Variations in Soil Blue Carbon Sequestration between Natural Mangrove Metapopulations and a Mixed Mangrove Plantation: A Case Study from the World’s Largest Contiguous Mangrove Forest
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
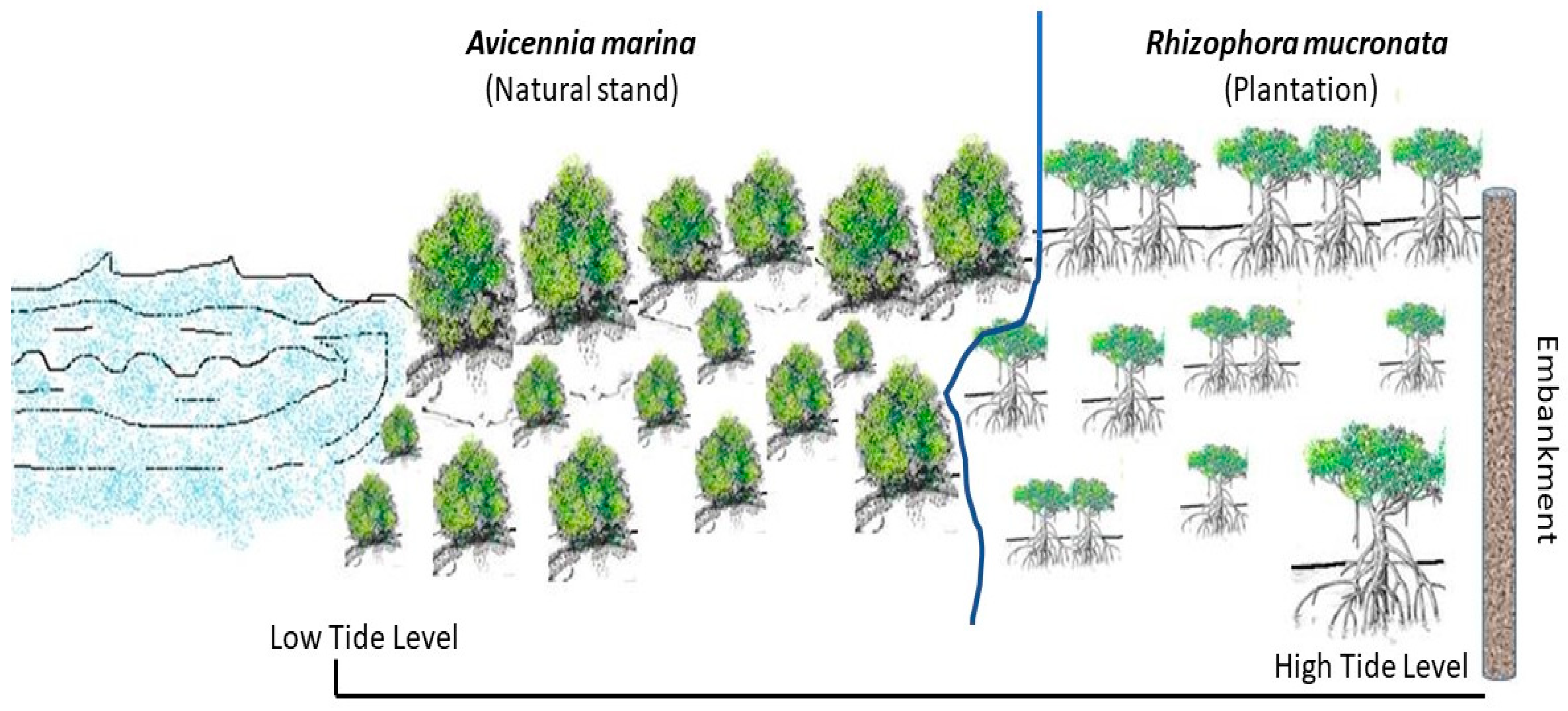
2.2. Plantation, Monitoring and Assessment Strategy
2.3. Soil Sampling and Analyses
2.4. Assessing the Health of the Ecosystem
2.5. Statistical Tests
3. Results
3.1. Biodiversity and Ecological Health Assessment
3.2. Soil Parameters
4. Discussion
4.1. Biodiversity and Ecological Health
4.2. Comparing the Ecosystem Function and Services
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Takagi, H. Long-term design of mangrove landfills as an effective tide attenuator under relative sea-level rise. Sustainability 2018, 10, 1045. [Google Scholar] [CrossRef]
- Alongi, D.M. Mangrove forests: Resilience, protection from tsunamis, and responses to global climate change. Estuar. Coast. Shelf Sci. 2008, 76, 1–13. [Google Scholar] [CrossRef]
- Chowdhury, A.; Naz, A.; Bhattacharyya, S.; Sanyal, P. Cost–benefit analysis of ‘Blue Carbon’sequestration by plantation of few key mangrove species at Sundarban Biosphere Reserve, India. Carbon Manag. 2018, 9, 575–586. [Google Scholar] [CrossRef]
- Trégarot, E.; Caillaud, A.; Cornet, C.C.; Taureau, F.; Catry, T.; Cragg, S.M.; Failler, P. Mangrove ecological services at the forefront of coastal change in the French overseas territories. Sci. Total Environ. 2021, 763, 143004. [Google Scholar] [CrossRef]
- Naz, A.; Chowdhury, A. Eco-Engineering and mangrove restoration methods to stabilize earthen embankments and establishing bio-shield against natural disasters: A case study from Sundarban Ramsar Wetland, India. In Assessing, Mapping and Modelling of Mangrove Ecosystem Services in the Asia-Pacific Region; Springer: Singapore, 2022; pp. 183–198. [Google Scholar]
- Barik, J.; Sanyal, P.; Ghosh, T.; Mukhopadhyay, S.K. Carbon stock and storage pattern in the Sundarbans mangrove forest, NE coast of India. Trop. Ecol. 2021, 62, 95–106. [Google Scholar] [CrossRef]
