Comparison of Life Traits in Two Bacterivorous Nematodes Suggest Different Ecological Strategies to Exploit Similar Habitats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Nematode Culture and Strains
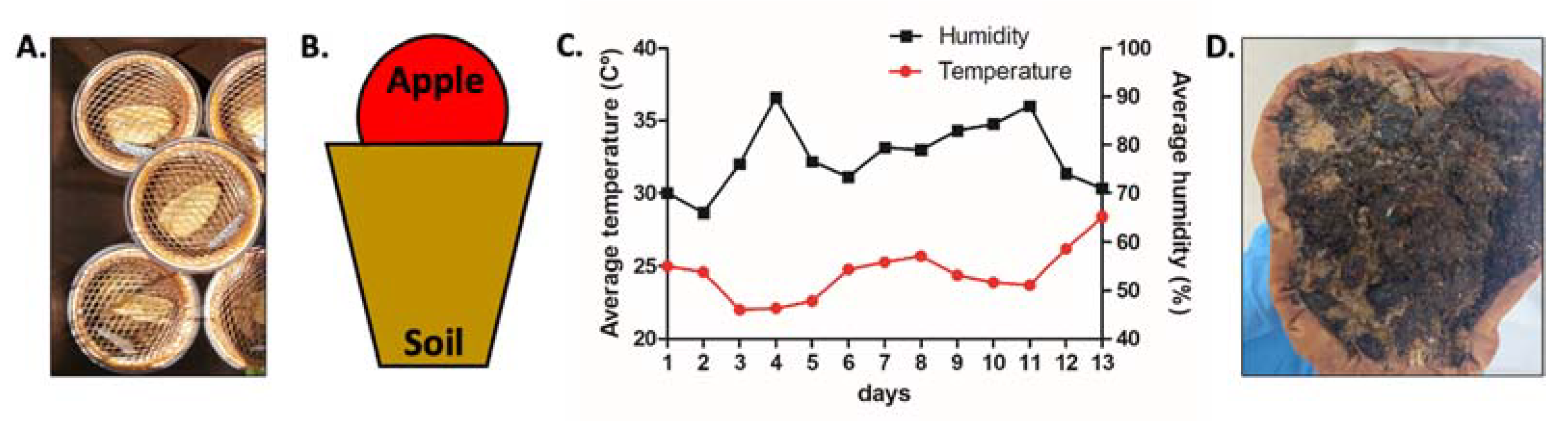
2.2. A. tricornis Cultivation and Isolation
2.3. Sequencing Analysis
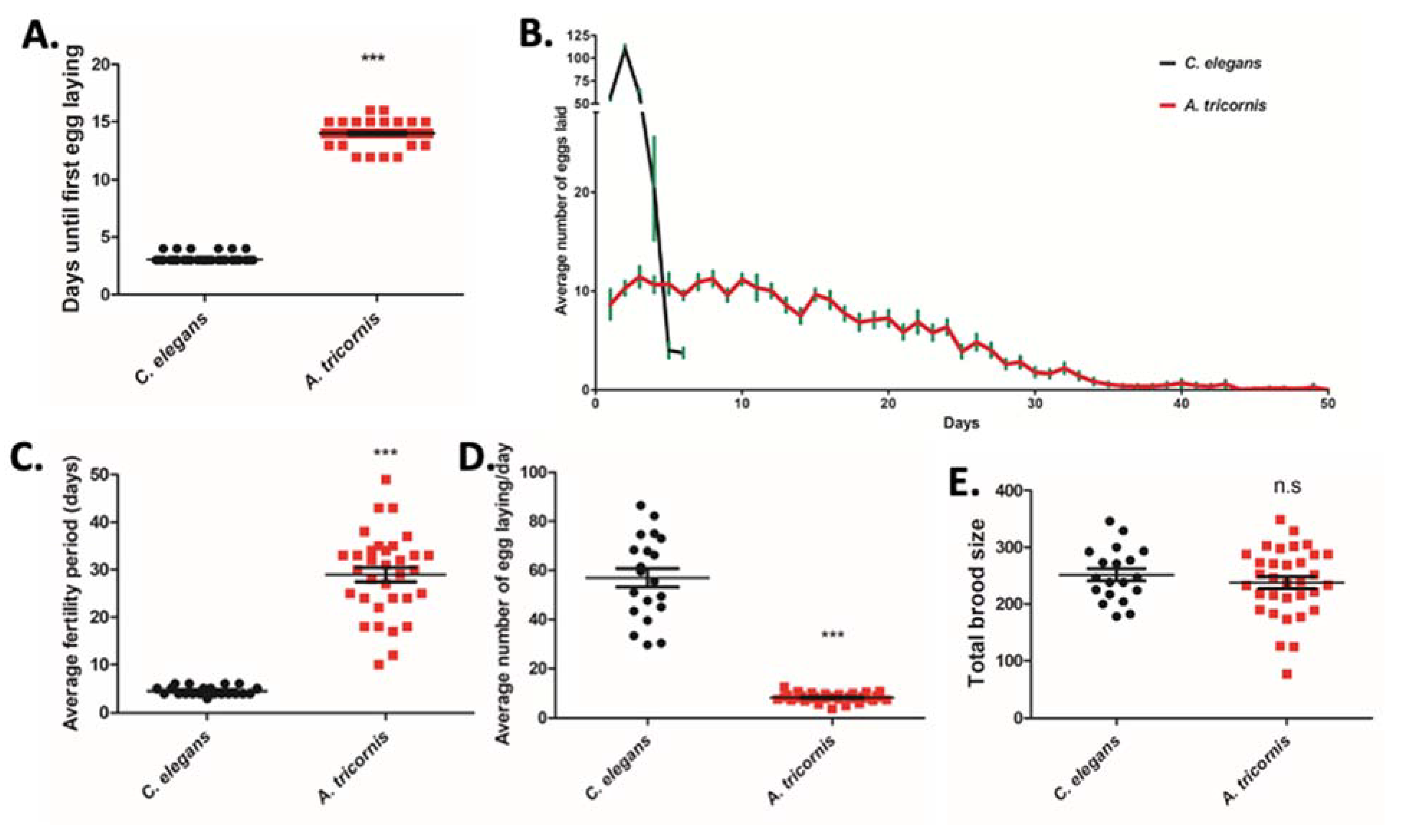
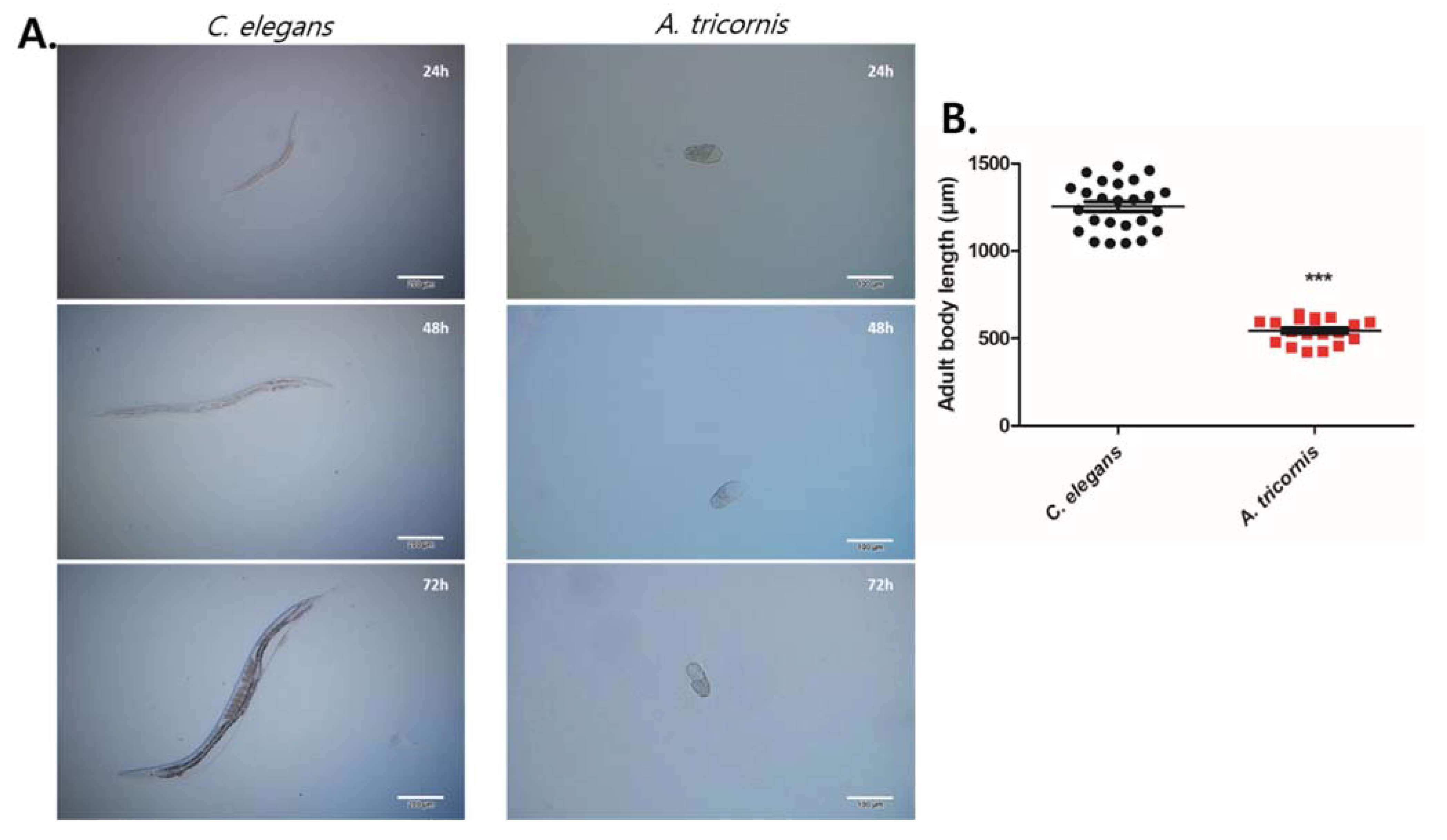
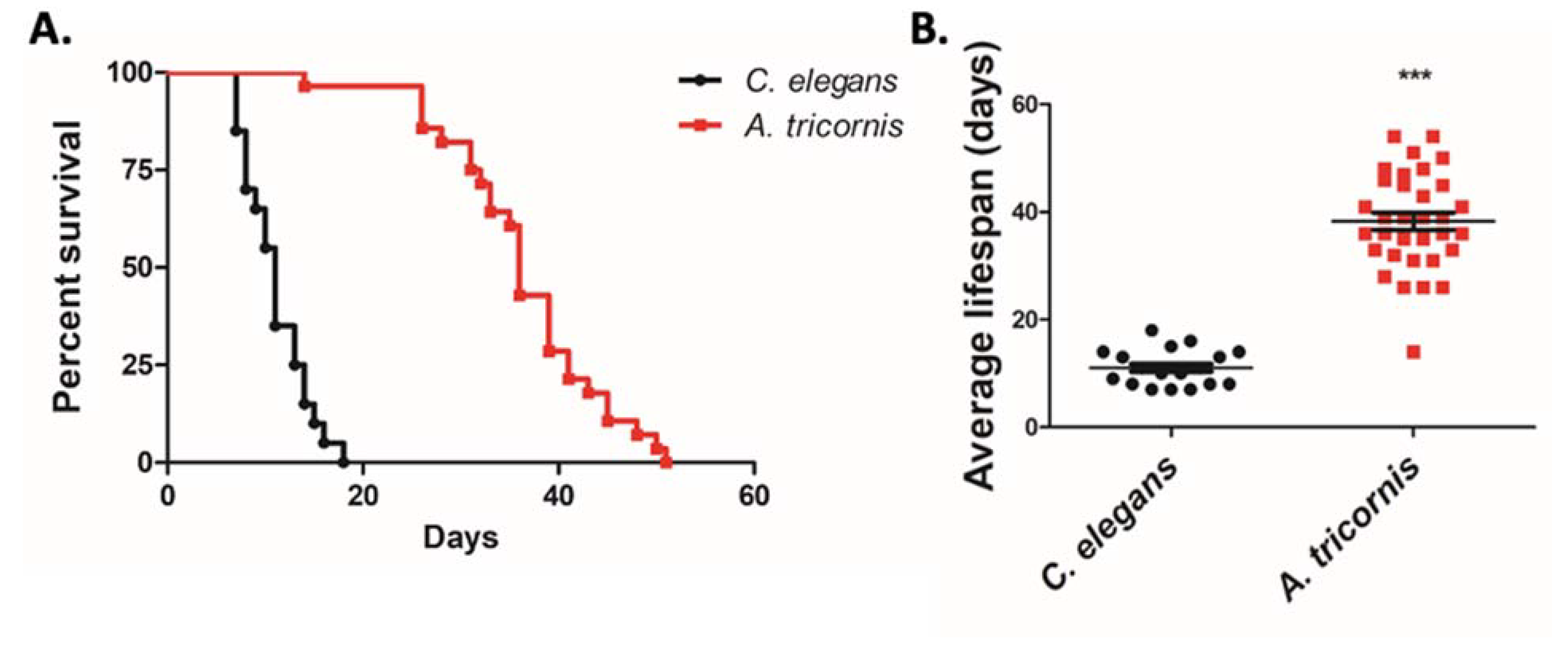
2.4. Lifespan, Egg Laying Assay, Brood Size, and Body Length Measurement
2.5. Measuring Fluorescence Accumulation
2.6. Pharyngeal Pumping
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blaxter, M. Imagining Sisyphus happy: DNA barcoding and the unnamed majority. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150329. [Google Scholar] [CrossRef] [PubMed]
- van den Hoogen, J.; Geisen, S.; Routh, D.; Ferris, H.; Traunspurger, W.; Wardle, D.A.; de Goede, R.G.M.; Adams, B.J.; Ahmad, W.; Andriuzzi, W.S.; et al. Soil nematode abundance and functional group composition at a global scale. Nature 2019, 572, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Bongers, T. The maturity index: An ecological measure of environmental disturbance based on nematode species composition. Oecologia 1990, 83, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Bongers, T.; Ferris, H. Nematode community structure as a bioindicator in environmental monitoring. Trends Ecol. Evol. 1999, 14, 224–228. [Google Scholar] [CrossRef]
