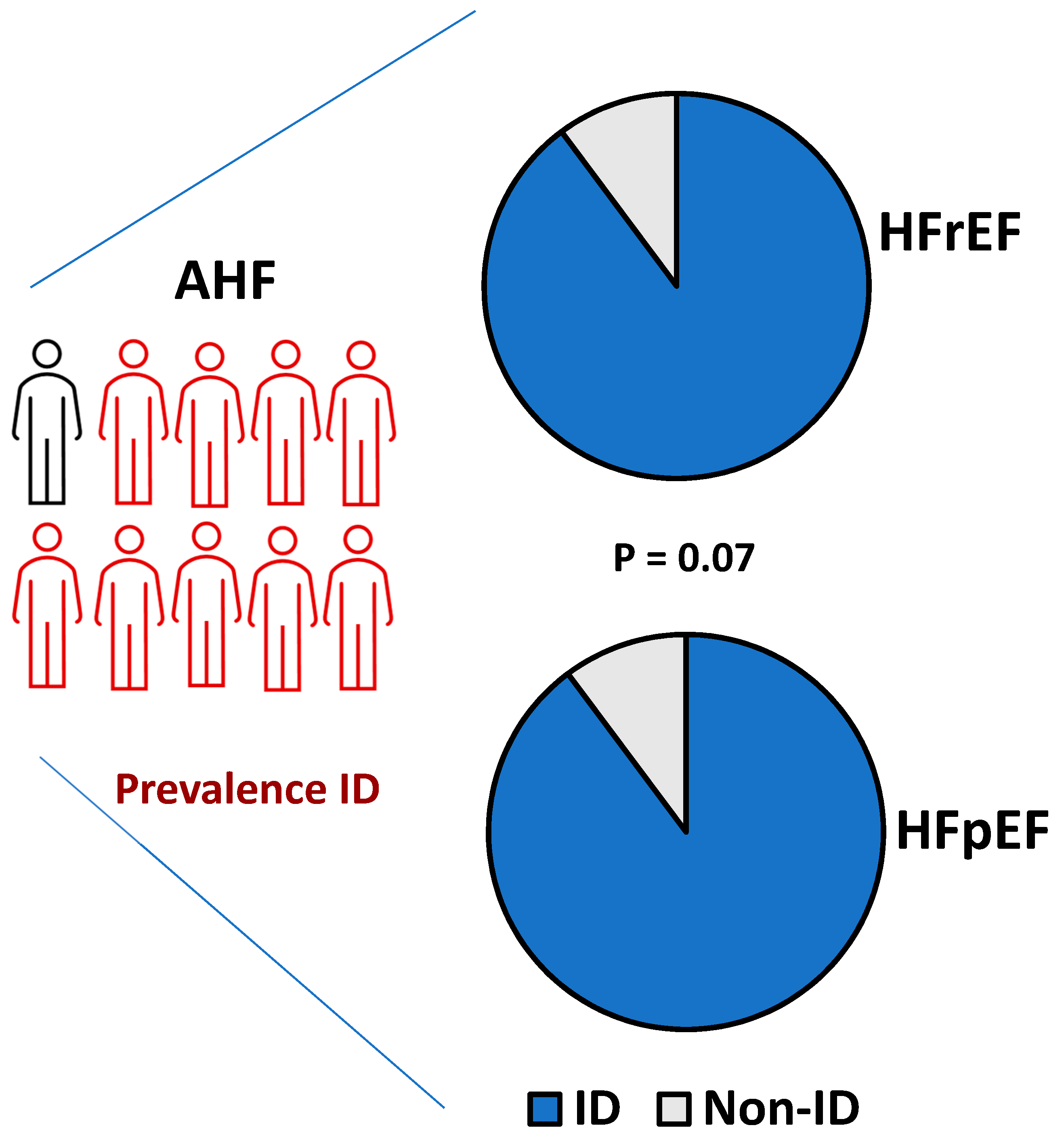
3.1. Prevalence and Clinical Profile of Patients
3.2. Effect of FCM Treatment on Morbidity and Mortality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Snipelisky, D.; Chaudhry, S.-P.; Stewart, G.C. The Many Faces of Heart Failure. Card. Electrophysiol. Clin. 2019, 11, 11–20. [Google Scholar] [CrossRef]
- Dharmarajan, K.; Rich, M.W. Epidemiology, Pathophysiology, and Prognosis of Heart Failure in Older Adults. Heart Fail. Clin. 2017, 13, 417–426. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [PubMed]
- Christiansen, M.N.; Køber, L.; Torp-Pedersen, C.; Gislason, G.H.; Schou, M.; Smith, J.G.; Vasan, R.S.; Andersson, C. Preheart failure comorbidities and impact on prognosis in heart failure patients: A nationwide study. J. Intern. Med. 2020, 287, 698–710. [Google Scholar] [CrossRef]
- Metra, M.; Zacà, V.; Parati, G.; Agostoni, P.; Bonadies, M.; Ciccone, M.; Cas, A.D.; Iacoviello, M.; Lagioia, R.; Lombardi, C.; et al. Cardiovascular and noncardiovascular comorbidities in patients with chronic heart failure. J. Cardiovasc. Med. 2011, 12, 76–84. [Google Scholar] [CrossRef]
- Anker, S.D.; Comin Colet, J.; Filippatos, G.; Willenheimer, R.; Dickstein, K.; Drexler, H.; Lüscher, T.F.; Bart, B.; Banasiak, W.; Niegowska, J.; et al. Ferric Carboxymaltose in Patients with Heart Failure and Iron Deficiency. N. Engl. J. Med. 2009, 361, 2436–2448. [Google Scholar] [CrossRef]
- Ponikowski, P.; Van Veldhuisen, D.J.; Comin-Colet, J.; Ertl, G.; Komajda, M.; Mareev, V.; McDonagh, T.; Parkhomenko, A.; Tavazzi, L.; Levesque, V.; et al. Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency. Eur. Heart J. 2015, 36, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Kirwan, B.-A.; van Veldhuisen, D.J.; Filippatos, G.; Comin-Colet, J.; Ruschitzka, F.; Lüscher, T.F.; Arutyunov, G.P.; Motro, M.; Mori, C.; et al. Effects of ferric carboxymaltose on hospitalisations and mortality rates in iron-deficient heart failure patients: An individual patient data meta-analysis. Eur. J. Heart Fail. 2018, 20, 125–133. [Google Scholar] [CrossRef]
- López-Vilella, R.; Lozano-Edo, S.; Arenas Martín, P.; Jover-Pastor, P.; Ezzitouny, M.; Sorolla Romero, J.; Almenar Bonet, L. Impact of intravenous ferric carboxymaltose on heart failure with preserved and reduced ejection fraction. ESC Heart Fail. 2021, 9, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Ponikowski, P.; Kirwan, B.-A.; Anker, S.D.; McDonagh, T.; Dorobantu, M.; Drozdz, J.; Fabien, V.; Filippatos, G.; Göhring, U.M.; Keren, A.; et al. Ferric carboxymaltose for iron deficiency at discharge after acute heart failure: A multicentre, double-blind, randomised, controlled trial. Lancet 2020, 396, 1895–1904. [Google Scholar] [CrossRef]
- McDonagh, T.; Damy, T.; Doehner, W.; Lam, C.S.; Sindone, A.; Van Der Meer, P.; Cohen-Solal, A.; Kindermann, I.; Manito, N.; Pfister, O.; et al. Screening, diagnosis and treatment of iron deficiency in chronic heart failure: Putting the 2016 European Society of Cardiology heart failure guidelines into clinical practice. Eur. J. Heart Fail. 2018, 20, 1664–1672. [Google Scholar] [CrossRef] [PubMed]
