Lactic Acid Permeability of Aquaporin-9 Enables Cytoplasmic Lactate Accumulation via an Ion Trap
Abstract
1. Introduction
2. Materials and Methods
2.1. Plasmids and Cloning
2.2. Yeast Transformation and Culture
2.3. Radiolabel Transport Assays
2.4. Statistical Analysis
3. Results
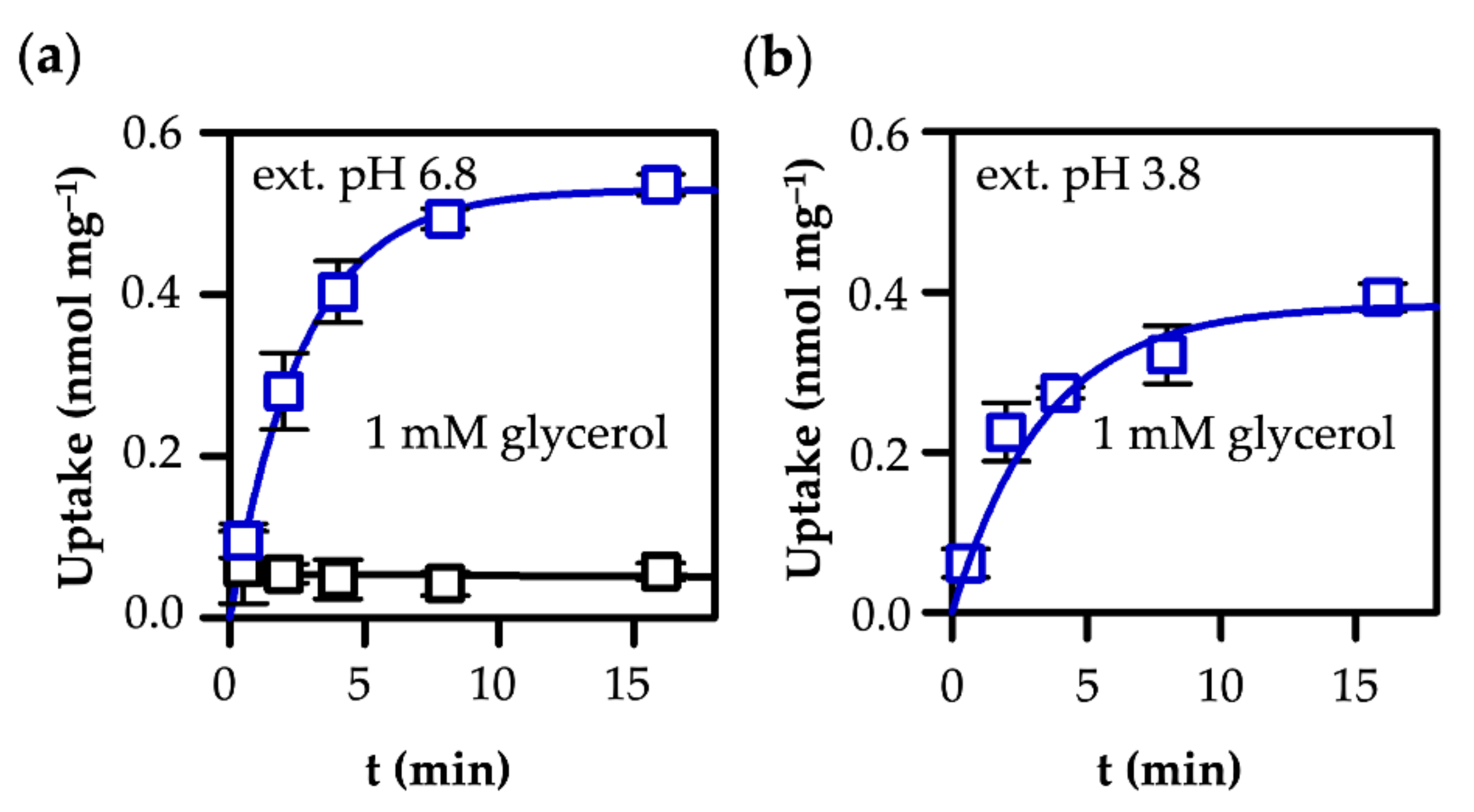
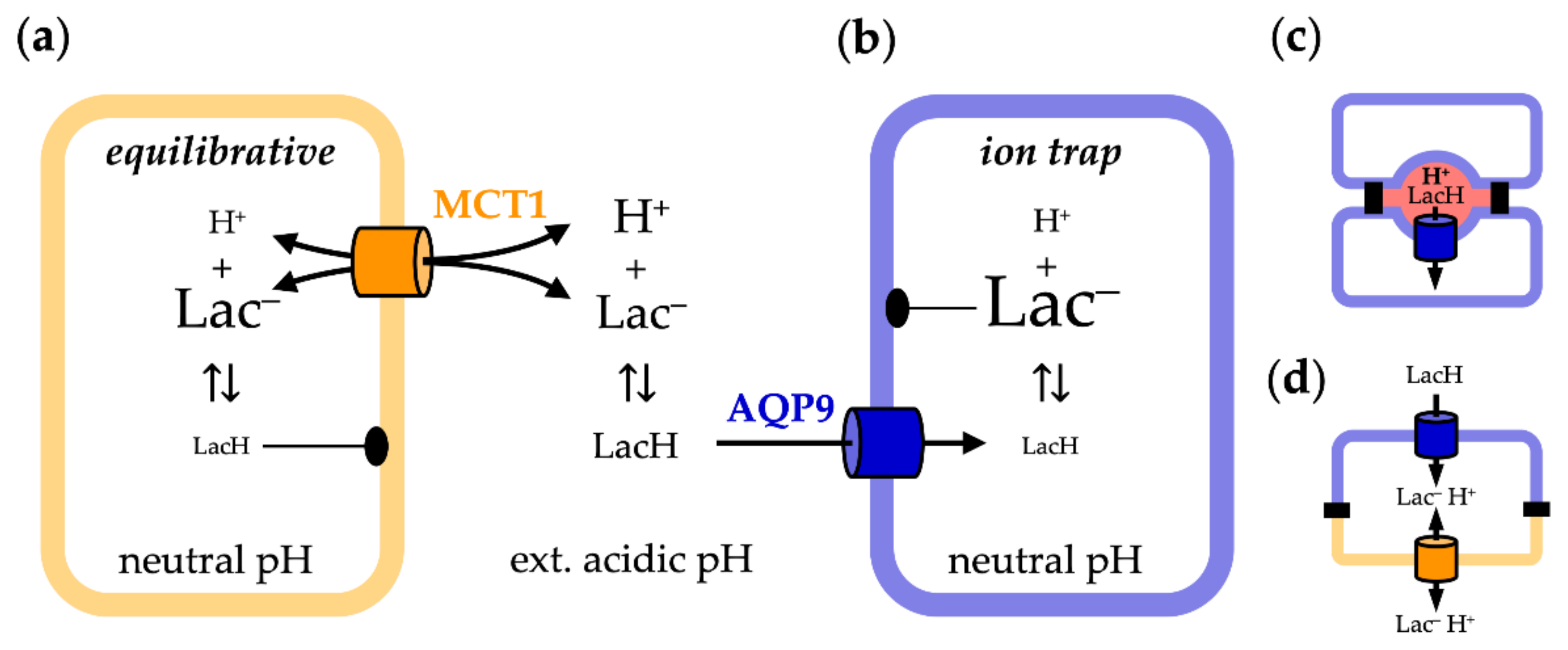
3.1. AQP9-Mediated Uptake of Glycerol Is Equilibrative and pH-Independent
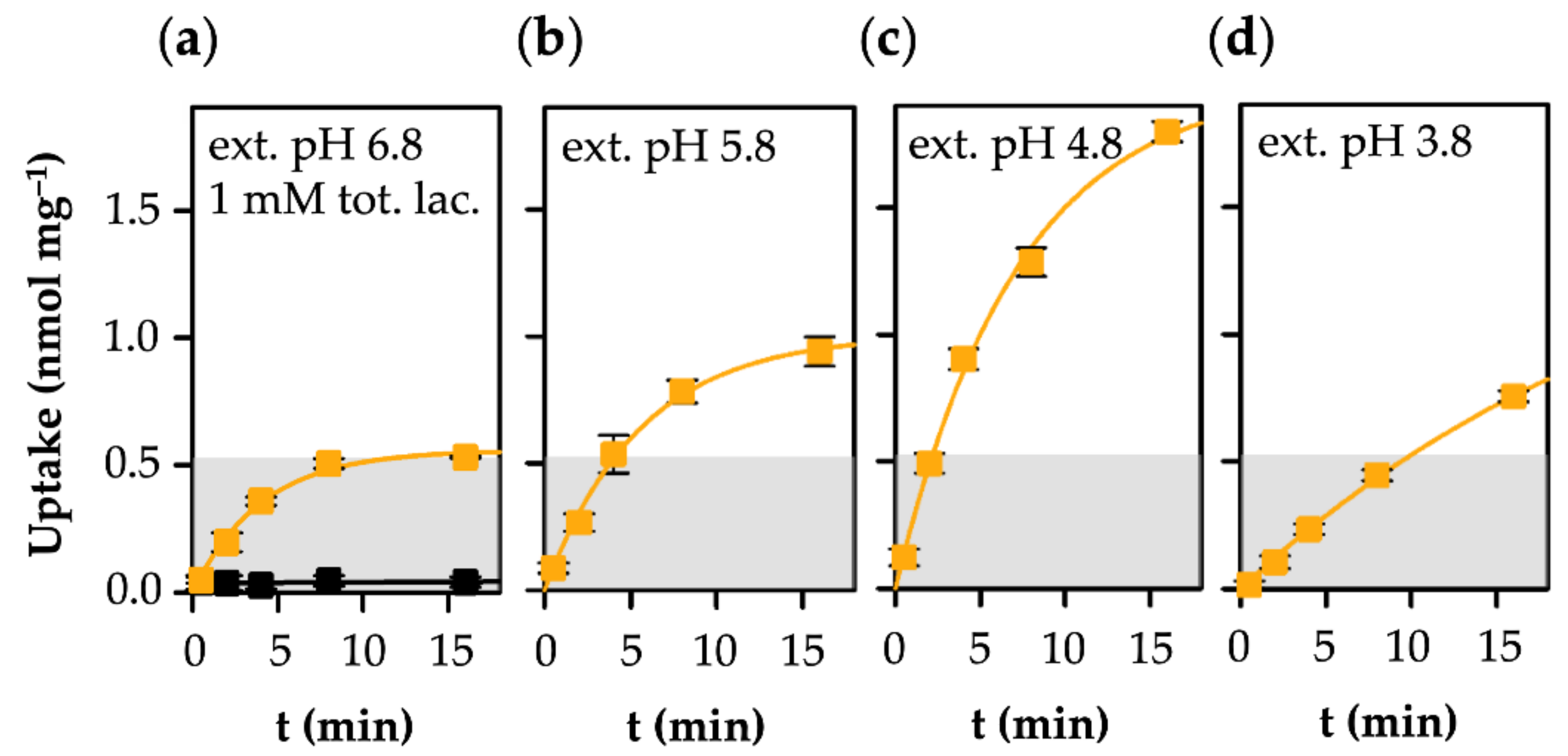
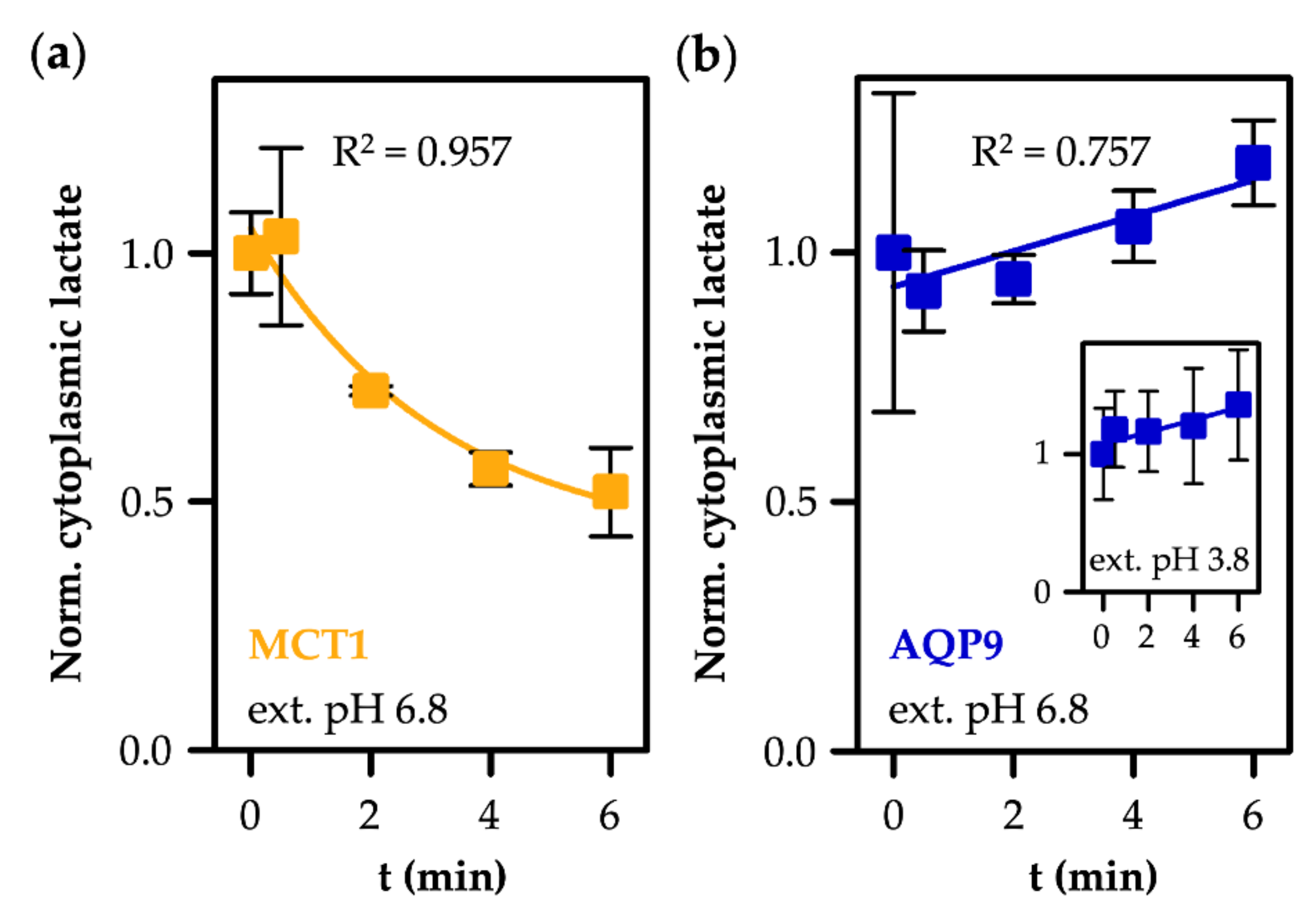
3.2. MCT1-Mediated Uptake of l-Lactate Is pH-Dependent
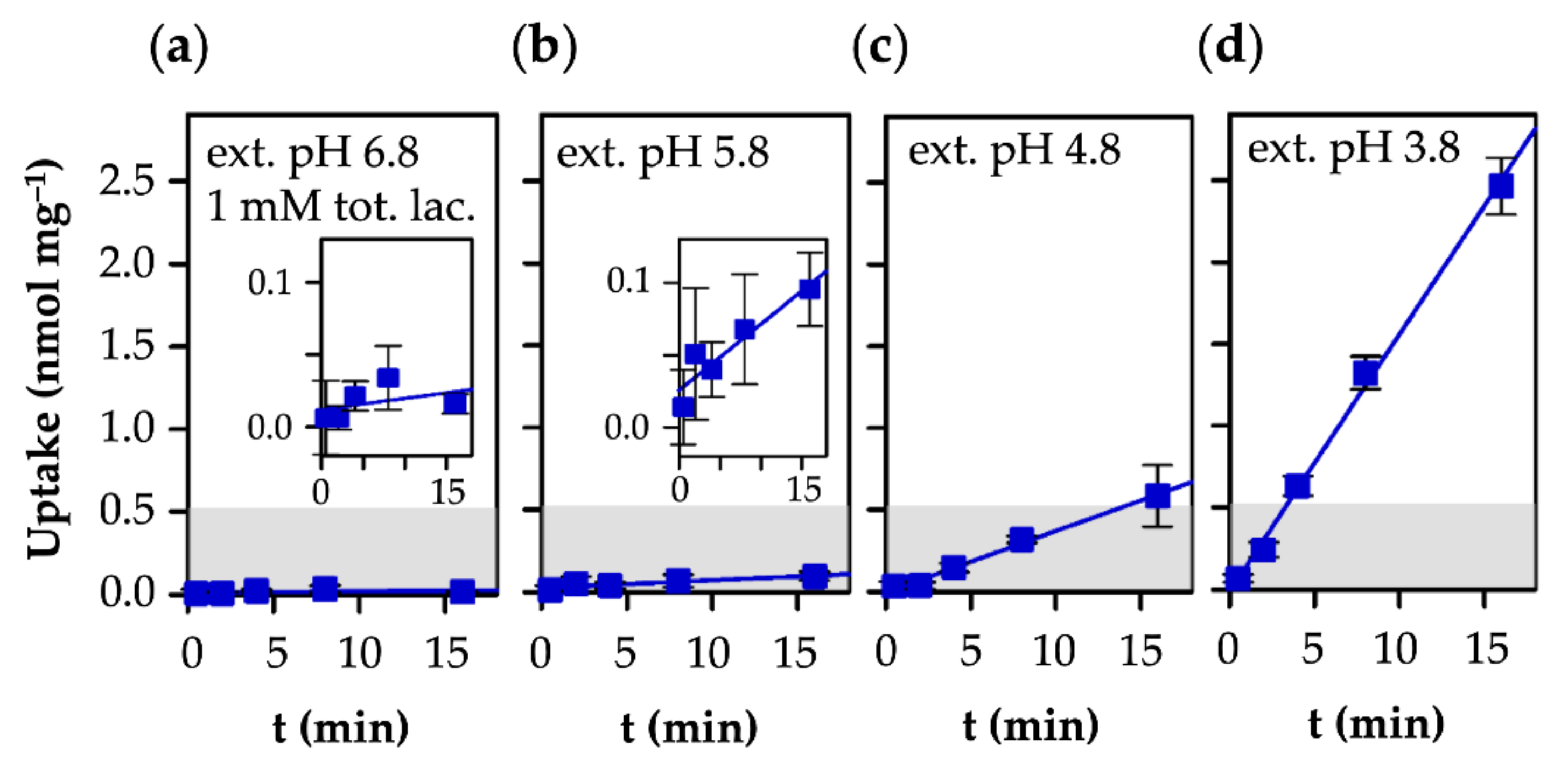
3.3. AQP9-Mediated Uptake of l-Lactic Acid Causes Large Accumulation of l-Lactate Anions
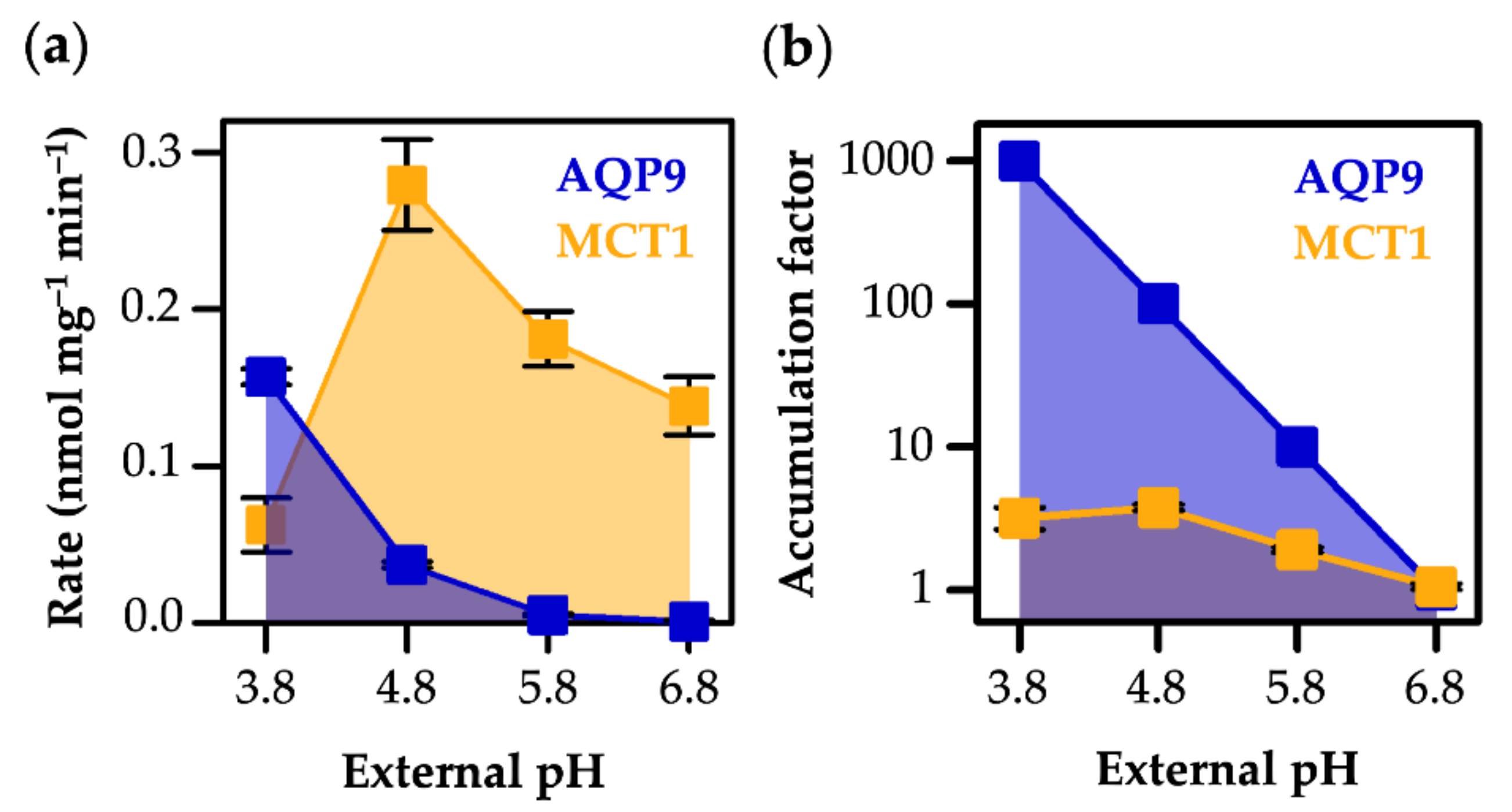
3.4. AQP9-Mediated l-Lactic Acid Permeability Establishes an Ion Trap
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- King, L.S.; Kozono, D.; Agre, P. From structure to disease: The evolving tale of aquaporin biology. Nat. Rev. Mol. Cell Biol. 2004, 5, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Verkman, A.S. More than just water channels: Unexpected cellular roles of aquaporins. J. Cell Sci. 2005, 118, 3225–3232. [Google Scholar] [CrossRef]
- Verkman, A.S.; Anderson, M.O.; Papadopoulos, M.C. Aquaporins: Important but elusive drug targets. Nat. Rev. Drug Discov. 2014, 13, 259–277. [Google Scholar] [CrossRef] [PubMed]