- Bongers, T.; Bongers, M. Functional diversity of nematodes. Appl. Soil Ecol. 1998, 10, 239–251. [Google Scholar] [CrossRef]
- Darby, B.J.; Neher, D.A.; Belnap, J. Soil nematode communities are ecologically more mature beneath late- than early-successional stage biological soil crusts. Appl. Soil Ecol. 2007, 35, 203–212. [Google Scholar] [CrossRef]
- Ferris, H.; Eyre, M.; Venette, R.C.; Lau, S.S. Population energetics of bacterial-feeding nematodes: Stage-specific development and fecundity rates. Soil Biol. Biochem. 1996, 28, 271–280. [Google Scholar] [CrossRef]
- Venette, R.C.; Ferris, H. Thermal constraints to population growth of bacterial-feeding nematodes. Soil Biol. Biochem. 1997, 29, 63–74. [Google Scholar] [CrossRef]
- Brenner, S. The genetics of Caenorhabditis elegans. Genetics 1974, 77, 71–94. [Google Scholar] [CrossRef]
- Frezal, L.; Felix, M.A. C. elegans outside the Petri dish. eLife 2015, 4, e05849. [Google Scholar] [CrossRef]
- Felix, M.A.; Braendle, C. The natural history of Caenorhabditis elegans. Curr. Biol. 2010, 20, R965–R969. [Google Scholar] [CrossRef] [PubMed]
- Kiontke, K.C.; Felix, M.A.; Ailion, M.; Rockman, M.V.; Braendle, C.; Penigault, J.B.; Fitch, D.H. A phylogeny and molecular barcodes for Caenorhabditis, with numerous new species from rotting fruits. BMC Evol. Biol. 2011, 11, 339. [Google Scholar] [CrossRef] [PubMed]
- Felix, M.A.; Duveau, F. Population dynamics and habitat sharing of natural populations of Caenorhabditis elegans and C. briggsae. BMC Biol. 2012, 10, 59. [Google Scholar] [CrossRef]
