Deep Sea Water-Dissolved Organic Matter Intake Improves Hyperlipidemia and Inhibits Thrombus Formation and Vascular Inflammation in High-Fat Diet Hamsters
Abstract
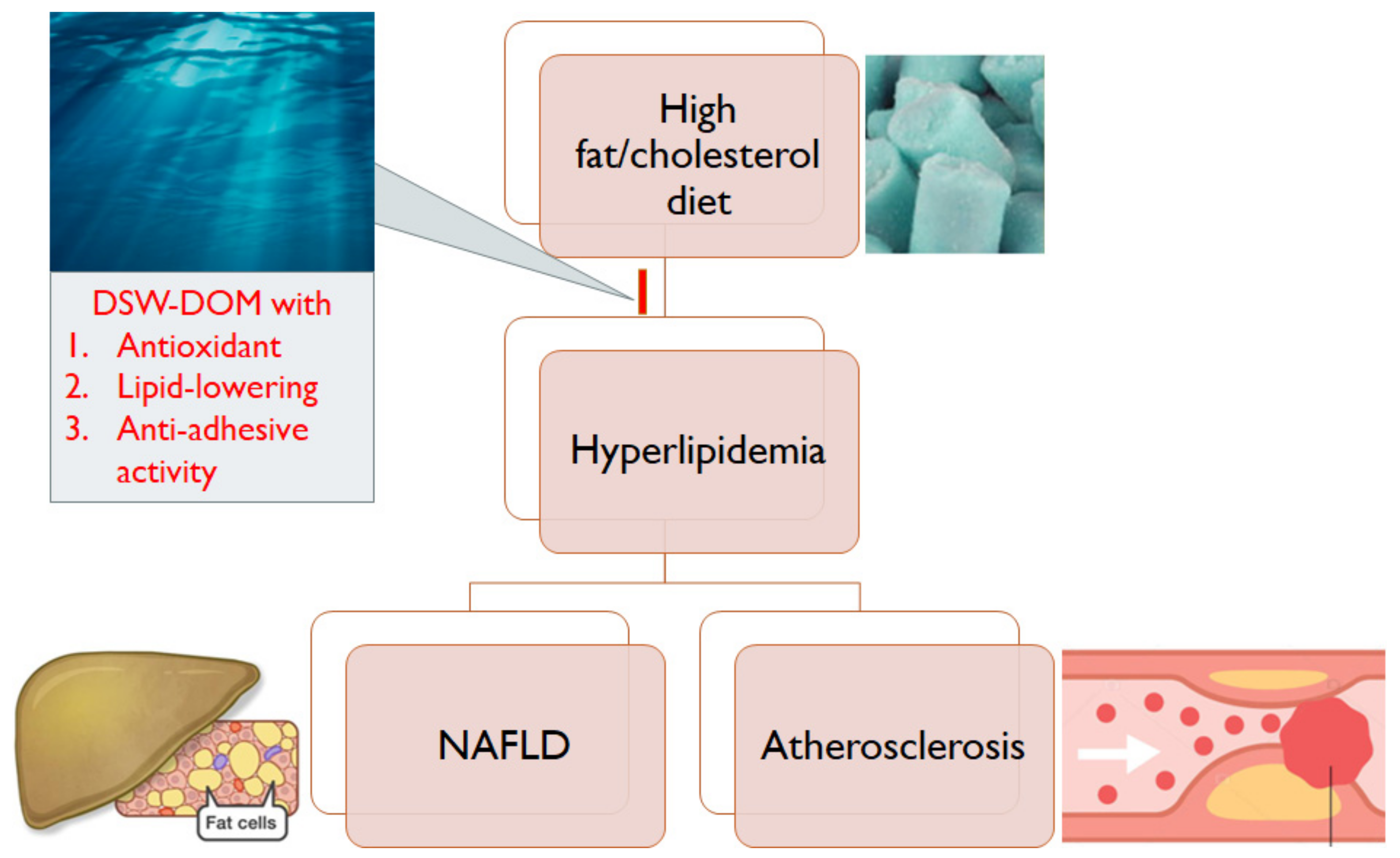
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Grouping
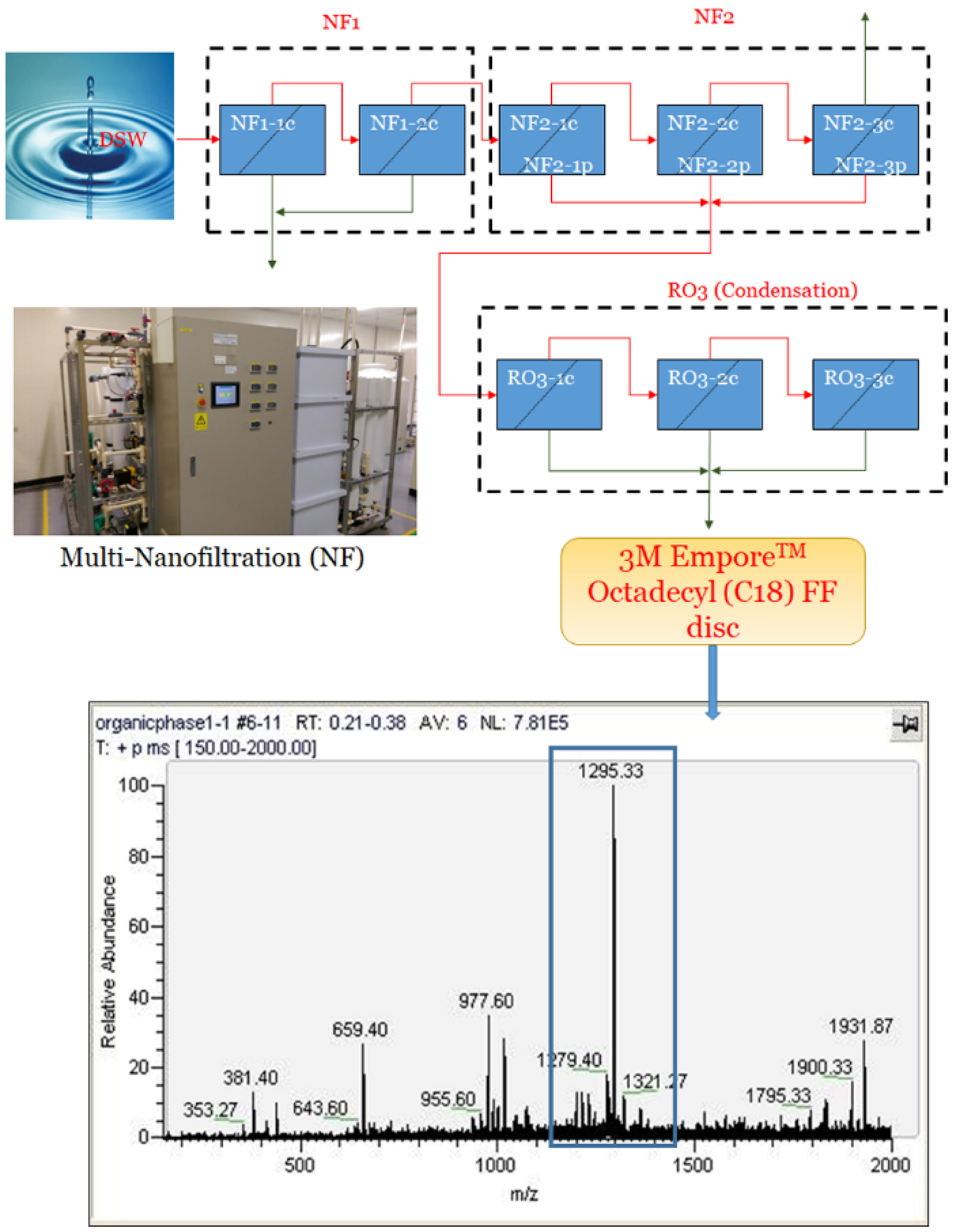
2.3. Preparation of Deep Sea Water-Dissolved Organic Matter (DSW-DOM)
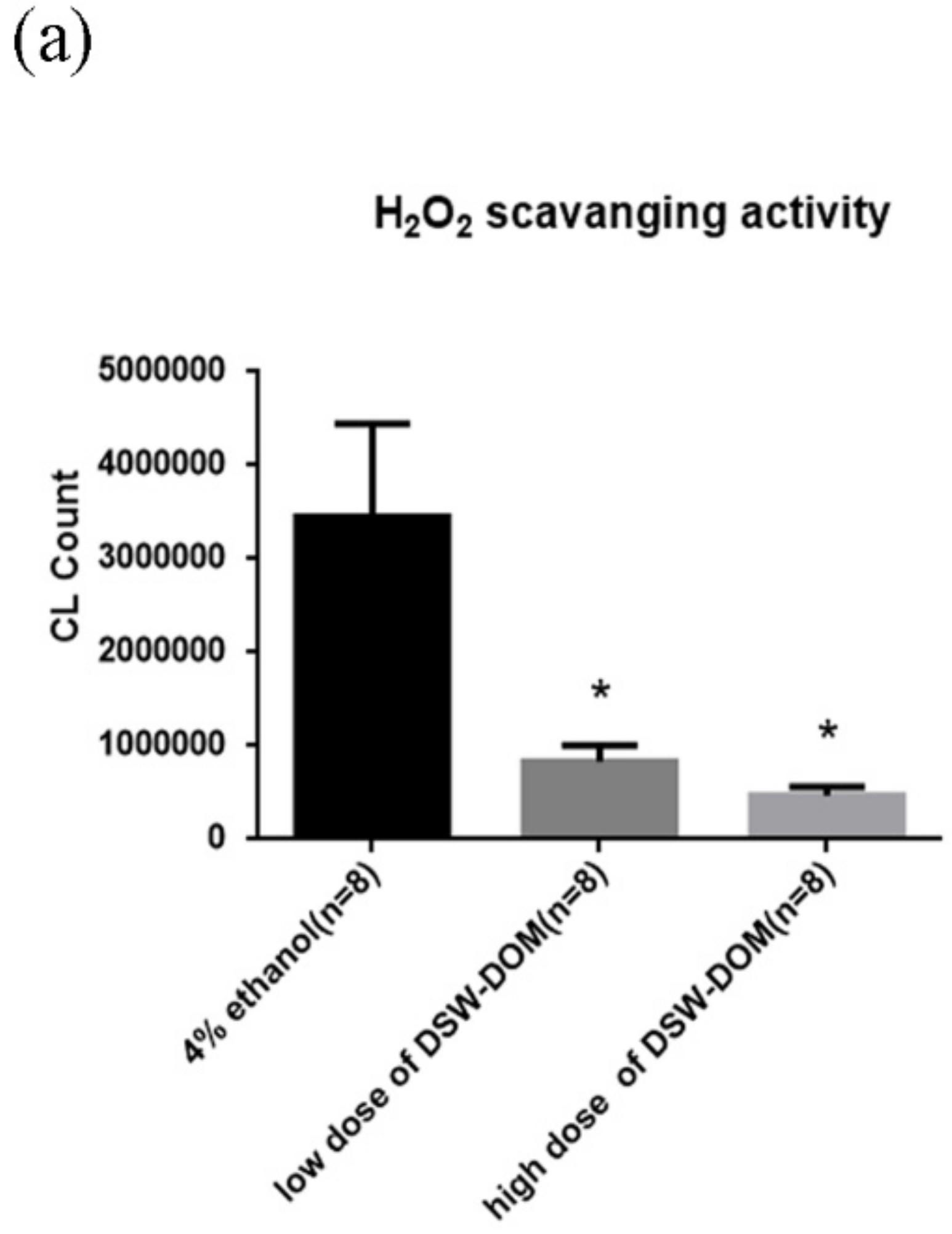
2.4. Hydrogen Peroxide (H2O2) Scavenging Capacity of DSW-DOM
2.5. Blood Lipid Analysis
2.6. Triglyceride (TG) Colorimetric Assay
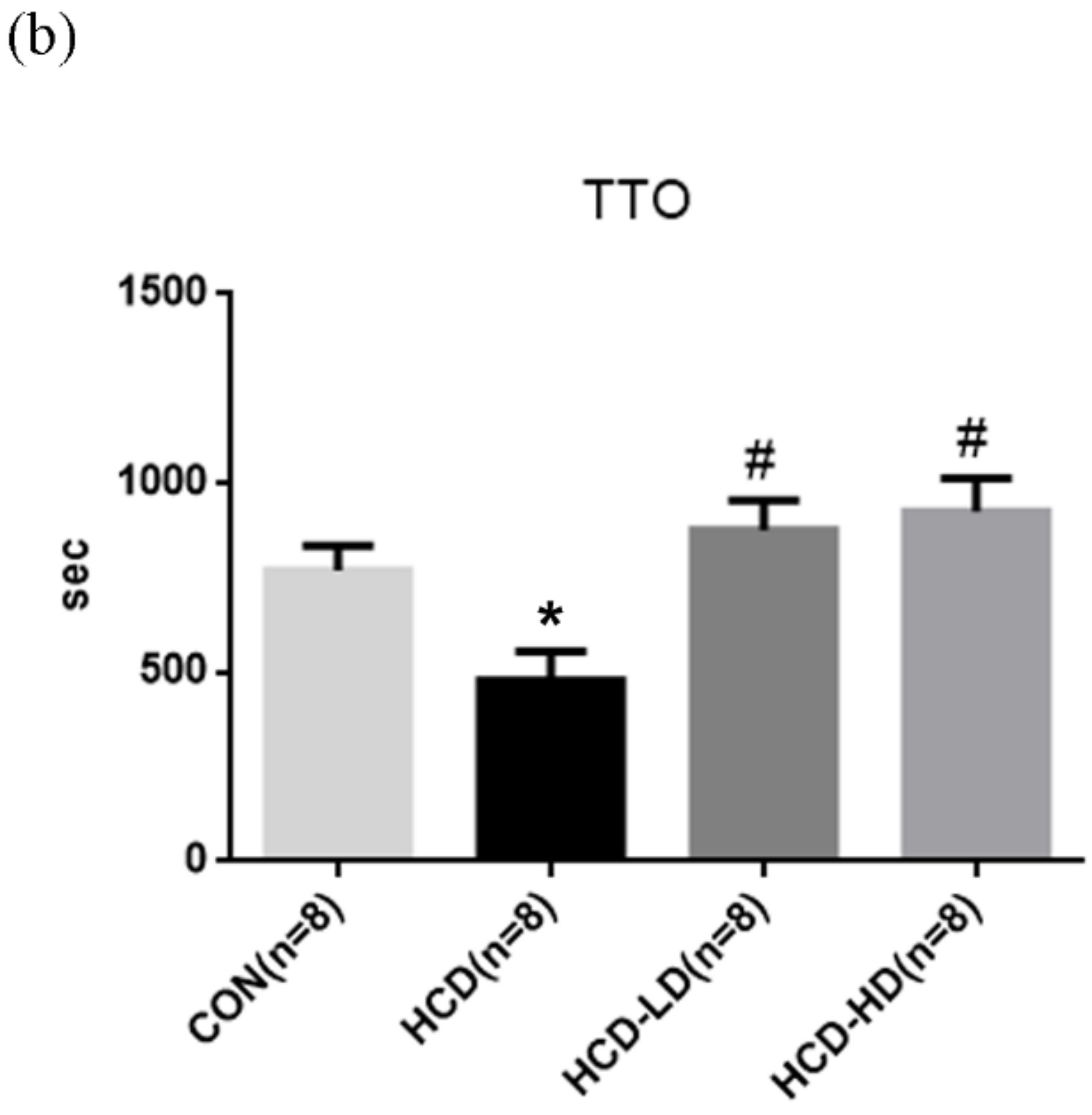
2.7. Ferric Chloride (FeCl3)-Induced Acute Arterial Thrombosis
2.8. Platelet Adhesiveness Detection
2.9. Blood Reactive Oxygen Species (ROS) Detection
2.10. Malondialdehyde (MDA) Assay
2.11. Histological Analysis
2.12. Oil Red O Stain
2.13. Immunohistochemistry
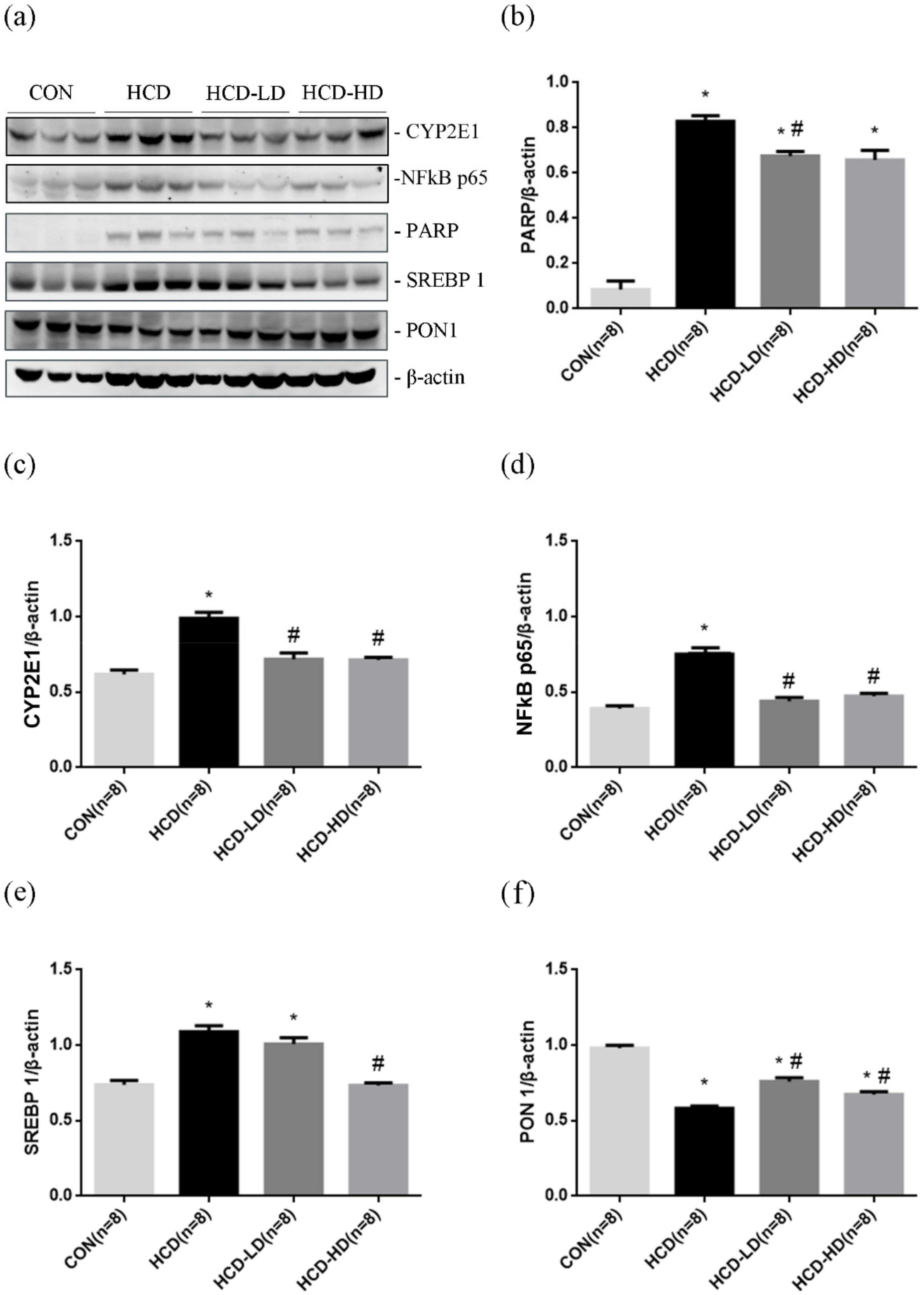
3. Western Blot
Statistical Analysis
4. Results
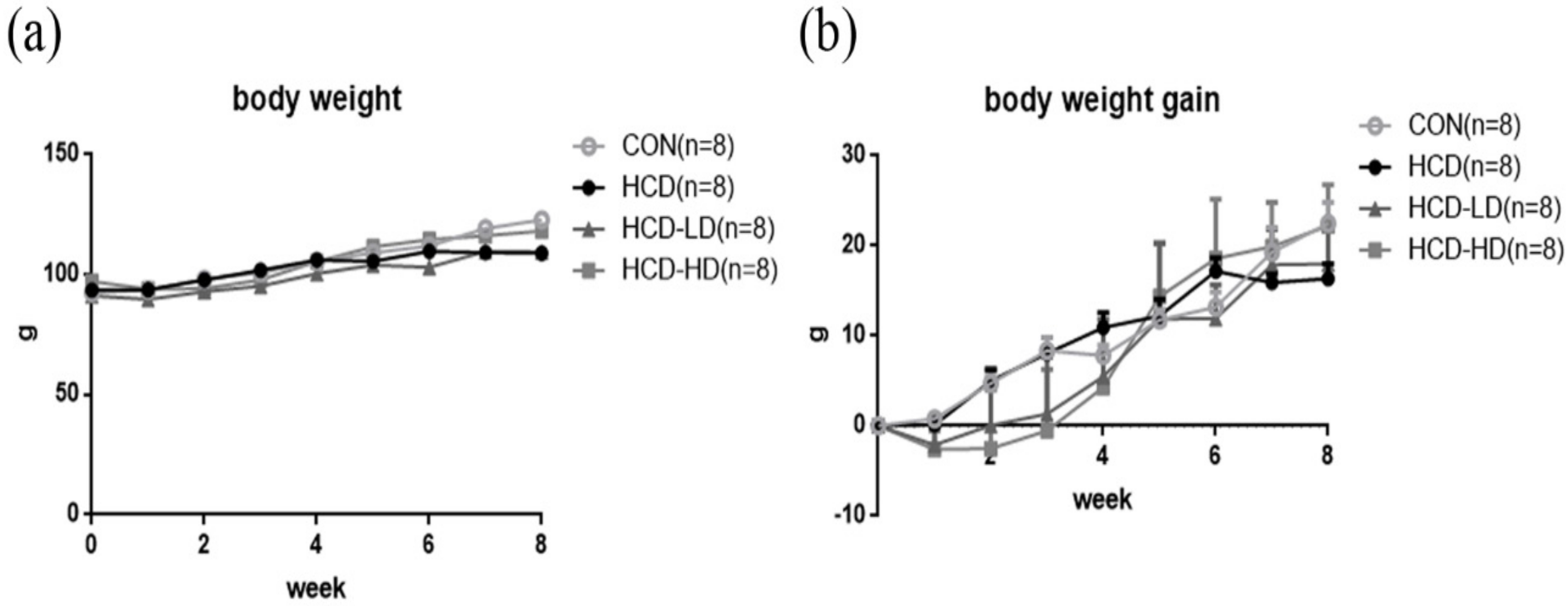
4.1. Body Weight was Not Significantly Different among Four Groups of Animals
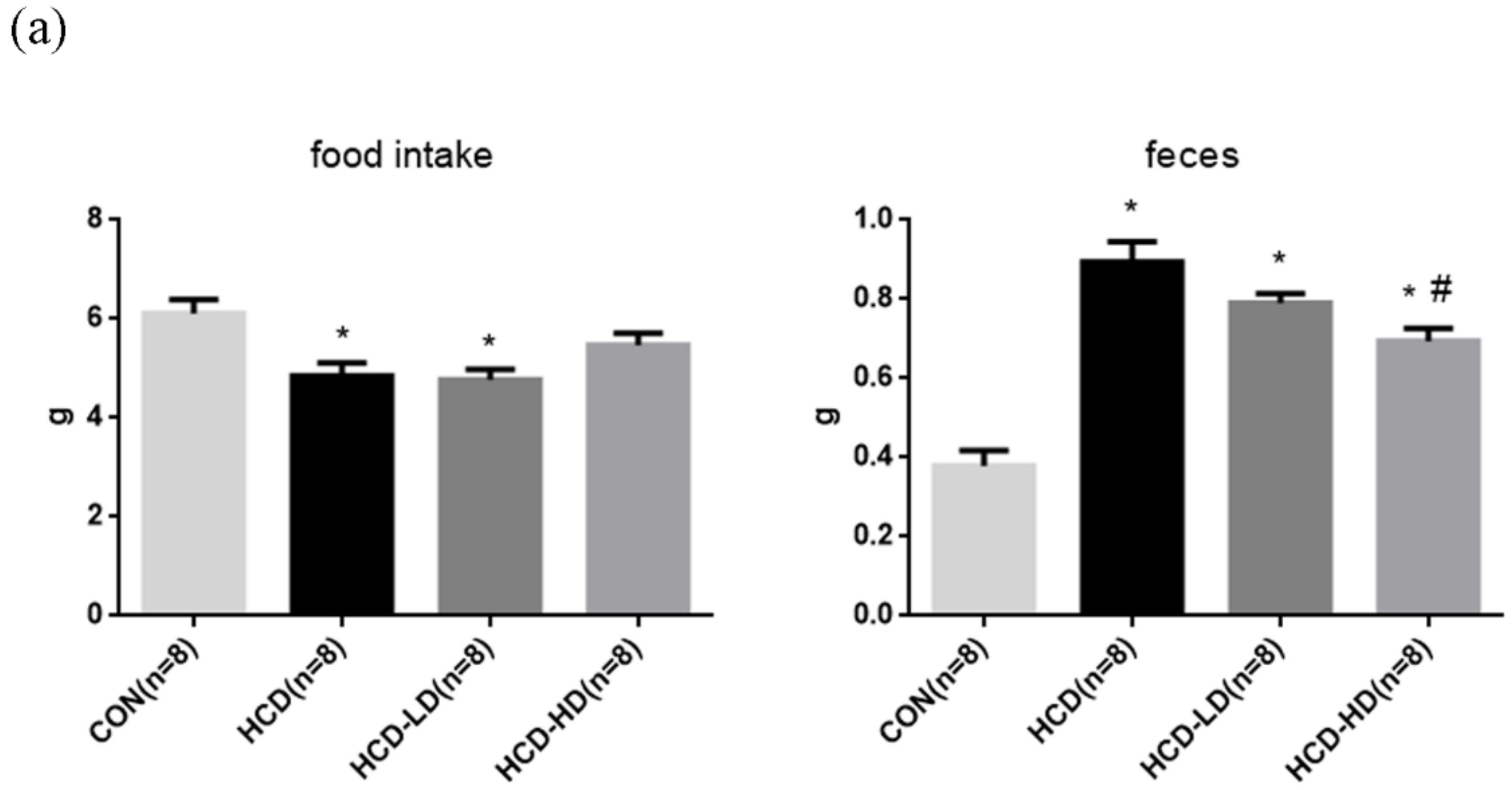
4.2. Food Intake and Feces
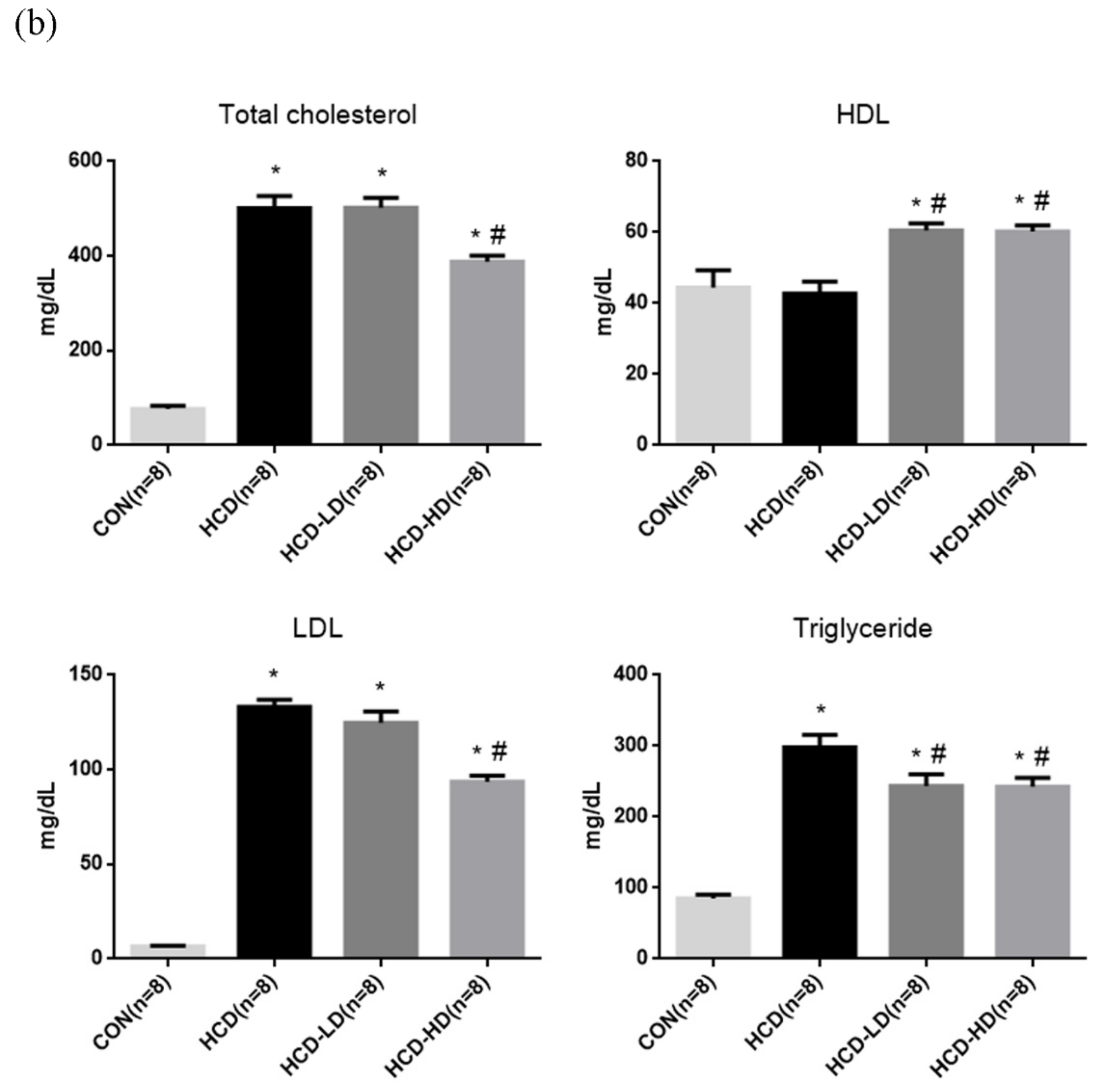
4.3. Lipid Profile
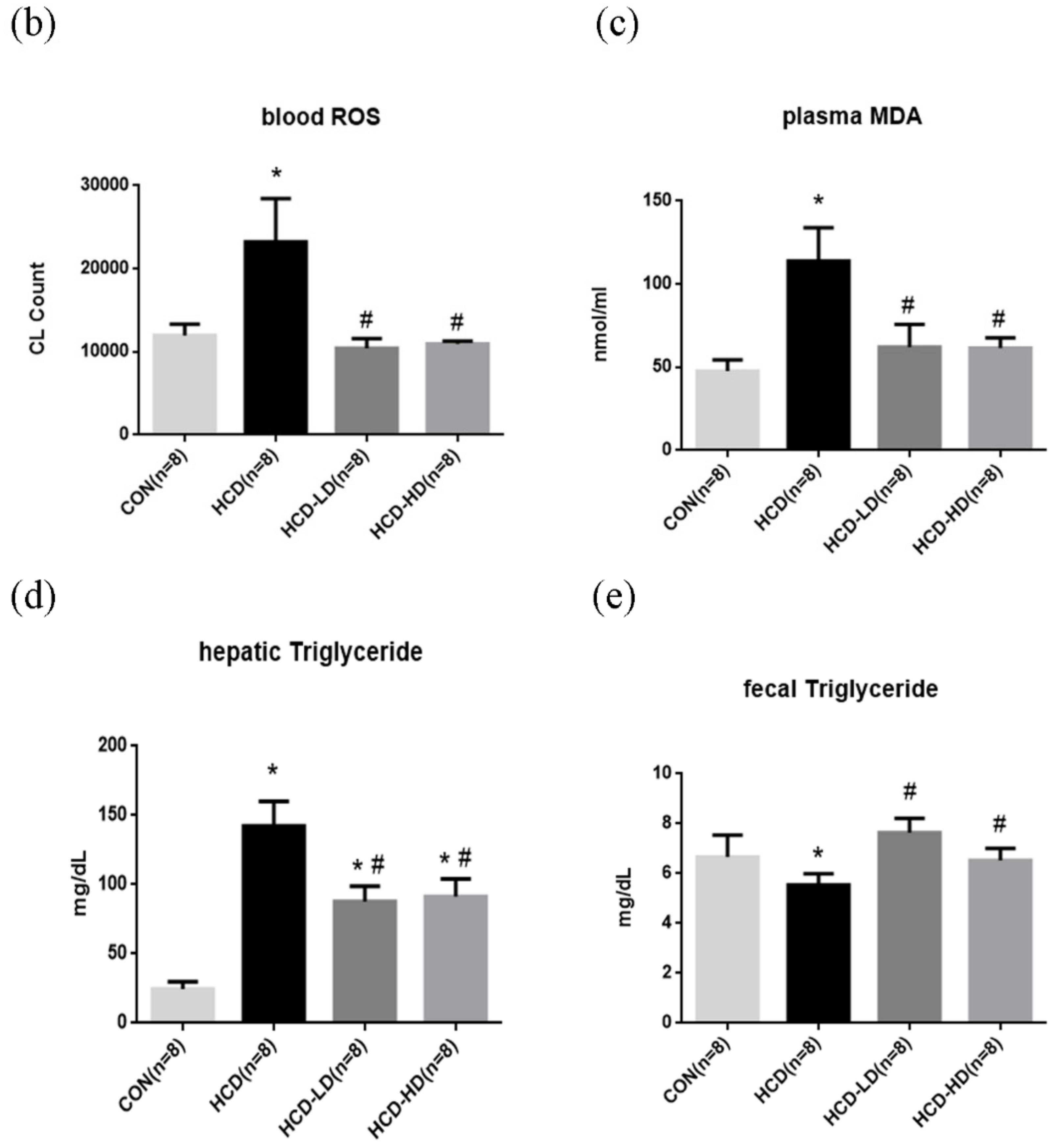
4.4. Hepatic and Fecal Triglyceride Concentration
4.5. DSW-DOM Can Efficiently Scavenge Hydrogen Peroxide (H2O2) In Vitro
4.6. DSW-DOM Significantly Reduces Blood Reactive Oxygen Species (ROS)
4.7. Malondialdehyde (MDA) Concentration in Plasma
4.8. DSW-DOM Significantly Reduces Hepatic Triglycerides via the Increased Fecal Excretion of Triglycerides
4.9. DSW-DOM Significantly Reduces the Oxidative and Inflammatory Parameters
4.10. DSW-DOM Significantly Delays the Acute Arterial Thrombosis Model of Time to Occlusion
4.11. DSW-DOM Efficiently Inhibits Platelet Adhesiveness Detection in Mesenteric Arterioles
4.12. DSW-DOM Efficiently Reduces Hepatic CYP2E1, Nfk p65, SREBP-1 and PARP and Increases PON1 Expression
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Statement of Ethics
Abbreviations
| NAFLD | Non-alcoholic fatty liver disease |
| CVD | Cardiovascular disease |
| TG | Triglyceride |
| DSW | Deep sea water |
| DSW-DOM | Deep sea water-dissolved organic matter |
| ox-LDL | Oxidized low-density lipoprotein |
| ROS | Reactive oxygen species |
| MDA | Malondialdehyde |
| LDL-C | Low-density lipoprotein cholesterol |
| VLDL-C | Very-low-density lipoprotein cholesterol |
| TC | Total cholesterol |
| MS | Metabolic syndrome |
| NASH | Nonalcoholic steatohepatitis |
| HCC | Hepatocellular carcinoma |
| LDL | Low-density lipoprotein |