- Beitz, E.; Golldack, A.; Rothert, M.; von Bülow, J. Challenges and achievements in the therapeutic modulation of aquaporin functionality. Pharmacol. Ther. 2015, 155, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Kondo, H.; Shimomura, I.; Kishida, K.; Kuriyama, H.; Makino, Y.; Nishizawa, H.; Matsuda, M.; Maeda, N.; Nagaretani, H.; Kihara, S.; et al. Human aquaporin adipose (AQPap) gene. Genomic structure, promoter analysis and functional mutation. Eur. J. Biochem. 2002, 269, 1814–1826. [Google Scholar] [CrossRef] [PubMed]
- Calamita, G.; Gena, P.; Ferri, D.; Rosito, A.; Rojek, A.; Nielsen, S.; Marinelli, R.A.; Frühbeck, G.; Svelto, M. Biophysical assessment of aquaporin-9 as principal facilitative pathway in mouse liver import of glucogenetic glycerol. Biol. Cell 2012, 104, 342–351. [Google Scholar] [CrossRef]
- Wu, B.; Beitz, E. Aquaporins with selectivity for unconventional permeants. Cell. Mol. Life Sci. 2007, 64, 2413–2421. [Google Scholar] [CrossRef] [PubMed]
- Almasalmeh, A.; Krenc, D.; Wu, B.; Beitz, E. Structural determinants of the hydrogen peroxide permeability of aquaporins. FEBS J. 2014, 281, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Hub, J.S.; de Groot, B.L. Does CO2 permeate through aquaporin-1? Biophys. J. 2006, 91, 842–848. [Google Scholar] [CrossRef]
- Ripoche, P.; Goossens, D.; Devuyst, O.; Gane, P.; Colin, Y.; Verkman, A.S.; Cartron, J.P. Role of RhAG and AQP1 in NH3 and CO2 gas transport in red cell ghosts: A stopped-flow analysis. Transfus. Clin. Biol. 2006, 13, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cohen, J.; Boron, W.F.; Schulten, K.; Tajkhorshid, E. Exploring gas permeability of cellular membranes and membrane channels with molecular dynamics. J. Struct. Biol. 2007, 157, 534–544. [Google Scholar] [CrossRef] [PubMed]