- Donato, D.C.; Kauffman, J.B.; Murdiyarso, D.; Kurnianto, S.; Stidham, M.; Kanninen, M. Mangroves among the most carbon-rich forests in the tropics. Nat. Geosci. 2011, 4, 293–297. [Google Scholar] [CrossRef]
- Thomas, N.; Lucas, R.; Bunting, P.; Hardy, A.; Rosenqvist, A.; Simard, M. Distribution and drivers of global mangrove forest change, 1996–2010. PLoS ONE 2017, 12, e0179302. [Google Scholar] [CrossRef]
- Bera, B.; Bhattacharjee, S.; Sengupta, N.; Shit, P.K.; Adhikary, P.P.; Sengupta, D.; Saha, S. Significant reduction of carbon stocks and changes of ecosystem service valuation of Indian Sundarban. Sci. Rep. 2022, 12, 7809. [Google Scholar] [CrossRef]
- Chowdhury, A.; Sanyal, P.; Maiti, S.K. Dynamics of mangrove diversity influenced by climate change and consequent accelerated sea level rise at Indian Sundarbans. Int. J. Global Warm. 2016, 9, 486–506. [Google Scholar] [CrossRef]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Maiti, S.K. Ecorestoration of Coal Mine Degraded Lands, 1st ed.; Springer: New Delhi, India, 2013. [Google Scholar]
- Subbiah, B.V.; Asija, G.L. A rapid procedure for the determination of available nitrogen in soils. Curr. Sci. 1956, 25, 259–260. [Google Scholar]
- Olsen, S.R. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate (No. 939); US Department of Agriculture: Washington, DC, USA, 1954. [Google Scholar]
- Tue, N.T.; Dung, L.V.; Nhuan, M.T.; Omori, K. Carbon storage of a tropical mangrove forest in Mui Ca Mau National Park, Vietnam. Catena 2014, 121, 119–126. [Google Scholar] [CrossRef]
- Simpson, E.H. Measurement of diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Laude, R. Statistics and partitioning of species diversity, and similarity among multiple communities. Oikos 1996, 76, 5–13. [Google Scholar]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–656. [Google Scholar] [CrossRef]