- Almenar-Bonet, L.; Blasco-Perio, M.; Laiz-Marro, L.; Camafort, M.; Buño-Soto, A.; Crespo-Leiro, M. Perfiles analíticos pre-configurados en insuficiencia cardiaca: Implementación y uso en el Sistema Nacional de Salud Español. Adv. Lab. Med. 2022, 3, 1–8. [Google Scholar] [CrossRef]
- Martin, R.C.; Lisi, D. Iron Deficiency in Heart Failure: Characteristics and Treatment. Curr. Geriatr. Rep. 2021, 10, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Beale, A.L.; Warren, J.L.; Roberts, N.; Meyer, P.; Townsend, N.P.; Kaye, D. Iron deficiency in heart failure with preserved ejection fraction: A systematic review and meta-analysis. Open Heart 2019, 6, e001012. [Google Scholar] [CrossRef] [PubMed]
- Yamani, N.; Ahmed, A.; Gosain, P.; Fatima, K.; Shaikh, A.T.; Qamar, H.; Shahid, I.; Arshad, M.S.; Almas, T.; Figueredo, V. Effect of iron supplementation in patients with heart failure and iron deficiency: A systematic review and meta-analysis. Int. J. Cardiol. Heart Vasc. 2021, 36, 100871. [Google Scholar] [CrossRef] [PubMed]
- Ismahel, H.; Ismahel, N. Iron replacement therapy in heart failure: A literature review. Egypt. Heart J. EHJ Off. Bull. Egypt. Soc. Cardiol. 2021, 73, 85. [Google Scholar] [CrossRef]
- Rizzo, C.; Carbonara, R.; Ruggieri, R.; Passantino, A.; Scrutinio, D. Iron Deficiency: A New Target for Patients With Heart Failure. Front. Cardiovasc. Med. 2021, 8, 709872. [Google Scholar] [CrossRef]
- Lewis, G.D.; Malhotra, R.; Hernandez, A.F.; McNulty, S.E.; Smith, A.; Felker, G.M. Effect of Oral Iron Repletion on Exercise Capacity in Patients With Heart Failure With Reduced Ejection Fraction and Iron Deficiency: The IRONOUT HF Randomized Clinical Trial. JAMA 2017, 317, 1958–1966. [Google Scholar] [CrossRef]
- Rocha, B.M.L.; Cunha, G.J.; Menezes Falcao, L.F. The Burden of Iron Deficiency in Heart Failure: Therapeutic Approach. J. Am. Coll. Cardiol. 2018, 71, 782–793. [Google Scholar] [CrossRef]
- Alcaide-Aldeano, A.; Garay, A.; Alcoberro, L.; Jiménez-Marrero, S.; Yun, S.; Tajes, M.; García-Romero, E.; Díez-López, C.; González-Costello, J.; Mateus-Porta, G.; et al. Iron Deficiency: Impact on Functional Capacity and Quality of Life in Heart Failure with Preserved Ejection Fraction. J. Clin. Med. 2020, 9, 1199. [Google Scholar] [CrossRef]
- Beale, A.; Carballo, D.; Stirnemann, J.; Garin, N.; Agoritsas, T.; Serratrice, J.; Kaye, D.; Meyer, P.; Carballo, S. Iron Deficiency in Acute Decompensated Heart Failure. J. Clin. Med. 2019, 8, 1569. [Google Scholar] [CrossRef] [PubMed]
- Fitzsimons, S.; Yeo, T.J.; Ling, L.H.; Sim, D.; Leong, K.T.G.; Yeo, P.S.D.; Ong, H.Y.; Jaufeerally, F.; Ng, T.P.; Poppe, K.; et al. Impact of change in iron status over time on clinical outcomes in heart failure according to ejection fraction phenotype. ESC Heart Fail. 2021, 8, 4572–4583. [Google Scholar] [CrossRef] [PubMed]
- Jankowska, E.A.; Von Haehling, S.; Anker, S.D.; Macdougall, I.C.; Ponikowski, P. Iron deficiency and heart failure: Diagnostic dilemmas and therapeutic perspectives. Eur. Heart J. 2013, 34, 816–829. [Google Scholar] [CrossRef]