- Missner, A.; Pohl, P. 110 years of the Meyer-Overton rule: Predicting membrane permeability of gases and other small compounds. Chemphyschem 2009, 10, 1405–1414. [Google Scholar] [CrossRef] [PubMed]
- Boron, W.F.; Endeward, V.; Gros, G.; Musa-Aziz, R.; Pohl, P. Intrinsic CO2 permeability of cell membranes and potential biological relevance of CO2 channels. Chemphyschem 2011, 12, 1017–1019. [Google Scholar] [CrossRef]
- Tsukaguchi, H.; Shayakul, C.; Berger, U.V.; Mackenzie, B.; Devidas, S.; Guggino, W.B.; van Hoek, A.N.; Hediger, M.A. Molecular characterization of a broad selectivity neutral solute channel. J. Biol. Chem. 1998, 273, 24737–24743. [Google Scholar] [CrossRef] [PubMed]
- Rothert, M.; Rönfeldt, D.; Beitz, E. Electrostatic attraction of weak monoacid anions increases probability for protonation and passage through aquaporins. J. Biol. Chem. 2017, 292, 9358–9364. [Google Scholar] [CrossRef]
- Deuticke, B. Monocarboxylate transport in erythrocytes. J. Membr. Biol. 1982, 70, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Halestrap, A.P. The SLC16 gene family—Structure, role and regulation in health and disease. Mol. Asp. Med. 2013, 34, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Mori, S.; Kurimoto, T.; Miki, A.; Maeda, H.; Kusuhara, S.; Nakamura, M. Aqp9 gene