| HDL | High-density lipoprotein |
| AMPK | AMP-activated protein kinase |
| CAD | Coronary artery disease |
| HDL-C | High-density lipoprotein cholesterol |
| TEAC | Trolox equivalent antioxidant capacity |
| LDL-R | LDL receptor |
| IGF-1R | Insulin-like growth factor 1 receptor |
| COX-1 | Cyclooxygenase 1 |
| HO-1 | Heme oxygenase-1 |
| CETP | Cholesteryl ester transport protein |
| IR | Insulin resistance |
| CON | Control group |
| HCD | High-fat/high-cholesterol diet group |
| HCD-LD | High-cholesterol diet with low dose of DSW-DOM |
| HCD-HD | High-cholesterol diet with high dose of DSW-DOM |
| C18 | Octadecyl |
| ddH2O | Double-distilled water |
| CL | Chemiluminescence |
| rcf | Relative centrifugal field |
| FeCl3 | Ferric chloride |
| TTO | Time to occlusion |
| rpm | Revolutions per minute |
| PGI2 | Prostacyclin |
| NaCl | Sodium chloride |
| Na2HPO4 | Sodium hydrogen phosphate |
| KCl | Potassium chloride |
| NaHCO3 | Sodium hydrogen carbonate |
| HEPES | 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid |
| BSA | Bovine serum albumin |
| PGI2 | Prostacyclin |
| AM | Acetoxymethyl |
| FP | Fluorescently-labeled platelets |
| H2SO4 | Sulfuric acid |
| BHT | Butylated hydroxytoluene |
| TBA | Thiobarbituric acid |
| ICAM-1 | Intercellular Adhesion Molecule 1 |
| PARP | Poly (ADP-ribose) polymerase |
| PON1 | Paraoxonase 1 |
| SREBP 1 | Sterol regulatory element-binding transcription factor 1 |
| VWF | Von Willebrand factor |
| HRP | Horseradish peroxidase |
| IgG | Immunoglobulin G |
| PBST | Phosphate buffer saline tween-20 |
| DAB | 3′,3′-diaminobenzendine |
| RIPA | Radio immunoprecipitation assay |
| BCA | Bicinchoninic acid |
| SDS-PAGE | Sodium dodecyl sulfate polyacrylamide gel electrophoresis |
| PVDF | Polyvinylidene difluoride |
| ECL | Enhanced chemiluminescence |
| HOCl | Hypochlorous acid |
| FFA | Free fatty acid |
| RCT | Reverse cholesterol transport |
| SEM | Standard error of the mean |
| ANOVA | Analysis of variance |
References
- Chait, A.; Brunzell, J. Acquired hyperlipidemia (secondary dyslipoproteinemias). Endocrinol. Metab. Clin. N. Am. 1990, 19, 259–278. [Google Scholar] [CrossRef]
- Tsai, T.-Y.; Chu, L.-H.; Lee, C.-L.; Pan, T.-M. Atherosclerosis-preventing activity of lactic acid bacteria-fermented milk− soymilk supplemented with Momordica charantia. J. Agric. Food Chem. 2009, 57, 2065–2071. [Google Scholar] [CrossRef]