- Samuel, B.S.; Rowedder, H.; Braendle, C.; Felix, M.A.; Ruvkun, G. Caenorhabditis elegans responses to bacteria from its natural habitats. Proc. Natl. Acad. Sci. USA 2016, 113, E3941–E3949. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Yucheol, L.; Park, J. First report and morphological description of two Acrobeloides species (Nematoda: Rhabditida: Cephalobidae) in South Korea. J. Species Res. 2021, 10, 405–411. [Google Scholar] [CrossRef]
- Williams, B.D.; Schrank, B.; Huynh, C.; Shownkeen, R.; Waterston, R.H. A genetic mapping system in Caenorhabditis elegans based on polymorphic sequence-tagged sites. Genetics 1992, 131, 609–624. [Google Scholar] [CrossRef]
- Floyd, R.; Abebe, E.; Papert, A.; Blaxter, M. Molecular barcodes for soil nematode identification. Mol. Ecol. 2002, 11, 839–850. [Google Scholar] [CrossRef]
- Haber, M.; Schungel, M.; Putz, A.; Muller, S.; Hasert, B.; Schulenburg, H. Evolutionary history of Caenorhabditis elegans inferred from microsatellites: Evidence for spatial and temporal genetic differentiation and the occurrence of outbreeding. Mol. Biol. Evol. 2005, 22, 160–173. [Google Scholar] [CrossRef]
- Berg, M.; Monnin, D.; Cho, J.; Nelson, L.; Crits-Christoph, A.; Shapira, M. TGFbeta/BMP immune signaling affects abundance and function of C. elegans gut commensals. Nat. Commun. 2019, 10, 604. [Google Scholar] [CrossRef]