deletion enhances retinal ganglion cell (RGC) death and dysfunction induced by optic nerve crush: Evidence that aquaporin 9 acts as an astrocyte-to-neuron lactate shuttle in concert with monocarboxylate transporters to support RGC function and survival. Mol. Neurobiol. 2020, 57, 4530–4548. [Google Scholar]
- Tan, G.; Sun, S.Q.; Yuan, D.L. Expression of the water channel protein aquaporin-9 in human astrocytic tumors: Correlation with pathological grade. J. Int. Med. Res. 2008, 36, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Warth, A.; Mittelbronn, M.; Hülper, P.; Erdlenbruch, B.; Wolburg, H. Expression of the water channel protein aquaporin-9 in malignant brain tumors. Appl. Immunohistochem. Mol. Morphol. 2007, 15, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Rambow, J.; Wu, B.; Rönfeldt, D.; Beitz, E. Aquaporins with anion/monocarboxylate permeability: Mechanisms, relevance for pathogen-host interactions. Front. Pharmacol. 2014, 5, 199. [Google Scholar] [CrossRef] [PubMed]
- Bader, A.; Beitz, E. Transmembrane facilitation of lactate/H+ instead of lactic acid is not a question of semantics but of cell viability. Membranes 2020, 10, 236. [Google Scholar] [CrossRef] [PubMed]
- Köpnick, A.L.; Jansen, A.; Geistlinger, K.; Epalle, N.H.; Beitz, E. Basigin drives intracellular accumulation of l-lactate by harvesting protons and substrate anions. PLoS ONE 2021, 16, e0249110. [Google Scholar] [CrossRef] [PubMed]