- Macreadie, P.I.; Anton, A.; Raven, J.A.; Beaumont, N.; Connolly, R.M.; Friess, D.A.; Duarte, C.M. The future of Blue Carbon science. Nat. Commun. 2019, 10, 1–13. [Google Scholar] [CrossRef]
- Singh, J.K. Structural characteristics of mangrove forest in different coastal habitats of Gulf of Khambhat arid region of Gujarat, west coast of India. Heliyon 2020, 6, e04685. [Google Scholar] [CrossRef]
- Jiang, Y.; Kang, M.; Zhu, Y.; Xu, G. Plant biodiversity patterns on Helan mountain, China. Acta Oecologica 2007, 32, 125–133. [Google Scholar] [CrossRef]
- Blasco, F.; Janodet, E.; Bellan, M.F. Natural hazards and mangroves in the Bay of Bengal. J. Coast. Res. 1994, 12, 277–288. [Google Scholar]
- Wetson, A.M.; Zörb, C.; John, E.A.; Flowers, T.J. High phenotypic plasticity of Suaeda maritima observed under hypoxic conditions in relation to its physiological basis. Ann. Bot. 2012, 109, 1027–1036. [Google Scholar] [CrossRef]
- MacArthur, R.H.; Wilson, E.O. The theory of island biogeography. In The Theory of Island Biogeography; Princeton University Press: Princeton, NJ, USA, 2016. [Google Scholar]
- Muñoz-Rojas, M. Soil quality indicators: Critical tools in ecosystem restoration. Curr. Opin. Environ. Sci. Health 2018, 5, 47–52. [Google Scholar] [CrossRef]
- Salmo, S.G.; Lovelock, C.; Duke, N.C. Vegetation and soil characteristics as indicators of restoration trajectories in restored mangroves. Hydrobiologia 2013, 720, 1–18. [Google Scholar] [CrossRef]
- Patel, N.T.; Gupta, A.; Pandey, A.N. Salinity tolerance of Avicennia marina (Forssk.) Vierh. from Gujarat coasts of India. Aquat. Bot. 2010, 93, 9–16. [Google Scholar] [CrossRef]
- Ahmed, A.; Ohlson, M.; Hoque, S.; Moula, M.G. Chemical composition of leaves of a mangrove tree (Sonneratia apetala Buch.-Ham.) and their correlation with some soil variables. Bangladesh J. Bot. 2010, 39, 61–69. [Google Scholar] [CrossRef]
- Hatje, V.; Masqué, P.; Patire, V.F.; Dórea, A.; Barros, F. Blue carbon stocks, accumulation rates, and associated spatial variability in Brazilian mangroves. Limnol. Oceanogr. 2021, 66, 321–334. [Google Scholar] [CrossRef]