- Baigent, C.; Blackwell, L.; Emberson, J.; Holland, L.; Reith, C.; Bhala, N.; Peto, R.; Barnes, E.; Keech, A.; Simes, J. Efficacy and Safety of More Intensive Lowering of LDL Cholesterol: A Meta-Analysis of Data from 170,000 Participants in 26 Randomised Trials; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Berry, J.D.; Dyer, A.; Cai, X.; Garside, D.B.; Ning, H.; Thomas, A.; Greenland, P.; Van Horn, L.; Tracy, R.P.; Lloyd-Jones, D.M. Lifetime Risks of Cardiovascular Disease. N. Engl. J. Med. 2012, 366, 321–329. [Google Scholar] [CrossRef]
- Harchaoui, K.; Visser, M.; Kastelein, J.; Stroes, E.; Dallinga-Thie, G. Triglycerides and cardiovascular risk. Curr. Cardiol. Rev. 2009, 5, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-S.; Kwon, Y.-S.; Kim, S.; Moon, D.-S.; Kim, H.J.; Nam, K.-S. Regulatory mechanism of mineral-balanced deep sea water on hypocholesterolemic effects in HepG2 hepatic cells. Biomed. Pharmacother. 2017, 86, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Mills, S.J.; Harrison, S.A. Comparison of the natural history of alcoholic and nonalcoholic fatty liver disease. Curr. Gastroenter. Rep. 2005, 7, 32–36. [Google Scholar] [CrossRef]
- Serviddio, G.; Bellanti, F.; Vendemiale, G. Free radical biology for medicine: Learning from nonalcoholic fatty liver disease. Free Radic. Biol. Med. 2013, 65, 952–968. [Google Scholar] [CrossRef]
- Zhu, X.; Yan, H.; Xia, M.; Chang, X.; Xu, X.; Wang, L.; Sun, X.; Lu, Y.; Bian, H.; Li, X. Metformin attenuates triglyceride accumulation in HepG2 cells through decreasing stearyl-coenzyme A desaturase 1 expression. Lipids Health Dis. 2018, 17, 1–9. [Google Scholar] [CrossRef]
- Adams, L.A.; Waters, O.R.; Knuiman, M.W.; Elliott, R.R.; Olynyk, J.K. NAFLD as a risk factor for the development of diabetes and the metabolic syndrome: An eleven-year follow-up study. Am. J. Gastroenterol. 2009, 104, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Wesolowski, S.R.; El Kasmi, K.C.; Jonscher, K.R.; Friedman, J.E. Developmental origins of NAFLD: A womb with a clue. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 81–96. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease—Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Ridker, P.M.; Hansson, G.K. Progress and challenges in translating the biology of atherosclerosis. Nature 2011, 473, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Davignon, J.; Ganz, P. Role of endothelial dysfunction in atherosclerosis. Circulation 2004, 109, III-27–III-32. [Google Scholar] [CrossRef]
- Korporaal, S.J.; Akkerman, J.-W.N. Platelet activation by low density lipoprotein and high density lipoprotein. Pathophysiol. Haemost. Thromb. 2006, 35, 270–280. [Google Scholar] [CrossRef]
- Gawaz, M.; Langer, H.; May, A.E. Platelets in inflammation and atherogenesis. J.Clin. Investig. 2005, 115, 3378–3384. [Google Scholar] [CrossRef]