- Yeo, T.J.; Yeo, P.S.D.; Hadi, F.A.; Cushway, T.; Lee, K.Y.; Yin, F.F.; Ching, A.; Li, R.; Loh, S.Y.; Lim, S.L.; et al. Single-dose intravenous iron in Southeast Asian heart failure patients: A pilot randomized placebo-controlled study (PRACTICE-ASIA-HF). ESC Heart Fail. 2018, 5, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Ponikowska, B.; Suchocki, T.; Paleczny, B.; Olesinska, M.; Powierza, S.; Borodulin-Nadzieja, L.; Reczuch, K.; von Haehling, S.; Doehner, W.; Anker, S.D.; et al. Iron Status and Survival in Diabetic Patients With Coronary Artery Disease. Diabetes Care 2013, 36, 4147–4156. [Google Scholar] [CrossRef] [PubMed]
- Núñez, J.; Miñana, G.; Cardells, I.; Palau, P.; Llàcer, P.; Fácila, L.; Almenar, L.; López-Lereu, M.P.; Monmeneu, J.V.; Amiguet, M.; et al. Noninvasive Imaging Estimation of Myocardial Iron Repletion Following Administration of Intravenous Iron: The Myocardial-IRON Trial. J. Am. Heart Assoc. 2020, 9, e014254. [Google Scholar] [CrossRef]
- Santas, E.; Miñana, G.; Cardells, I.; Palau, P.; Llàcer, P.; Fácila, L.; Almenar, L.; López-Lereu, M.P.; Monmeneu, J.V.; Sanchis, J.; et al. Short-term changes in left and right systolic function following ferric carboxymaltose: A substudy of the Myocardial-IRON trial. ESC Heart Fail. 2020, 7, 4222–4230. [Google Scholar] [CrossRef]
- van Veldhuisen, D.J.; Ponikowski, P.; van der Meer, P.; Metra, M.; Böhm, M.; Doletsky, A.; Cohen-Solal, A. Effect of Ferric Carboxymaltose on Exercise Capacity in Patients With Chronic Heart Failure and Iron Deficiency. Circulation 2017, 136, 1374–1383. [Google Scholar] [CrossRef]
- Martens, P.; Dupont, M.; Dauw, J.; Nijst, P.; Herbots, L.; Dendale, P.; Vandervoort, P.; Bruckers, L.; Tang, W.H.W.; Mullens, W. The effect of intravenous ferric carboxymaltose on cardiac reverse remodelling following cardiac resynchronization therapy—The IRON-CRT trial. Eur. Heart J. 2021, 42, 4905–4914. [Google Scholar] [CrossRef]
- Dalal, J.; Katekhaye, V.; Jain, R. Effect of ferric carboxymaltose on hospitalization and mortality outcomes in chronic heart failure: A meta-analysis. Indian Heart J. 2017, 69, 736–741. [Google Scholar] [CrossRef]
- Barandiaran Aizpurua, A.; Sanders-van Wijk, S.; Brunner-La Rocca, H.P.; Henkens, M.T.; Weerts, J.; Spanjers, M.H.; van Empel, V.P. Iron deficiency impacts prognosis but less exercise capacity in heart failure with preserved ejection fraction. ESC Heart Fail 2021, 8, 1304–1313. [Google Scholar] [CrossRef] [PubMed]
- Kasner, M.; Aleksandrov, A.S.; Westermann, D.; Lassner, D.; Gross, M.; Von Haehling, S.; Tschöpe, C. Functional iron deficiency and diastolic function in heart failure with preserved ejection fraction. Int. J. Cardiol. 2013, 168, 4652–4657. [Google Scholar] [CrossRef] [PubMed]
- Martens, P.; Verbrugge, F.; Nijst, P.; Dupont, M.; Tang, W.W.; Mullens, W. Impact of Iron Deficiency on Response to and Remodeling After Cardiac Resynchronization Therapy. Am. J. Cardiol. 2017, 119, 65–70. [Google Scholar] [CrossRef]
- Núñez, J.; Monmeneu, J.V.; Mollar, A.; Núñez, E.; Bodí, V.; Miñana, G.; García-Blas, S.; Santas, E.; Agüero, J.; Chorro, F.J.; et al. Left ventricular ejection fraction recovery in patients with heart failure treated with intravenous iron: A pilot study. ESC Heart Fail. 2016, 3, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Graham, F.J.; Pellicori, P.; Ford, I.; Petrie, M.C.; Kalra, P.R.; Cleland, J.G. Intravenous iron for heart failure with evidence of iron deficiency: A meta-analysis of randomised trials. Clin. Res. Cardiol. Off. J. Ger. Card. Soc. 2021, 110, 1299–1307. [Google Scholar] [CrossRef] [PubMed]