- Soares-Silva, I.; Paiva, S.; Diallinas, G.; Casal, M. The conserved sequence NXX[S/T]HX[S/T]QDXXXT of the lactate/pyruvate:H(+) symporter subfamily defines the function of the substrate translocation pathway. Mol. Membr. Biol. 2007, 24, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Gietz, R.D.; Schiestl, R.H.; Willems, A.R.; Woods, R.A. Studies on the transformation of intact yeast cells by the LiAc/SS-DNA/PEG procedure. Yeast 1995, 11, 355–360. [Google Scholar] [CrossRef]
- Wu, B.; Rambow, J.; Bock, S.; Holm-Bertelsen, J.; Wiechert, M.; Blancke Soares, A.; Spielmann, T.; Beitz, E. Identity of a Plasmodium lactate/H(+) symporter structurally unrelated to human transporters. Nat. Commun. 2015, 6, 6284. [Google Scholar] [CrossRef]
- Köpnick, A.L.; Geistlinger, K.; Beitz, E. Cysteine 159 delineates a hinge region of the alternating access monocarboxylate transporter 1 and is targeted by cysteine-modifying inhibitors. FEBS J. 2021, 288, 6052–6062. [Google Scholar] [CrossRef] [PubMed]
- Gabba, M.; Frallicciardi, J.; van ’t Klooster, J.; Henderson, R.; Syga, Ł.; Mans, R.; van Maris, A.J.A.; Poolman, B. Weak acid permeation in synthetic lipid vesicles and across the yeast plasma membrane. Biophys. J. 2020, 118, 422–434. [Google Scholar] [CrossRef]
- Cherry, J.M.; Hong, E.L.; Amundsen, C.; Balakrishnan, R.; Binkley, G.; Chan, E.T.; Christie, K.R.; Costanzo, M.C.; Dwight, S.S.; Engel, S.R.; et al. Saccharomyces Genome Database: The genomics resource of budding yeast. Nucleic Acids Res. 2012, 40, D700–D705. [Google Scholar] [CrossRef]