- Yamada, Y.; Doi, T.; Hamakubo, T.; Kodama, T. Scavenger receptor family proteins: Roles for atherosclerosis, host defence and disorders of the central nervous system. Cell. Mol. Life Sci. CMLS 1998, 54, 628–640. [Google Scholar] [CrossRef] [PubMed]
- Lusis, A.J. Atherosclerosis. Nature 2000, 407, 233. [Google Scholar] [CrossRef]
- Nakasone, T.; Akeda, S. The application of deep sea water in Japan. In Proceedings of the Twenty-Eighth UJNR Aquaculture Panel Symposium, Kihei, HI, USA, 10–12 November 1999; pp. 69–75. [Google Scholar]
- Miyamura, M.; Yoshioka, S.; Hamada, A.; Takuma, D.; Yokota, J.; Kusunose, M.; Kyotani, S.; Kawakita, H.; Odani, K.; TsUTSUI, Y. Difference between deep seawater and surface seawater in the preventive effect of atherosclerosis. Biol. Pharm. Bull. 2004, 27, 1784–1787. [Google Scholar] [CrossRef]
- Kimata, H.; Tai, H.; Nakagawa, K.; Yokoyama, Y.; Nakajima, H.; Ikegami, Y. Improvement of skin symptoms and mineral imbalance by drinking deep sea water in patients with atopic eczema/dermatitis syndrome (AEDS). Acta Med.-Hrad. Kral. 2002, 45, 83–84. [Google Scholar] [CrossRef]
- Hwang, H.S.; Kim, H.A.; Lee, S.H.; Yun, J.W. Anti-obesity and antidiabetic effects of deep sea water on ob/ob mice. Mar. Biotechnol. 2009, 11, 531–539. [Google Scholar] [CrossRef]
- Yoshioka, S.; Hamada, A.; Cui, T.; Yokota, J.; Yamamoto, S.; Kusunose, M.; Miyamura, M.; Kyotani, S.; Kaneda, R.; Tsutsui, Y. Pharmacological activity of deep-sea water: Examination of hyperlipemia prevention and medical treatment effect. Biol. Pharm. Bull. 2003, 26, 1552–1559. [Google Scholar] [CrossRef] [PubMed]
- Katsuda, S.-I.; Yasukawa, T.; Nakagawa, K.; Miyake, M.; Yamasaki, M.; Katahira, K.; Mohri, M.; Shimizu, T.; Hazama, A. Deep-sea water improves cardiovascular hemodynamics in Kurosawa and Kusanagi-Hypercholesterolemic (KHC) rabbits. Biol. Pharm. Bull. 2008, 31, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.-S.; Chang, Y.-Y.; Hsu, C.-L.; Lin, H.-W.; Chang, M.-H.; Chen, J.-W.; Chen, S.-S.; Chen, Y.-C. Alleviative effects of deep-seawater drinking water on hepatic lipid accumulation and oxidation induced by a high-fat diet. J. Chin. Med. Assoc. 2013, 76, 95–101. [Google Scholar] [CrossRef]
- Ha, B.G.; Shin, E.J.; Park, J.-E.; Shon, Y.H. Anti-diabetic effect of balanced deep-sea water and its mode of action in high-fat diet induced diabetic mice. Mar. Drugs 2013, 11, 4193–4212. [Google Scholar] [CrossRef]