- Jankowska, E.A.; Kirwan, B.-A.; Kosiborod, M.; Butler, J.; Anker, S.D.; McDonagh, T.; Dorobantu, M.; Drozdz, J.; Filippatos, G.; Keren, A.; et al. The effect of intravenous ferric carboxymaltose on health-related quality of life in iron-deficient patients with acute heart failure: The results of the AFFIRM-AHF study. Eur. Heart J. 2021, 42, 3011–3020. [Google Scholar] [CrossRef]
- Núñez, J.; Domínguez, E.; Ramón, J.M.; Núñez, E.; Sanchis, J.; Santas, E.; Heredia, R.; González, J.; Miñana, G.; López, L.; et al. Iron deficiency and functional capacity in patients with advanced heart failure with preserved ejection fraction. Int. J. Cardiol. 2016, 207, 365–367. [Google Scholar] [CrossRef]
- González-Costello, J.; Comín-Colet, J.; Lupón, J.; Enjuanes, C.; de Antonio, M.; Fuentes, L.; Moliner-Borja, P.; Farré, N.; Zamora, E.; Manito, N.; et al. Importance of iron deficiency in patients with chronic heart failure as a predictor of mortality and hospitalizations: Insights from an observational cohort study. BMC Cardiovasc. Disord. 2018, 18, 206. [Google Scholar] [CrossRef]
- Nunez, J.; Garcia-Blas, S.; Comin-Colet, J. Iron deficiency and risk of early readmission following hospitalization for acute heart failure. Reply. Eur. J. Heart Fail 2016, 18, 881. [Google Scholar] [CrossRef][Green Version]
- Martens, P.; Nijst, P.; Verbrugge, F.H.; Smeets, K.; Dupont, M.; Mullens, W. Impact of iron deficiency on exercise capacity and outcome in heart failure with reduced, mid-range and preserved ejection fraction. Acta Cardiol. 2018, 73, 115–123. [Google Scholar] [CrossRef]
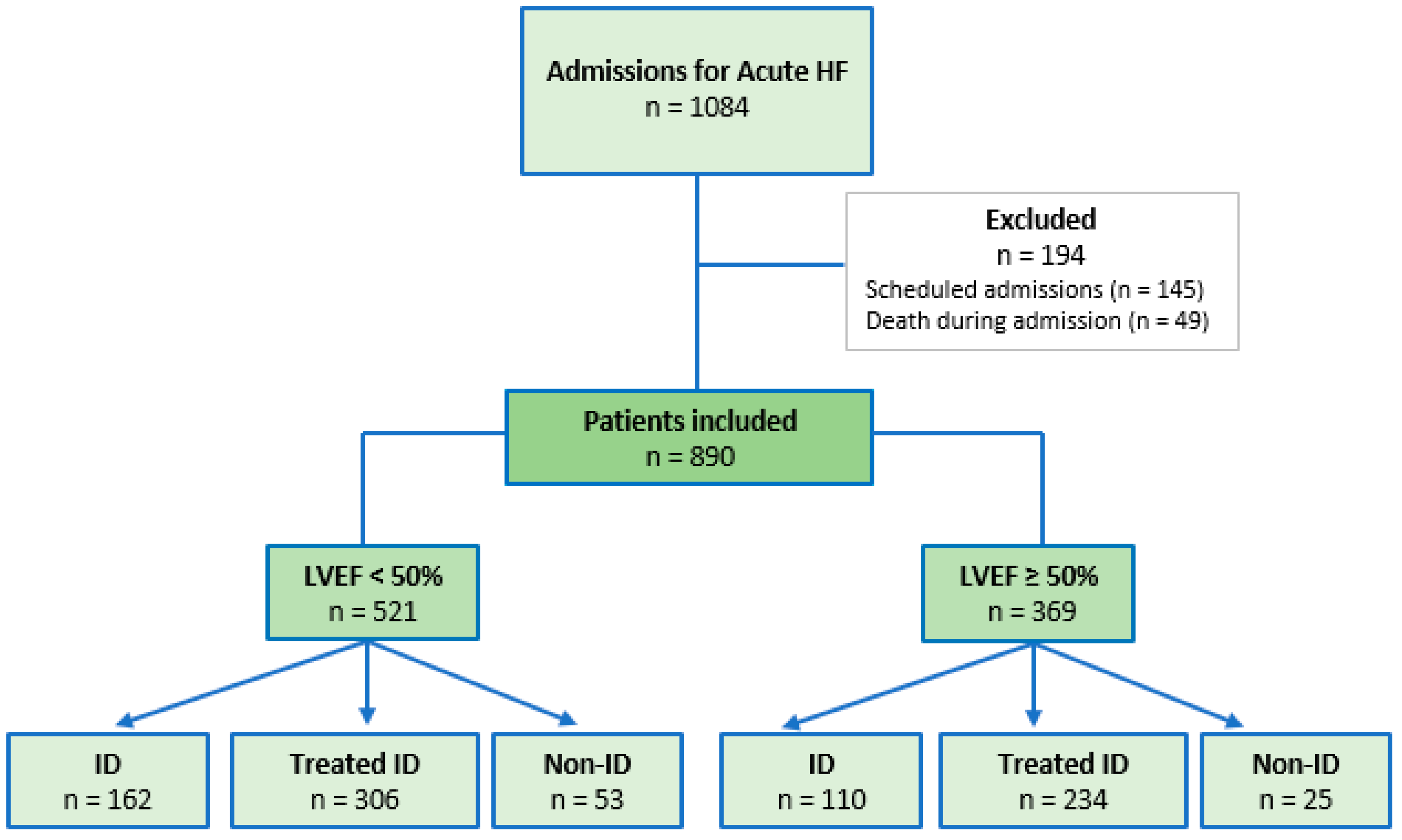
| ID n = 162 | Treated ID n = 306 | No ID n = 53 | p | |
|---|---|---|---|---|
| Patient history (n, %) | ||||
| Age (years) (*) | 73.0 ± 12.1 | 73.4 ± 10.4 | 68.2 ± 12.3 | 0.007 |
| Male | 109 (67.3) | 211 (69.0) | 34 (64.2) | 0.769 |
| Baseline heart disease (n, %) | ||||
| IHD | 63 (38.9) | 122 (40.0) | 21 (39.6) | 0.979 |
| VHD | 26 (16.0) | 49 (16.0) | 8 (15.1) | 0.982 |
| AF | 15 (9.3) | 15 (4.9) | 4 (7.5) | 0.183 |
| DCM | 35 (21.6) | 70 (22.9) | 12 (22.6) | 0.952 |
| HT | 17 (10.5) | 40 (13.1) | 7 (13.2) | 0.705 |
| Other | 6 (3.7) | 10 (3.3) | 2 (3.8) | 0.812 |
| History (n, %) | ||||
| CVS | 36 (22.2) | 61 (19.9) | 8 (15.1) | 0.527 |
| HT | 125 (77.2) | 229 (74.8) | 29 (54.7) | 0.004 |
| Dyslipidemia | 100 (61.7) | 180 (58.8) | 24 (45.3) | 0.105 |
| DM | 96 (59.3) | 170 (55.6) | 21 (39.6) | 0.043 |
| Smoking | 23 (14.2) | 35 (11.4) | 4 (7.5) | 0.399 |
| Alcoholism | 6 (3.7) | 19 (6.2) | 2 (3.8) | 0.451 |
| COPD | 26 (16.0) | 58 (19.0) | 8 (15.1) | 0.644 |
| SAHS | 24 (14.8) | 43 (14.1) | 9 (17.0) | 0.852 |
| Obesity (BMI > 30 kg/m2) | 39 (24.1) | 71 (23.2) | 12 (22.6) | 0.938 |
| Renal failure | 37 (22.8) | 60 (19.6) | 10 (18.9) | 0.678 |
| Hypothyroidism | 7 (4.3) | 28 (9.2) | 3 (5.7) | 0.143 |
| AF | 82 (50.6) | 156 (51.0) | 29 (54.7) | 0.865 |
| Stroke | 15 (9.3) | 35 (11.4) | 4 (7.5) | 0.593 |
| PVD | 16 (9.9) | 36 (11.8) | 3 (5.7) | 0.387 |