- Thorne, G. The genus Acrobeles von Linstow. Trans. Am. Microsc. Soc. 1925, 44, 40. [Google Scholar] [CrossRef]
- Bostrom, S. A scanning electron microscope study of some species of terrestrial nematodes from Spitzbergen. Nematologica 1987, 33, 9. [Google Scholar] [CrossRef]
- De Ley, P.; Geraert, E.; Coomans, A. Seven cephalobids from Senegal (Nematoda: Rhabditida). J. Afr. Zool. 1990, 104, 287–304. [Google Scholar]
- Bostrom, S.; Holovachov, O. Description of Acrobeloides uberrinus Anderson, 1965 and A. syrtisus Yeates, 1967 (Rhabditida: Cephalobidae) with a discussion of the taxonomic status of A. tricornis (Thorne, 1925) Thorne, 1937 and A. setosus Brzeski, 1962. J. Nematode Morphol. Syst. 2012, 13, 97–106. [Google Scholar]
- Mikola, J.; Sulkava, P. Responses of microbial-feeding nematodes to organic matter distribution and predation in experimental soil habitat. Soil. Biol. Biochem. 2001, 33, 811–817. [Google Scholar] [CrossRef]
- Laakso, J.; Setala, H. Population- and ecosystem-level effects of predation on microbial-feeding nematodes. Oecologia 1999, 120, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Lahl, V.; Sadler, B.; Schierenberg, E. Egg development in parthenogenetic nematodes: Variations in meiosis and axis formation. Int. J. Dev. Biol. 2006, 50, 393–398. [Google Scholar] [CrossRef]
- Avery, L. The genetics of feeding in Caenorhabditis elegans. Genetics 1993, 133, 897–917. [Google Scholar] [CrossRef]