- Fu, Z.-Y.; Yang, F.L.; Hsu, H.-W.; Lu, Y.-F. Drinking deep seawater decreases serum total and low-density lipoprotein–cholesterol in hypercholesterolemic subjects. J. Med. Food 2012, 15, 535–541. [Google Scholar] [CrossRef]
- Hsu, C.-L.; Chang, Y.-Y.; Chiu, C.-H.; Yang, K.-T.; Wang, Y.; Fu, S.-G.; Chen, Y.-C. Cardiovascular protection of deep-seawater drinking water in high-fat/cholesterol fed hamsters. Food Chem. 2011, 127, 1146–1152. [Google Scholar] [CrossRef]
- Sheu, M.-J.; Chou, P.-Y.; Lin, W.-H.; Pan, C.-H.; Chien, Y.-C.; Chung, Y.-L.; Liu, F.-C.; Wu, C.-H. Deep sea water modulates blood pressure and exhibits hypolipidemic effects via the AMPK-ACC pathway: An in vivo study. Mar. Drugs 2013, 11, 2183–2202. [Google Scholar] [CrossRef]
- Chang, M.H.; Tzang, B.S.; Yang, T.Y.; Hsiao, Y.C.; Yang, H.C.; Chen, Y.C. Effects of Deep-Seawater on Blood Lipids and Pressure in High-Cholesterol Dietary Mice. J. Food Biochem. 2011, 35, 241–259. [Google Scholar] [CrossRef]
- He, S.; Hao, J.; Peng, W.; Qiu, P.; Li, C.; Guan, H. Modulation of lipid metabolism by deep-sea water in cultured human liver (HepG2) cells. Mar. Biotech. 2014, 16, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Nani, M.; Zura, S.; Majid, F.; Jaafar, A.; Mahdzir, A.; Musa, M. Potential health benefits of deep sea water: A review. Evid.-Based Complement. Altern. Med. 2016, 2016, 6520475. [Google Scholar]
- Shen, J.L.; Hsu, T.C.; Chen, Y.C.; Hsu, J.D.; Yang, L.C.; Tsai, F.J.; Li, C.C.; Cheng, Y.W.; Huang, C.Y.; Tzang, B.S. Effects of deep-sea water on cardiac abnormality in high-cholesterol dietary mice. J. Food Biochem. 2012, 36, 1–11. [Google Scholar] [CrossRef]
- Radhakrishnan, G.; Yamamoto, M.; Maeda, H.; Nakagawa, A.; KatareGopalrao, R.; Okada, H.; Nishimori, H.; Wariishi, S.; Toda, E.; Ogawa, H. Intake of dissolved organic matter from deep seawater inhibits atherosclerosis progression. Biochem. Biophys. Res. Commun. 2009, 387, 25–30. [Google Scholar] [CrossRef]
- Huang, W.-C.; Lin, C.-L.; Hsu, Y.-J.; Chiu, Y.-S.; Chen, Y.-M.; Wu, M.-F.; Huang, C.-C.; Wang, M.-F. Inulin and fibersol-2 combined have hypolipidemic effects on high cholesterol diet-induced hyperlipidemia in hamsters. Molecules 2016, 21, 313. [Google Scholar] [CrossRef]
- Dillard, A.; Matthan, N.R.; Lichtenstein, A.H. Use of hamster as a model to study diet-induced atherosclerosis. Nutr. Metab. 2010, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Dorfman, S.E.; Smith, D.E.; Osgood, D.P.; Lichtenstein, A.H. Study of diet-induced changes in lipoprotein metabolism in two strains of Golden-Syrian hamsters. J. Nutr. 2003, 133, 4183–4188. [Google Scholar] [CrossRef][Green Version]