| Clinical characteristics (n, %) | ||||
| No. of previous admissions (*) | 0.8 ± 0.6 | 0. 8 ± 0.6 | 0.7 ± 0.5 | 0.506 |
| de novo HF | 63 (38.9) | 103 (33.7) | 18 (34.01) | 0.518 |
| FC (NYHA) | ||||
| I | 37 (22.8) | 65 (21.3) | 11 (20.8) | 0.910 |
| II | 79 (48.8) | 142 (46.4) | 25 (47.2) | 0.959 |
| III | 45 (27.8) | 94 (30.7) | 17 (32.1) | 0.754 |
| IV | 1 (0.6) | 5 (1.6) | 0 (0.0) | 0.438 |
| Cause of decompensation | ||||
| Arrhythmia | 35 (21.6) | 64 (20.9) | 12 (22.6) | 0.955 |
| Infectious | 18 (11.1) | 36 (11.8) | 6 (11.4) | 0.977 |
| Ischemic | 15 (9.3) | 26 (8.5) | 5 (9.4) | 0.551 |
| Disease progression | 59 (36.4) | 113 (36.9) | 20 (37.7) | 0.984 |
| Unknown | 30 (18.5) | 67 (21.9) | 8 (15.1) | 0.430 |
| HT | 5 (3.1) | 8 (2.6) | 2 (3.8) | 0.881 |
| Hemodynamic pattern | ||||
| Pulmonary congestion | 113 (69.8) | 203 (66.3) | 35 (66.0) | 0.737 |
| Systemic pulmonary congestion | 17 (10.5) | 30 (9.8) | 7 (13.2) | 0.753 |
| Systemic congestion | 30 (18.5) | 69 (22.5) | 9 (17.0) | 0.460 |
| Low output | 2 (1.2) | 4 (1.3) | 2 (3.8) | 0.376 |
| Echocardiography (n, %) | ||||
| LVEF (*) | 32.7 ± 9.6 | 33.4 ± 10.2 | 37.3 ± 10.4 | 0.014 |
| RV function | ||||
| Normal | 112 (69.1) | 179 (58.5) | 36 (68.0) | 0.055 |
| Mild depression | 11 (6.8) | 24 (7.8) | 4 (7.5) | 0.919 |
| Moderate depression | 24 (14.8) | 43 (14.1) | 8 (15.1) | 0.964 |
| Severe depression | 15 (9.3) | 18 (5.9) | 5 (9.4) | 0.335 |
| ID n = 110 | Treated ID n = 234 | No ID n = 25 | p | |
|---|---|---|---|---|
| Patient history (n, %) | ||||
| Age (years) (*) | 77.9 ± 9.9 | 79.9 ± 7.3 | 72.9 (9.3) | <0.0001 |
| Male | 33 (30.0) | 83 (35.5) | 7 (28.0) | 0.509 |
| Baseline heart disease (n, %) | ||||
| IHD | 4 (3.6) | 18 (7.7) | 2 (8.0) | 0.46 |
| VHD | 53 (48.2) | 102 (43.6) | 10 (40.0) | 0.644 |
| AF | 12 (10.9) | 20 (8.5) | 3 (12.0) | 0.714 |
| DCM | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 |
| HT | 33 (30.0) | 85 (36.3) | 9 (40.0) | 0.508 |
| Other | 8 (7.3) | 9 (3.8) | 1 (4.0) | 0.379 |
| History (n, %) | ||||
| CVS | 14 (12.7) | 42 (17.9) | 4 (16.0) | 0.472 |
| HT | 95 (86.4) | 213 (91.1) | 20 (80.0) | 0.150 |
| Dyslipidemia | 59 (53.6) | 135 (57.7) | 15 (60.0) | 0.732 |
| DM | 46 (41.8) | 73 (31.2) | 5 (20.0) | 0.05 |
| Smoking | 12 (10.9) | 12 (5.1) | 2 (8.0) | 0.146 |
| Alcoholism | 7 (6.4) | 13 (5.6) | 1 (4.0) | 0.871 |
| COPD | 24 (21.8) | 54 (23.1) | 5 (20.0) | 0.921 |
| SAHS | 15 (13.6) | 44 (18.8) | 4 (16.0) | 0.489 |
| Obesity (BMI > 30 kg/m2) | 28 (25.5) | 45 (19.2) | 5 (20.0) | 0.415 |
| Renal failure | 42 (38.2) | 75 (32.1) | 10 (40.0) | 0.383 |
| Hypothyroidism | 16 (14.5) | 36 (15.4) | 4 (16.0) | 0.973 |
| AF | 74 (67.3) | 163 (69.7) | 20 (80.0) | 0.458 |
| Stroke | 16 (14.5) | 36 (15.4) | 4 (16.0) | 0.973 |
| PVD | 1 (0.9) | 3 (1.3) | 0 (0.0) | 0.822 |
| Clinical characteristics (n, %) | ||||
| No. of previous admissions (*) | 0.9 ± 0.5 | 0.9 ± 0.7 | 0.8 ± 0.6 | 0.753 |
| de novo HF | 42 (38.2) | 75 (32.1) | 10 (40.0) | 0.446 |
| FC (NYHA) | ||||
| I | 11 (10.0) | 18 (7.7) | 3 (12.0) | 0.645 |
| II | 68 (61.8) | 142 (60.7) | 15 (60.0) | 0.975 |
| III | 22 (20.0) | 53 (22.6) | 5 (20.0) | 0.828 |
| IV | 9 (8.2) | 21 (9.0) | 2 (8.0) | 0.963 |