- Ratul, S.B.; Gu, X.; Qiao, P.; Sagala, F.W.; Nan, S.; Islam, N.; Chen, L. Blue carbon sequestration following mangrove restoration: Evidence from a carbon neutral case in China. Ecosyst. Health Sustain. 2022, 8, 2101547. [Google Scholar] [CrossRef]
- Gu, J.; Wu, J. Blue carbon effects of mangrove restoration in subtropics where Spartina alterniflora invaded. Ecol. Eng. 2023, 186, 106822. [Google Scholar] [CrossRef]
- Adame, M.F.; Najera, E.; Lovelock, C.E.; Brown, C.J. Avoided emissions and conservation of scrub mangroves: Potential for a Blue Carbon project in the Gulf of California, Mexico. Biol. Lett. 2018, 14, 20180400. [Google Scholar] [CrossRef]
- Sidik, F.; Supriyanto, B.; Krisnawati, H.; Muttaqin, M.Z. Mangrove conservation for climate change mitigation in Indonesia. Wiley Interdiscip. Rev. Clim. Chang. 2018, 9, e529. [Google Scholar] [CrossRef]
- Chen, L.; Zeng, X.; Tam, N.F.; Lu, W.; Luo, Z.; Du, X.; Wang, J. Comparing carbon sequestration and stand structure of monoculture and mixed mangrove plantations of Sonneratia caseolaris and S. apetala in Southern China. For. Ecol. Manag. 2012, 284, 222–229. [Google Scholar] [CrossRef]
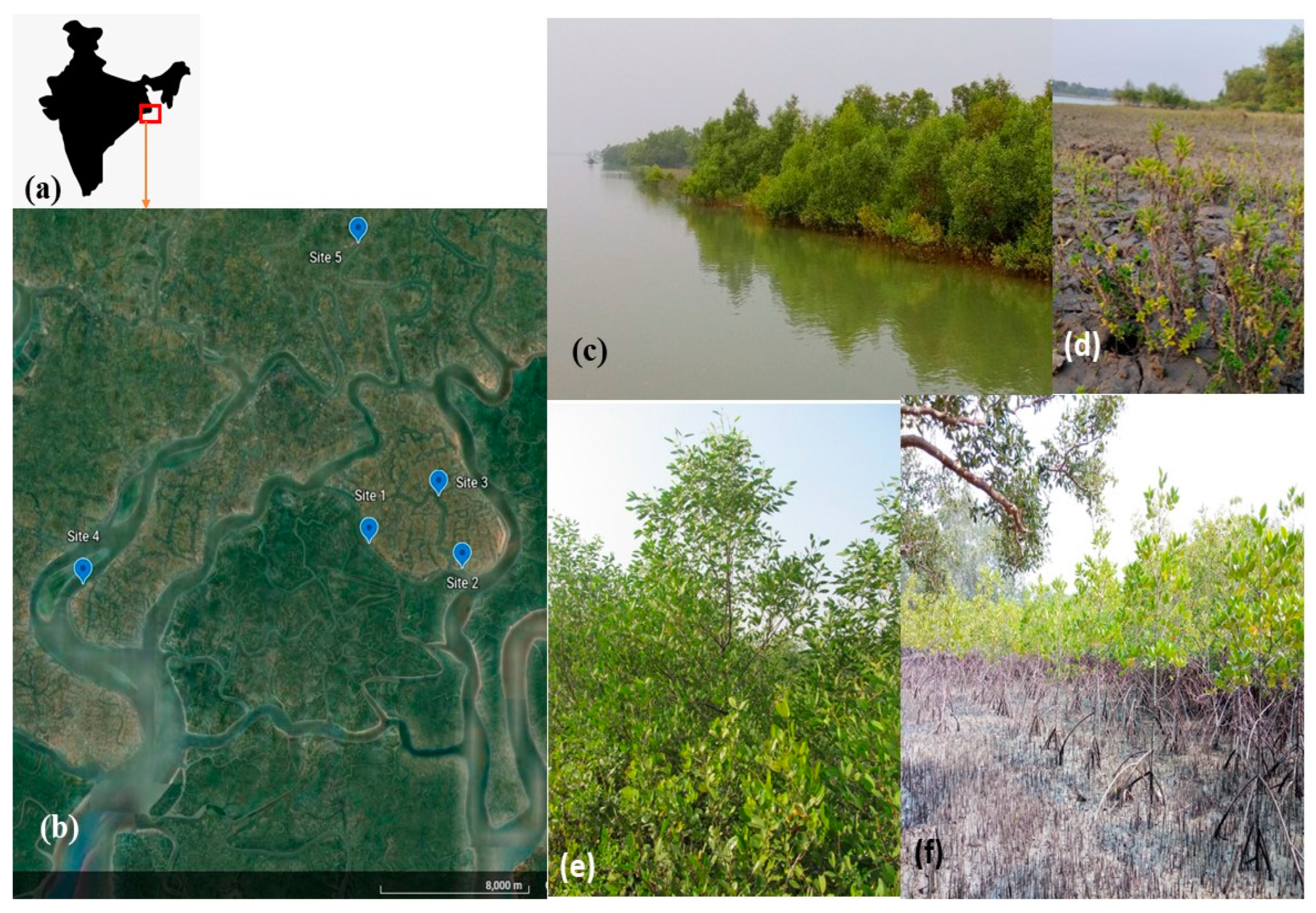
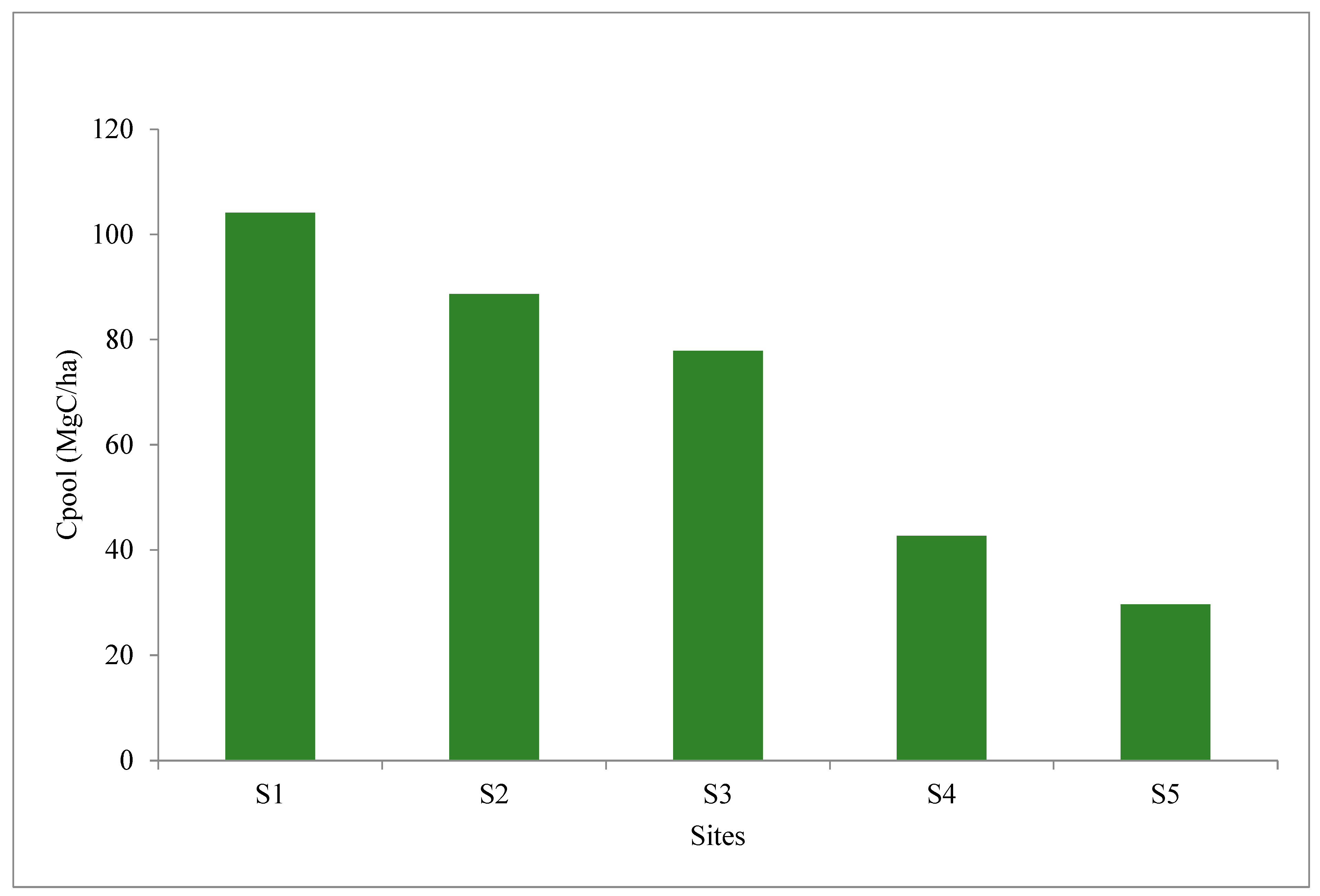
| Sites | Name of Monitoring Location | Latitude and Longitudes | Approximate Linear Distance from Nearest Conserved Mangrove Forest Patch—Sajnekhali Sanctuary (km) | Remarks |
|---|---|---|---|---|
| S1 | Plantation site | 22.1013272° N, 88.855648° E | 0.3 | The plantation comprises of Rhizophora mucronata, executed in 2012. The plantation site is behind a natural Avicennia marina plantation site, as depicted in detail in Figure 2. Hence, the study site is an association between naturally grown A. marina and planted R. mucronata. |
| S2 | Lahiripur | 22.0912318° N, 88.9027087° E | 0.3 | Near to the Lahiripur Revenue village. The patch is dominated by Avicennia marina. |