- Srivastava, R.A.K.; He, S. Anti-hyperlipidemic and insulin sensitizing activities of fenofibrate reduces aortic lipid deposition in hyperlipidemic Golden Syrian hamster. Mol. Cell. Biochem. 2010, 345, 197–206. [Google Scholar] [CrossRef]
- Dalbøge, L.S.; Pedersen, P.J.; Hansen, G.; Fabricius, K.; Hansen, H.B.; Jelsing, J.; Vrang, N. A hamster model of diet-induced obesity for preclinical evaluation of anti-obesity, anti-diabetic and lipid modulating agents. PLoS ONE 2015, 10, e0135634. [Google Scholar] [CrossRef]
- Chen, D.-L.; Chen, T.-W.; Chien, C.-T.; Li, P.-C. Intravenous low redox potential saline attenuates FeCl3-induced vascular dysfunction via downregulation of endothelial H2O2, CX3CL1, intercellular adhesion molecule-1, and p53 expression. Transl. Res. 2011, 157, 306–319. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.A.; Welsh, D.C.; Bickel, D.J.; Lynch, J.J., Jr.; Lyle, E.A. Differential effects of sodium nitroprusside and hydralazine in a rat model of topical FeCl3-induced carotid artery thrombosis. Thromb. Res. 2003, 111, 59–64. [Google Scholar] [CrossRef]
- Eckly, A.; Hechler, B.; Freund, M.; Zerr, M.; Cazenave, J.P.; Lanza, F.; Mangin, P.; Gachet, C. Mechanisms underlying FeCl3-induced arterial thrombosis. J. Thromb. Haemost. 2011, 9, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-C.; Yao, C.-A.; Lin, Y.-R.; Yang, J.-C.; Chien, C.-T. Deep-sea water containing selenium provides intestinal protection against duodenal ulcers through the upregulation of Bcl-2 and thioredoxin reductase 1. PLoS ONE 2014, 9, e96006. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pessayre, D.; Berson, A.; Fromenty, B.; Mansouri, A. Mitochondria in Steatohepatitis. In Seminars in Liver Disease: 2001; Thieme Medical Publishers, Inc.: New York, NY, USA, 2001; pp. 57–70. [Google Scholar]
- Sviridov, D.; Nestel, P. Dynamics of reverse cholesterol transport: Protection against atherosclerosis. Atherosclerosis 2002, 161, 245–254. [Google Scholar] [CrossRef]
- Grundy, S.M. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Final Report. Circulation 2002, 106, 3143–3421. [Google Scholar] [CrossRef]
- Barter, P.J.; Caulfield, M.; Eriksson, M.; Grundy, S.M.; Kastelein, J.J.; Komajda, M.; Lopez-Sendon, J.; Mosca, L.; Tardif, J.-C.; Waters, D.D. Effects of torcetrapib in patients at high risk for coronary events. N. Engl. J. Med. 2007, 357, 2109–2122. [Google Scholar] [CrossRef]
- Investigators, A.-H. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N. Engl. J. Med. 2011, 365, 2255–2267. [Google Scholar] [CrossRef]