| Cause of decompensation | ||||
| Arrhythmia | 21 (19.1) | 51 (21.8) | 4 (16.0) | 0.711 |
| Infectious | 3 (2.7) | 9 (3.8) | 2 (8.0) | 0.459 |
| Ischemic | 2 (1.8) | 4 (1.7) | 2 (8.0) | 0.116 |
| Disease progression | 52 (47.3) | 115 (49.1) | 12 (48.0) | 0.984 |
| Unknown | 6 (5.5) | 8 (3.4) | 1 (4.0) | 0.672 |
| HT | 26 (23.6) | 41 (17.5) | 4 (16.0) | 0.371 |
| Hemodynamic pattern | ||||
| Pulmonary congestion | 76 (69.1) | 160 (68.4) | 18 (72.0) | 0.931 |
| Systemic pulmonary congestion | 21 (19.1) | 48 (20.5) | 5 (20.0) | 0.954 |
| Systemic congestion | 13 (11.8) | 22 (9.4) | 2 (8.0) | 0.738 |
| Low output | 0 (0.0) | 4 (1.7) | 0 (0.0) | 0.832 |
| Echocardiography (n, %) | ||||
| LVEF (*) | 63.2 ± 7.5 | 62.0 ± 7.0 | 60.0 ± 6.3 | 0.094 |
| RV function | ||||
| Normal | 82 (74.5) | 174 (74.4) | 20 (80.0) | 0.824 |
| Mild depression | 19 (17.3) | 44 (18.8) | 5 (20.0) | 0.414 |
| Moderate depression | 8 (7.3) | 10 (4.3) | 0 (0.0) | 0.234 |
| Severe depression | 1 (0.9) | 6 (2.6) | 0 (0.0) | 0.445 |
| ID n = 162 | Treated ID n = 306 | No ID n = 53 | p | |
|---|---|---|---|---|
| Laboratory tests on admission (#) | ||||
| Urea (mg/dL) | 40.0 (46.0) | 46.0 (85.0) | 39.0 (42.0) | 0.614 |
| Creatinine (mg/dL) | 1.03 (0.44) | 1.06 (1.36) | 1.08 (1.74) | 0.953 |
| GFR (mL/min/1.73 m2) | 58.6 (31.0) | 56.0 (69.9) | 58.0 (50.4) | 0.893 |
| Bilirubin (mg/dL) | 1.0 (0.5) | 1.1 (0.6) | 0.9 (0.5) | 0.863 |
| AST (U/L) | 23.0 (9.0) | 21.0 (14.0) | 22.8 (10.1) | 0.203 |
| ALT (U/L) | 22.6 (18.8) | 23.0 (17.3) | 24.0 (19.1) | 0.885 |
| TnT(u) (ng/mL) | 45.9 (28.3) | 44.0 (36.7) | 48.1 (29.6) | 0.658 |
| NT-proBNP (pg/mL) | 5762 (3870) | 5398 (3207) | 6296 (2748) | 0.158 |
| Sodium (mEq/L) | 140.5 (7.0) | 142.0 (7.3) | 151.5 (7.1) | 0.101 |
| Potassium (mEq/L) | 4.2 (0.8) | 4.1 (1.4) | 3.9 (0.7) | 0.270 |
| Hemoglobin (g/dL) | 13.9 (2.0) | 13.2 (4.9) | 13.4 (4.1) | 0.219 |
| Hematocrit (%) | 40.6 (7.8) | 40.7 (11.9) | 40.2 (10.4) | 0.951 |
| Uric acid (mg/dL) | 8.8 (4.7) | 8.5 (4.4) | 8.6 (3.7) | 0.785 |
| Cholesterol-HDL (mg/dL) | 42.1 (23.3) | 43.0 (16.3) | 44.5 (17.9) | 0.713 |
| Cholesterol-LDL (mg/dL) | 79.2 (24.7) | 74.0 (42.6) | 76.5 (21.6) | 0.332 |
| Triglycerides (mg/dL) | 133.5 (84.1) | 148.7 (62.0) | 151.5 (79.4) | 0.068 |
| Ferritin (ng/mL) | 166.1 (135.2) | 156.0 (89.1) | 531.5 (223.2) | <0.0001 |
| TSAT (%) | 16.3 (6.0) | 18.0 (10.9) | 25.0 (16.1) | <0.0001 |
| HbA1c (%) | 6.3 (0.8) | 6.3 (0.9) | 6.5 (0.6) | 0.263 |
| CA125 (U/mL) | 73.0 (62.9) | 73.9 (60.6) | 72.4 (69.2) | 0.980 |
| Discharge treatment (n, %) | ||||
| ACEI/ARB II | 99 (60.7) | 198 (64.7) | 34 (64.2) | 0.0001 |
| Beta-blockers | 105 (64.4) | 195 (63.7) | 33 (62.3) | 0.940 |
| ARNI | 41 (25.3) | 70 (22.9) | 11 (20.8) | 0.747 |
| MRA | 68 (41.7) | 138 (45.1) | 23 (43.4) | 0.773 |
| SGLT2i | 37 (22.7) | 77 (25.2) | 11 (20.8) | 0.722 |
| Ivabradine | 19 (11.7) | 52 (17.0) | 8 (15.1) | 0.319 |
| Digoxin | 52 (31.9) | 83 (27.1) | 18 (34.0) | 0.394 |
| Loop diuretics | 157 (96.3) | 282 (92.2) | 49 (92.5) | 0.123 |
| Thiazides | 26 (16.0) | 46 (15.0) | 6 (11.3) | 0.730 |
| Acetazolamide | 3 (1.8) | 9 (2.9) | 1 (1.9) | 0.738 |
| Tolvaptan | 6 (3.7) | 18 (5.9) | 2 (3.8) | 0.537 |