- Reed, R.H.; Chudek, J.A.; Foster, R.; Gadd, G.M. Osmotic significance of glycerol accumulation in exponentially growing yeasts. Appl. Environ. Microbiol. 1987, 53, 2119–2123. [Google Scholar] [CrossRef]
- Tamás, M.J.; Luyten, K.; Sutherland, F.C.; Hernandez, A.; Albertyn, J.; Valadi, H.; Li, H.; Prior, B.A.; Kilian, S.G.; Ramos, J.; et al. Fps1p controls the accumulation and release of the compatible solute glycerol in yeast osmoregulation. Mol. Microbiol. 1999, 31, 1087–1104. [Google Scholar] [CrossRef] [PubMed]
- de Groot, B.L.; Grubmüller, H. Water permeation across biological membranes: Mechanism and dynamics of aquaporin-1 and GlpF. Science 2001, 294, 2353–2357. [Google Scholar] [CrossRef] [PubMed]
- Carmelo, V.; Santos, H.; Sá-Correia, I. Effect of extracellular acidification on the activity of plasma membrane ATPase and on the cytosolic and vacuolar pH of Saccharomyces cerevisiae. Biochim. Biophys. Acta 1997, 1325, 63–70. [Google Scholar] [CrossRef][Green Version]
- Poznanski, J.; Szczesny, P.; Ruszczyńska, K.; Zielenkiewicz, P.; Paczek, L. Proteins contribute insignificantly to the intrinsic buffering capacity of yeast cytoplasm. Biochem. Biophys. Res. Commun. 2013, 430, 741–744. [Google Scholar] [CrossRef]
- Petelenz-Kurdziel, E.; Eriksson, E.; Smedh, M.; Beck, C.; Hohmann, S.; Goksör, M. Quantification of cell volume changes upon hyperosmotic stress in Saccharomyces cerevisiae. Integr. Biol. 2011, 3, 1120–11216. [Google Scholar] [CrossRef]