- Tsompanidi, E.M.; Brinkmeier, M.S.; Fotiadou, E.H.; Giakoumi, S.M.; Kypreos, K.E. HDL biogenesis and functions: Role of HDL quality and quantity in atherosclerosis. Atherosclerosis 2010, 208, 3–9. [Google Scholar] [CrossRef]
- Litvinov, D.; Mahini, H.; Garelnabi, M. Antioxidant and anti-inflammatory role of paraoxonase 1: Implication in arteriosclerosis diseases. N. Am. J. Med. Sci. 2012, 4, 523–532. [Google Scholar] [PubMed]
- Haraguchi, Y.; Toh, R.; Hasokawa, M.; Nakajima, H.; Honjo, T.; Otsui, K.; Mori, K.; Miyamoto-Sasaki, M.; Shinohara, M.; Nishimura, K. Serum myeloperoxidase/paraoxonase 1 ratio as potential indicator of dysfunctional high-density lipoprotein and risk stratification in coronary artery disease. Atherosclerosis 2014, 234, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Kostapanos, M.S.; Elisaf, M.S. High density lipoproteins and type 2 diabetes: Emerging concepts in their relationship. World J. Exp. Med. 2014, 4, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Stancu, C.S.; Carnuta, M.G.; Sanda, G.M.; Toma, L.; Deleanu, M.; Niculescu, L.S.; Sasson, S.; Simionescu, M.; Sima, A.V. Hyperlipidemia-induced hepatic and small intestine ER stress and decreased paraoxonase 1 expression and activity is associated with HDL dysfunction in Syrian hamsters. Mol. Nutr. Food Res. 2015, 59, 2293–2302. [Google Scholar] [CrossRef]
- Pessayre, D.; Mansouri, A.; Fromenty, B.V. Mitochondrial dysfunction in steatohepatitis. Am. J. Physiol.-Gastrointest. Liver Physiol. 2002, 282, G193–G199. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, I.; Shimamoto, N.; Saiki, K.; Furujo, M.; Osaw, K. Lipid Peroxidation in Hepatic Fibrosis. In Lipid Peroxidation; BoD—Books on Demand: Norderstedt, Germany, 2012. [Google Scholar]
- Kojima, H.; Sakurai, S.; Uemura, M.; Fukui, H.; Morimoto, H.; Tamagawa, Y. Mitochondrial abnormality and oxidative stress in nonalcoholic steatohepatitis. Alcohol. Clin. Exp. Res. 2007, 31, S61–S66. [Google Scholar] [CrossRef]
- Wang, Y.; Ausman, L.M.; Russell, R.M.; Greenberg, A.S.; Wang, X.-D. Increased apoptosis in high-fat diet–induced nonalcoholic steatohepatitis in rats is associated with c-Jun NH2-terminal kinase activation and elevated proapoptotic Bax. J. Nutr. 2008, 138, 1866–1871. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Quan, X.-B.; Zeng, W.-J.; Yang, X.-O.; Wang, M.-J. Mechanism of Hepatocyte Apoptosis. J. Cell Death 2016, 9, JCD.S39824. [Google Scholar] [CrossRef] [PubMed]
- Savage, D.B.; Choi, C.S.; Samuel, V.T.; Liu, Z.-X.; Zhang, D.; Wang, A.; Zhang, X.-M.; Cline, G.W.; Yu, X.X.; Geisler, J.G. Reversal of diet-induced hepatic steatosis and hepatic insulin resistance by antisense oligonucleotide inhibitors of acetyl-CoA carboxylases 1 and 2. J. Clin. Investig. 2006, 116, 817–824. [Google Scholar] [CrossRef]