| Potassium supplement | 23 (14.1) | 49 (16.0) | 9 (17.0) | 0.836 |
| Hypokalemic therapy | 6 (3.7) | 9 (2.9) | 2 (3.8) | 0.885 |
| Antiplatelet agents | 52 (31.9) | 107 (35.0) | 17 (32.1) | 0.792 |
| Anticoagulants | 97 (59.5) | 208 (68.0) | 32 (60.4) | 0.172 |
| OAD (No SGLT2i) | 62 (38.0) | 135 (44.1) | 25 (47.2) | 0.371 |
| Nitrates | 16 (9.8) | 46 (15.0) | 8 (15.1) | 0.289 |
| Antiarrhythmic | 36 (22.1) | 61 (19.9) | 10 (18.9) | 0.802 |
| Statins | 97 (59.5) | 153 (50.0) | 28 (52.8) | 0.125 |
| Calcium antagonists | 41 (25.2) | 67 (21.9) | 11 (20.8) | 0.655 |
| Pulmonary vasodilator | 3 (1.8) | 3 (1.0) | 1 (1.9) | 0.691 |
| Alopurinol | 42 (25.8) | 67 (21.9) | 11 (20.8) | 0.565 |
| ID n = 110 | Treated ID n = 234 | No ID n = 25 | p | |
|---|---|---|---|---|
| Laboratory tests on admission analytics (#) | ||||
| Urea (mg/dL) | 67.0 (32.0) | 63.0 (36.3) | 66.0 (42.0) | 0.605 |
| Creatinine (mg/dL) | 0.91 (0.68) | 1.04 (0.65) | 1.07 (1.28) | 0.262 |
| GFR (mL/min/1.73 m2) | 60.0 (57.0) | 58.0 (25.0) | 57.0 (67.0) | 0.897 |
| Bilirubin (mg/dL) | 1.1 (0.6) | 1.1 (0.9) | 1.0 (0.5) | 0.833 |
| AST (U/L) | 19.0 (16.1) | 17.0 (16.4) | 18.5 (18.8) | 0.548 |
| ALT (U/L) | 23.0 (19.0) | 18.0 (21.0) | 20.5 (23.0) | 0.109 |
| TnT(u) (ng/mL) | 45.3 (48.3) | 49.0 (11.3) | 49.3 (43.4) | 0.551 |
| NT-proBNP (pg/mL) | 5710 (4905) | 5513 (4837) | 7953 (7390) | 0.074 |
| Sodium (mEq/L) | 139.0 (6.5) | 140.0 (6.0) | 139 (4.0) | 0.292 |
| Potassium (mEq/L) | 4.0 (0.6) | 4.1 (0.9) | 3.9 (0.4) | 0.331 |
| Hemoglobin (g/dL) | 13.0 (4.9) | 12.2 (2.0) | 12.1 (1.3) | 0.076 |
| Hematocrit (%) | 40.6 (12.7) | 38.6 (11.3) | 37.3 (11.2) | 0.233 |
| Uric acid (mg/dL) | 8.2 (4.0) | 8.1 (4.4) | 8.5 (4.6) | 0.900 |
| Cholesterol-HDL (mg/dL) | 41.0 (17.9) | 40.0 (15.4) | 40.0 (17.7) | 0.865 |
| Cholesterol-LDL (mg/dL) | 77.0 (36.9) | 70.0 (34.2) | 72.0 (32.6) | 0.224 |
| Triglycerides (mg/dL) | 69.0 (69.0) | 65.0 (53.0) | 88.0 (74.8) | 0.182 |
| Ferritin (ng/mL) | 103 (64.03) | 94.0 (34.1) | 406.0 (102.0) | <0.0001 |
| TSAT (%) | 15.0 (5.2) | 12.0 (3.0) | 22.0 (2.0) | <0.0001 |
| HbA1c (%) | 5.8 (1.7) | 5.9 (0.7) | 5.6 (1.4) | 0.377 |
| CA125 (U/mL) | 50.1 (75.6) | 53.0 (73.4) | 64.0 (63.2) | 0.694 |
| Discharge treatment (n, %) | ||||
| ACEI/ARB II | 61 (55.5) | 131 (60.0) | 15 (60.0) | 0.917 |
| Beta-blockers | 66 (60.0) | 133 (56.8) | 15 (60.0) | 0.839 |
| ARNI | 3 (2.7) | 5 (2.1) | 1 (4.0) | 0.825 |
| MRA | 38 (34.5) | 70 (29.9) | 8 (32.0) | 0.688 |
| SGLT2i | 29 (26.4) | 54 (23.1) | 6 (24.0) | 0.802 |
| Ivabradine | 2 (1.8) | 9 (3.8) | 1 (4.0) | 0.599 |
| Digoxin | 25 (22.7) | 51 (21.8) | 6 (24.0) | 0.958 |
| Loop diuretics | 101 (91.8) | 218 (93.2) | 24 (96.0) | 0.746 |
| Thiazides | 26 (23.6) | 51 (21.8) | 6 (24.0) | 0.914 |
| Acetazolamide | 1 (0.9) | 3 (1.3) | 1 (4.0) | 0.477 |
| Tolvaptan | 7 (6.4) | 12 (5.1) | 2 (8.0) | 0.787 |
| Potassium supplement | 11 (10.0) | 12 (5.1) | 3 (12.0) | 0.156 |
| Hypokalemic therapy | 10 (6.1) | 11 (4.7) | 3 (12.0) | 0.157 |
| Antiplatelet agents | 24 (21.8) | 48 (20.5) | 6 (24.0) | 0.901 |
| Anticoagulants | 67 (60.9) | 147 (62.8) | 16 (64.0) | 0.929 |
| OAD (No SGLT2i) | 33 (30.0) | 68 (29.1) | 8 (32.0) | 0.947 |
| Nitrates | 13 (11.8) | 21 (9.0) | 3 (12.0) | 0.675 |