- Geary, T.G.; Sims, S.M.; Thomas, E.M.; Vanover, L.; Davis, J.P.; Winterrowd, C.A.; Klein, R.D.; Ho, N.F.; Thompson, D.P. Haemonchus contortus: Ivermectin-induced paralysis of the pharynx. Exp. Parasitol. 1993, 77, 88–96. [Google Scholar] [CrossRef]
- Klass, M.R. Aging in the nematode Caenorhabditis elegans: Major biological and environmental factors influencing life span. Mech. Ageing Dev. 1977, 6, 413–429. [Google Scholar] [CrossRef]
- Dolgin, E.S.; Felix, M.A.; Cutter, A.D. Hakuna Nematoda: Genetic and phenotypic diversity in African isolates of Caenorhabditis elegans and C. briggsae. Heredity 2008, 100, 304–315. [Google Scholar] [CrossRef]
- Estifanos, T.K.; Traunspurger, W.; Peters, L. Selective feeding in nematodes: A stable isotope analysis of bacteria and algae as food sources for free-living nematodes. Nematology 2013, 15, 1–13. [Google Scholar] [CrossRef]
- Sohlenius, B. Growth and Reproduction of a Nematode Acrobeloides sp. Cultivated on Agar. Oikos 1973, 24, 27. [Google Scholar] [CrossRef]
- Liu, T.; Li, Y.; Xu, J.; Yan, X.; Li, H.; Whalen, J.K.; Hu, F. Bacterial traits and quality contribute to the diet choice and survival of bacterial-feeding nematodes. Soil Biol. Biochem. 2017, 115, 467–474. [Google Scholar] [CrossRef]
- Ferris, H.; Bongers, T.; de Goede, R.G.M. A framework for soil food web diagnostics: Extension of the nematode faunal analysis concept. Appl. Soil. Ecol. 2001, 18, 13–29. [Google Scholar] [CrossRef]

| Body Length (µm) | Growth Duration from Egg to Adult (Days) | Fertility Period (Days) | Eggs Laid/Day | Total Brood Size | Pharyngeal Pump/min | Lifespan (Days) | |