- Bröer, S.; Schneider, H.P.; Bröer, A.; Rahman, B.; Hamprecht, B.; Deitmer, J.W. Characterization of the monocarboxylate transporter 1 expressed in Xenopus laevis oocytes by changes in cytosolic pH. Biochem. J. 1998, 333, 167–174. [Google Scholar] [CrossRef]
- Bosshart, P.D.; Kalbermatter, D.; Bonetti, S.; Fotiadis, D. Mechanistic basis of l-lactate transport in the SLC16 solute carrier family. Nat. Commun. 2019, 10, 2649. [Google Scholar] [CrossRef]
- Wang, N.; Jiang, X.; Zhang, S.; Zhu, A.; Yuan, Y.; Xu, H.; Lei, J.; Yan, C. Structural basis of human monocarboxylate transporter 1 inhibition by anti-cancer drug candidates. Cell 2020, 8674, 31615–31619. [Google Scholar] [CrossRef]
- Preston, R.A.; Murphy, R.F.; Jones, E.W. Assay of vacuolar pH in yeast and identification of acidification-defective mutants. Proc. Natl. Acad. Sci. USA 1989, 86, 7027–7031. [Google Scholar] [CrossRef]
- Marlar, S.; Jensen, H.H.; Login, F.H.; Nejsum, L.N. Aquaporin-3 in cancer. Int. J. Mol. Sci. 2017, 18, 2106. [Google Scholar] [CrossRef]
- Bienert, G.P.; Desguin, B.; Chaumont, F.; Hols, P. Channel-mediated lactic acid transport: A novel function for aquaglyceroporins in bacteria. Biochem. J. 2013, 454, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.D.R.; Walloch, P.; Höger, B.; Beitz, E. Aquaporins with lactate/lactic acid permeability at physiological pH conditions. Biochimie 2021, 188, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Hui, S.; Ghergurovich, J.M.; Morscher, R.J.; Jang, C.; Teng, X.; Lu, W.; Esparza, L.A.; Reya, T.; Zhan, L.; Guo, Y.J.; et