| Antiarrhythmic | 22 (20.0) | 44 (18.8) | 3 (12.0) | 0.950 |
| Statins | 59 (5.4) | 129 (55.1) | 12 (48.0) | 0.786 |
| Calcium antagonists | 41 (37.3) | 94 (40.2) | 10 (40.0) | 0.874 |
| Pulmonary vasodilator | 2 (1.8) | 7 (3.0) | 0 (0.0) | 0.576 |
| Alopurinol | 21 (19.1) | 54 (23.1) | 6 (24.0) | 0.684 |
| Heart Failure with Reduced Ejection Fraction | ||||
| ID n = 162 | Treated ID n = 306 | No ID n = 53 | p | |
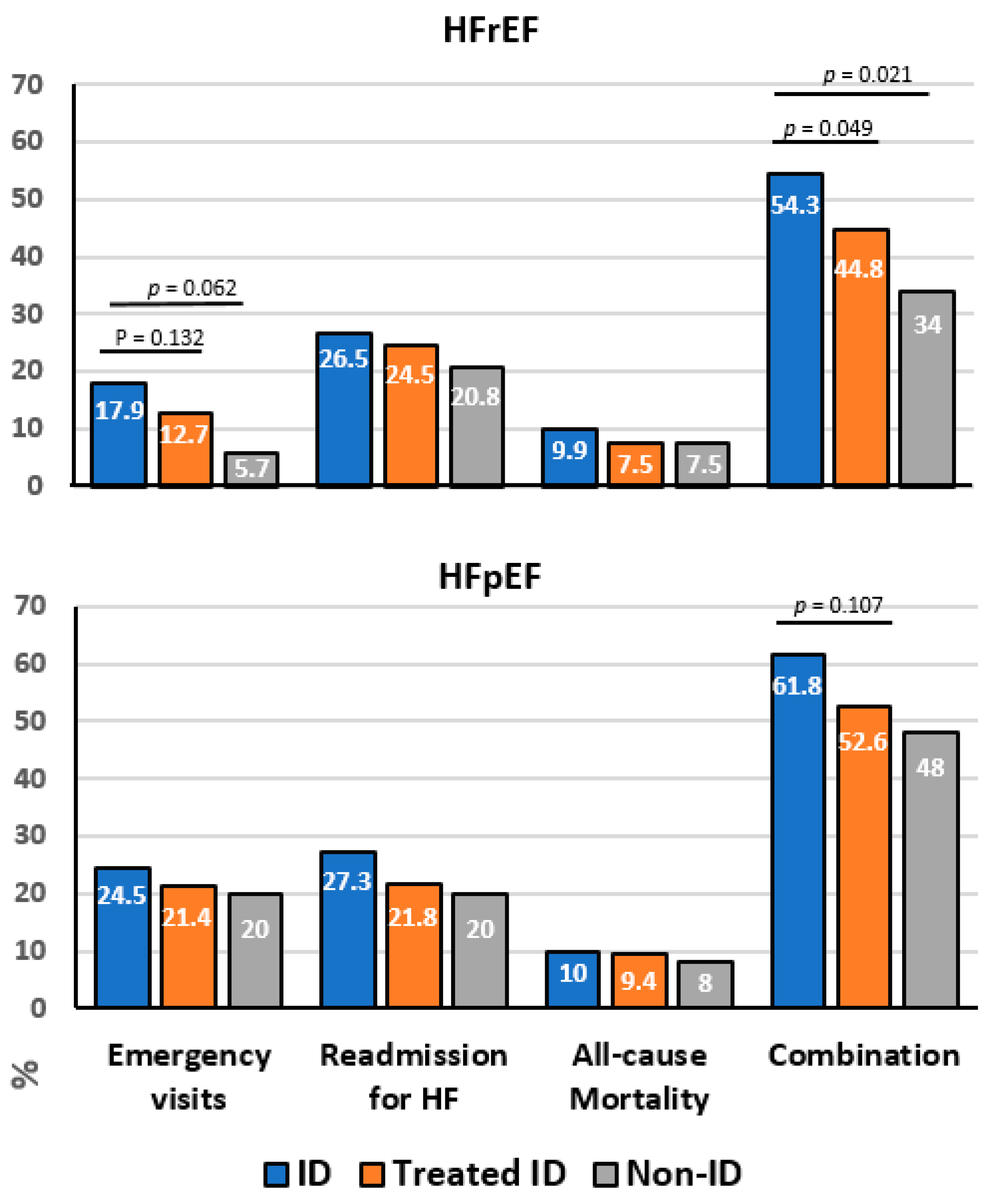
| Emergency visits (n, %) | 29 (17.9) | 39 (12.7) | 3 (5.7) | 0.062 a 0.132 b |
| Re-admission for HF (n, %) | 43 (26.5) | 75 (24.5) | 11 (20.8) | 0.690 a 0.630 b |
| All-cause mortality (n, %) | 16 (9.9) | 23 (7.5) | 4 (7.5) | 0.664 a 0.379 b |
| Combination (n, %) | 88 (54.3) | 137 (44.8) | 18 (34.0) | 0.021a 0.049b |
| Heart Failure with Preserved Ejection Fraction | ||||
| ID n = 110 | Treated ID n = 234 | No ID n = 25 | p | |
| Emergency visits (n, %) | 27 (24.5) | 50 (21.4) | 5 (20.0) | 0.773 a 0.510 b |
| Re-admission for HF (n, %) | 30 (27.3) | 51 (21.8) | 5 (20.0) | 0.492 a 0.264 b |
| All-cause mortality (n, %) | 11 (10.0) | 22 (9.4) | 2 (8.0) | 0.951 a 0.861 b |
| Combination (n, %) | 68 (61.8) | 123 (52.6) | 12 (48.0) | 0.210 a 0.107 b |
| Heart Failure with Reduced Ejection Fraction | |||||
| ID | Treated ID | ARR | RRR | NNT | |
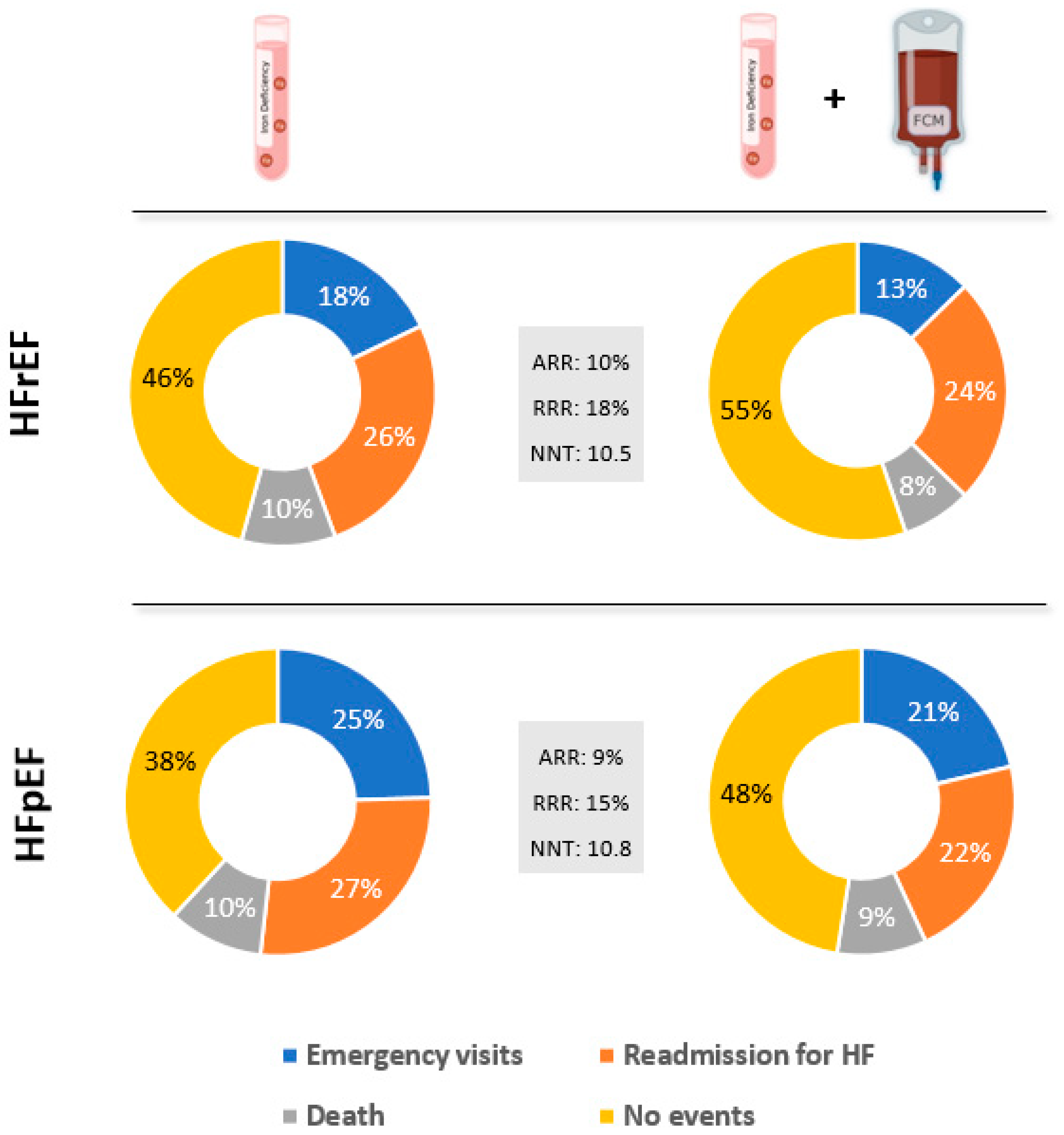
| Emergency visits (n, %) | 29 (17.9) | 39 (12.7) | 5% | 29% | 19.4 |
| Re-admission for HF (n, %) | 43 (26.5) | 75 (24.5) | 2% | 8% | 49.2 |
| All-cause mortality (n, %) | 16 (9.9) | 23 (7.5) | 2% | 24% | 42.4 |
| Combination (n, %) | 88 (54.3) | 137 (44.8) | 10% | 18% | 10.5 |
| Heart Failure with Preserved Ejection Fraction | |||||
| ID | Treated ID | ARR | RRR | NNT | |
| Emergency visits (n, %) | 27 (24.5) | 50 (21.4) | 3% | 13% | 31.5 |
| Re-admission for HF (n, %) | 30 (27.3) | 51 (21.8) | 5% | 20% | 18.3 |
| All-cause mortality (n, %) | 11 (10.0) | 22 (9.4) | 1% | 6% | 167.1 |
| Combination (n, %) | 68 (61.8) | 123 (52.6) | 9% | 15% | 10.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Vilella, R.; Donoso Trenado, V.; Jover Pastor, P.; Sánchez-Lázaro, I.; Martínez Dolz, L.; Almenar Bonet, L. Why Iron Deficiency in Acute Heart Failure Should Be Treated: A Real-World Clinical Practice Study. Life 2022, 12, 1828. https://doi.org/10.3390/life12111828
López-Vilella R, Donoso Trenado V, Jover Pastor P, Sánchez-Lázaro I, Martínez Dolz L, Almenar Bonet L. Why Iron Deficiency in Acute Heart Failure Should Be Treated: A Real-World Clinical Practice Study. Life. 2022; 12(11):1828. https://doi.org/10.3390/life12111828
Chicago/Turabian StyleLópez-Vilella, Raquel, Víctor Donoso Trenado, Pablo Jover Pastor, Ignacio Sánchez-Lázaro, Luis Martínez Dolz, and Luis Almenar Bonet. 2022. "Why Iron Deficiency in Acute Heart Failure Should Be Treated: A Real-World Clinical Practice Study" Life 12, no. 11: 1828. https://doi.org/10.3390/life12111828
APA StyleLópez-Vilella, R., Donoso Trenado, V., Jover Pastor, P., Sánchez-Lázaro, I., Martínez Dolz, L., & Almenar Bonet, L. (2022). Why Iron Deficiency in Acute Heart Failure Should Be Treated: A Real-World Clinical Practice Study. Life, 12(11), 1828. https://doi.org/10.3390/life12111828