| S3 | Poroshmoni sub-center | 22.1210751° N, 88.8902425° E | 3.7 | The area is in a creek locally called ‘Dutta River’. It has a 60% Relative density for Avicennia marina, while Sonneretia caseolaris constitute another 20%. This is a natural association of Avicennia-Sonneretia. |
| S4 | Amlamethi Mudflat | 22.0807666° N, 88.7105938° E | 9 | Mudflat in Bali Island of Gosaba block under 80% relative density of Phoenix paludosa, the mangrove palm. |
| S5 | Chotto-Mollakhali | 22.2281325° N, 88.8487385° E | 12 | Near to the revenue village of Chotto-Mollakhali. Dominance of halophyte-Suaeda maritima |
| Site 1 | Site 2 | Site 3 | Site 4 | Site 5 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | Family | RD | F | RD | F | RD | F | RD | F | RD | F |
| Acanthus ilicifolius L. | Acanthaceae | 8 | 100 | 8 | 100 | 06 | 60 | 10 | 60 | 4 | 60 |
| Avicennia marina (Forssk.) Vierh | Acanthaceae | 24 | 60 | 80 | 100 | 55 | 100 | 16 | 100 | 13 | 40 |
| Bruguera sexangula (Lour.) Poir. | Rhizophoraceae | 4 | 60 | 1 | 20 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ceriops tagal (Perr.) C.B. Robinson | Rhizophoraceae | 1 | 40 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Phoenix paludosa Roxb. | Arecaceae | 0 | 40 | 0 | 0 | 4 | 60 | 63 | 100 | 4 | 80 |
| Proteresia coarctata (Roxb.) Tateoka | Poaceae | 4 | 0 | 12 | 40 | 13 | 40 | 10 | 20 | 10 | 40 |
| Rhizophora mucronata Lamk. | Rhizophoraceae | 60 | 100 | 0 | 0 | 0 | 40 | 0 | 0 | 0 | 0 |
| Sonneretia caseolaris (L.) Engl. | Sonnetiaceae | 0 | 0 | 0 | 0 | 21 | 100 | 0 | 0 | 0 | 0 |
| Suaeda maritima (L.) Dumort. | Amaranthaceae | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 69 | 100 |
| Site 1 | Site 2 | Site 3 | Site 4 | Site 5 | |
|---|---|---|---|---|---|
| Simpson’s Index of Dominance | 0.41 | 0.66 | 0.36 | 0.43 | 0.5 |
| Simpson’s Index of Diversity | 0.59 | 0.34 | 0.64 | 0.57 | 0.5 |
| Shannon-Weiner Index | 1.13 | 0.65 | 1.23 | 1.05 | 1.0 |
| Sand | Silt | Clay | pH | SOC (%) | BD (g/cm3) | P (mg/kg) | Salinity (ppt) | N (mg/kg) | |
|---|---|---|---|---|---|---|---|---|---|