al. Glucose feeds the TCA cycle via circulating lactate. Nature 2017, 551, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Galeffi, F.; Turner, D.A. Exploiting metabolic differences in glioma therapy. Curr. Drug Discov. Technol. 2012, 9, 280–293. [Google Scholar] [CrossRef] [PubMed]
- Jelen, S.; Parm Ulhøi, B.; Larsen, A.; Frøkiær, J.; Nielsen, S.; Rützler, M. AQP9 expression in glioblastoma multiforme tumors is limited to a small population of astrocytic cells and CD15(+)/CalB(+) leukocytes. PLoS ONE 2013, 8, e75764. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geistlinger, K.; Schmidt, J.D.R.; Beitz, E. Lactic Acid Permeability of Aquaporin-9 Enables Cytoplasmic Lactate Accumulation via an Ion Trap. Life 2022, 12, 120. https://doi.org/10.3390/life12010120
Geistlinger K, Schmidt JDR, Beitz E. Lactic Acid Permeability of Aquaporin-9 Enables Cytoplasmic Lactate Accumulation via an Ion Trap. Life. 2022; 12(1):120. https://doi.org/10.3390/life12010120
Chicago/Turabian StyleGeistlinger, Katharina, Jana D. R. Schmidt, and Eric Beitz. 2022. "Lactic Acid Permeability of Aquaporin-9 Enables Cytoplasmic Lactate Accumulation via an Ion Trap" Life 12, no. 1: 120. https://doi.org/10.3390/life12010120
APA StyleGeistlinger, K., Schmidt, J. D. R., & Beitz, E. (2022). Lactic Acid Permeability of Aquaporin-9 Enables Cytoplasmic Lactate Accumulation via an Ion Trap. Life, 12(1), 120. https://doi.org/10.3390/life12010120