|---|---|---|---|---|---|---|---|
| C. elegans | 1254 ± 28.04 | 3.04 ± 0.018 | 4.57 ± 0.190 | 56.96 ± 3.838 | 251.6 ± 10.86 | 110.5 ± 7.258 | 11.05 ± 0.720 |
| A. tricornis | 543.0 ± 16.29 | 14.03 ± 0.085 | 28.97 ± 1.538 | 8.36 ± 0.296 | 238.0 ± 10.60 | 30.20 ± 9.721 | 38.27 ± 1.581 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, J.-H.; Indong, R.A.; Alcantara, A.V., Jr.; Yoon, K.-h.; Lee, J.I. Comparison of Life Traits in Two Bacterivorous Nematodes Suggest Different Ecological Strategies to Exploit Similar Habitats. Life 2022, 12, 1516. https://doi.org/10.3390/life12101516
Moon J-H, Indong RA, Alcantara AV Jr., Yoon K-h, Lee JI. Comparison of Life Traits in Two Bacterivorous Nematodes Suggest Different Ecological Strategies to Exploit Similar Habitats. Life. 2022; 12(10):1516. https://doi.org/10.3390/life12101516
Chicago/Turabian StyleMoon, Je-Hyun, Rocel Amor Indong, Alfredo V. Alcantara, Jr., Kyoung-hye Yoon, and Jin I. Lee. 2022. "Comparison of Life Traits in Two Bacterivorous Nematodes Suggest Different Ecological Strategies to Exploit Similar Habitats" Life 12, no. 10: 1516. https://doi.org/10.3390/life12101516
APA StyleMoon, J.-H., Indong, R. A., Alcantara, A. V., Jr., Yoon, K.-h., & Lee, J. I. (2022). Comparison of Life Traits in Two Bacterivorous Nematodes Suggest Different Ecological Strategies to Exploit Similar Habitats. Life, 12(10), 1516. https://doi.org/10.3390/life12101516