- Steinberg, D. Low density lipoprotein oxidation and its pathobiological significance. J. Biol. Chem. 1997, 272, 20963–20966. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, D. The LDL modification hypothesis of atherogenesis: An update. J. Lipid Res. 2009, 50, S376–S381. [Google Scholar] [CrossRef]
- Spady, D.; Woollett, L.; Dietschy, J. Regulation of plasma LDL-cholesterol levels by dietary cholesterol and fatty acids. Ann. Rev. Nutr. 1993, 13, 355–381. [Google Scholar] [CrossRef] [PubMed]
- Harmon, J.T.; Tandon, N.N.; Hoeg, J.M.; Jamieson, G. Thrombin binding and response in platelets from patients with dyslipoproteinemias: Increased stimulus-response coupling in type II hyperlipoproteinemia. Blood 1986, 68, 498–505. [Google Scholar] [CrossRef]
- Magwenzi, S.; Woodward, C.; Wraith, K.S.; Aburima, A.; Raslan, Z.; Jones, H.; McNeil, C.; Wheatcroft, S.; Yuldasheva, N.; Febbriao, M.; et al. Oxidized LDL activates blood platelets through CD36/NOX2–mediated inhibition of the cGMP/protein kinase G signaling cascade. Blood 2015, 125, 2693–2703. [Google Scholar] [CrossRef]
- Huo, Y.; Schober, A.; Forlow, S.B.; Smith, D.F.; Hyman, M.C.; Jung, S.; Littman, D.R.; Weber, C.; Ley, K. Circulating activated platelets exacerbate atherosclerosis in mice deficient in apolipoprotein E. Nat. Med. 2003, 9, 61–67. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.-C.; Cheng, Y.-H.; Chen, K.-H.; Chien, C.-T. Deep Sea Water-Dissolved Organic Matter Intake Improves Hyperlipidemia and Inhibits Thrombus Formation and Vascular Inflammation in High-Fat Diet Hamsters. Life 2022, 12, 82. https://doi.org/10.3390/life12010082
Wu C-C, Cheng Y-H, Chen K-H, Chien C-T. Deep Sea Water-Dissolved Organic Matter Intake Improves Hyperlipidemia and Inhibits Thrombus Formation and Vascular Inflammation in High-Fat Diet Hamsters. Life. 2022; 12(1):82. https://doi.org/10.3390/life12010082
Chicago/Turabian StyleWu, Chia-Chun, Yu-Hsuan Cheng, Kuo-Hsin Chen, and Chiang-Ting Chien. 2022. "Deep Sea Water-Dissolved Organic Matter Intake Improves Hyperlipidemia and Inhibits Thrombus Formation and Vascular Inflammation in High-Fat Diet Hamsters" Life 12, no. 1: 82. https://doi.org/10.3390/life12010082
APA StyleWu, C.-C., Cheng, Y.-H., Chen, K.-H., & Chien, C.-T. (2022). Deep Sea Water-Dissolved Organic Matter Intake Improves Hyperlipidemia and Inhibits Thrombus Formation and Vascular Inflammation in High-Fat Diet Hamsters. Life, 12(1), 82. https://doi.org/10.3390/life12010082