| S1 | 6.8 ± 0.5 a | 36.2 ± 4.9 a | 57.0 ± 5.2 d | 7.6 ± 0.1 a | 1.9 ± 0.12 c | 1.8 ± 0.1 b | 9.4 ± 1.2 b | 9.3 ± 0.4 a | 74.2 ± 3.5 b |
| S2 | 10.4 ± 0.8 c | 40.4 ± 1.8 ab | 49.2 ± 1.6 c | 7.4 ± 0.1 a | 1.8 ± 0.09 c | 1.7 ± 0.05 b | 8.5 ± 0.7 b | 8.9 ± 0.3 a | 72.4 ± 8.2 b |
| S3 | 8.7 ± 1.4 b | 64.2 ± 3.7 c | 27.1 ± 3.6 a | 7.8 ± 0.2 a | 1.0 ± 0.06 b | 1.5 ± 0.1 a | 9.0 ± 0.5 b | 7.9 ± 0.26 a | 83.6 ± 4.5 c |
| S4 | 19.3 ± 1.1 d | 44.8 ± 2.6 b | 35.9 ± 3.5 b | 11.3 ± 1.2 b | 0.8 ± 0.11 a | 1.8 ± 0.08 b | 5.0 ± 0.5 a | 16.5 ± 1.5 b | 45.2 ± 3.3 a |
| S5 | 19.0 ± 0.8 a | 60.8 ± 2.3 a | 20.2 ± 1.6 d | 11.7 ± 0.6 a | 0.7 ± 0.05 c | 1.4 ± 0.1 b | 3.0 ± 0.2 b | 18.0 ± 2.4 a | 37.2 ± 3.5 b |
| F value | 97.8 | 28.9 | 32.5 | 29.8 | 78.54 | 8.4 | 14.7 | 65.6 | 25.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chowdhury, A.; Naz, A.; Maiti, S.K. Variations in Soil Blue Carbon Sequestration between Natural Mangrove Metapopulations and a Mixed Mangrove Plantation: A Case Study from the World’s Largest Contiguous Mangrove Forest. Life 2023, 13, 271. https://doi.org/10.3390/life13020271
Chowdhury A, Naz A, Maiti SK. Variations in Soil Blue Carbon Sequestration between Natural Mangrove Metapopulations and a Mixed Mangrove Plantation: A Case Study from the World’s Largest Contiguous Mangrove Forest. Life. 2023; 13(2):271. https://doi.org/10.3390/life13020271
Chicago/Turabian StyleChowdhury, Abhiroop, Aliya Naz, and Subodh Kumar Maiti. 2023. "Variations in Soil Blue Carbon Sequestration between Natural Mangrove Metapopulations and a Mixed Mangrove Plantation: A Case Study from the World’s Largest Contiguous Mangrove Forest" Life 13, no. 2: 271. https://doi.org/10.3390/life13020271
APA StyleChowdhury, A., Naz, A., & Maiti, S. K. (2023). Variations in Soil Blue Carbon Sequestration between Natural Mangrove Metapopulations and a Mixed Mangrove Plantation: A Case Study from the World’s Largest Contiguous Mangrove Forest. Life, 13(2), 271. https://doi.org/10.3390/life13020271







