The Female Pelvic Floor Fascia Anatomy: A Systematic Search and Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Search Strategy
2.3. Inclusion and Exclusion Criteria
2.4. Study Selection and Data Collection Process
2.5. Data Extraction
2.6. Risk of Bias in Individual Studies
2.7. Data Synthesis
3. Results
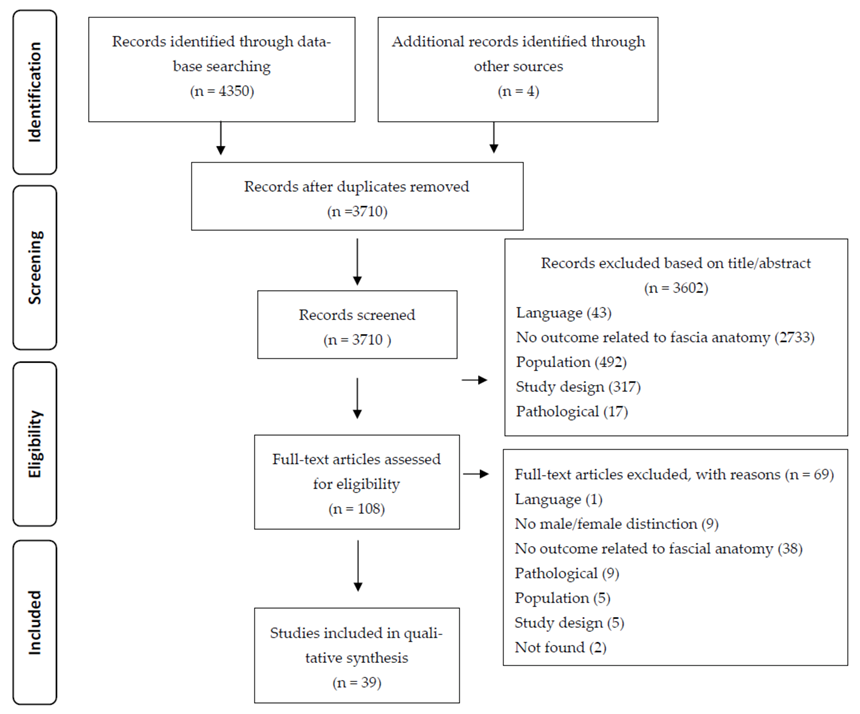
3.1. Study Selection
3.2. Study Characteristics
3.3. Risk of Bias (ROB)
3.4. Anatomical Structures Described
3.4.1. Perineal Membrane (PM)
3.4.2. Perineal Body (PB)
3.4.3. Endopelvic Fascia
3.4.4. Pubourethral and Pubovesical Ligament
3.4.5. Tendinous Arch of Pelvic Fascia (TAPF)
3.4.6. Tendinous Arch of Levator Ani (TALA)
3.4.7. Pubocervical Fascia
3.4.8. Rectovaginal Fascia (RVF)
3.4.9. Tendinous Arch of Rectovaginal Fascia
3.4.10. Rectovaginal Ligament
3.4.11. Rectosacral Fascia and the Inferior Fascia of the Pelvic Diaphragm
3.4.12. Paracolpium
4. Discussion
4.1. Membranous Layer of the Perineal Subcutaneous Tissue
4.2. Perineal Membrane (PM)
4.3. Perineal Body (PB)
4.4. Endopelvic Fascia
4.5. Particular Areas of the Endopelvic Fascia
4.5.1. Pubourethral and Pubovesical Ligaments
4.5.2. Tendinous Arch of Pelvic Fascia (TAPF)
4.5.3. Tendinous Arch of Levator Ani (TALA)
4.5.4. Internal Obturator Fascia
4.5.5. Pubocervical Fascia
4.5.6. Rectovaginal Fascia (RVF)
4.5.7. Rectosacral Fascia and Inferior Fascia of the Pelvic Diaphragm
4.5.8. Paracolpium
4.6. Limitations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. List of Keywords Used for the Search in Medline and Scopus Databases
Appendix B. List of Keywords Used for Gray Literature Search in ProQuest Database
References
- Gyhagen, M.; Åkervall, S.; Milsom, I. Clustering of pelvic floor disorders 20 years after one vaginal or one cesarean birth. Int. Urogynecol. J. 2015, 26, 1115–1121. [Google Scholar] [CrossRef] [PubMed]
- Kepenekci, I.; Keskinkilic, B.; Akinsu, F.; Cakir, P.; Elhan, A.H.; Erkek, A.B.; Kuzu, M.A. Prevalence of Pelvic Floor Disorders in the Female Population and the Impact of Age, Mode of Delivery, and Parity. Dis. Colon Rectum 2011, 54, 85–94. [Google Scholar] [CrossRef]
- Nygaard, I. Prevalence of Symptomatic Pelvic Floor Disorders in US Women. JAMA 2008, 300, 1311–1316. [Google Scholar] [CrossRef]
- Rortveit, G.; Subak, L.L.; Thom, D.H.; Creasman, J.M.; Vittinghoff, E.; Eeden, S.K.V.D.; Brown, J.S. Urinary Incontinence, Fecal Incontinence and Pelvic Organ Prolapse in a Population-Based, Racially Diverse Cohort. Female Pelvic Med. Reconstr. Surg. 2010, 16, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.M.; Lukacz, E.S.; Nager, C.W.; Hsu, J.-W.Y.; Luber, K.M. Prevalence and Co-Occurrence of Pelvic Floor Disorders in Community-Dwelling Women. Obstet. Gynecol. 2008, 111, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.M.; Matthews, C.A.; Conover, M.; Pate, V.; Funk, M.J. Lifetime Risk of Stress Urinary Incontinence or Pelvic Organ Prolapse Surgery. Obstet. Gynecol. 2014, 123, 1201–1206. [Google Scholar] [CrossRef] [PubMed]
- Smith, F.J.; Holman, C.D.J.; Moorin, R.; Tsokos, N. Lifetime Risk of Undergoing Surgery for Pelvic Organ Prolapse. Obstet. Gynecol. 2010, 116, 1096–1100. [Google Scholar] [CrossRef]
- Van der Woude, D.A.; Pijnenborg, J.; de Vries, J. Health status and quality of life in postpartum women: A systematic review of associated factors. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 185, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Norton, P.A. Pelvic Floor Disorders: The Role of Fascia and Ligaments. Clin. Obstet. Gynecol. 1993, 36, 926–938. [Google Scholar] [CrossRef]
- Morin, M.; Binik, Y.M.; Bourbonnais, D.; Khalifé, S.; Ouellet, S.; Bergeron, S. Heightened Pelvic Floor Muscle Tone and Altered Contractility in Women With Provoked Vestibulodynia. J. Sex. Med. 2017, 14, 592–600. [Google Scholar] [CrossRef]
- Thibault-Gagnon, S.; Morin, M. Active and Passive Components of Pelvic Floor Muscle Tone in Women with Provoked Vestibulodynia: A Perspective Based on a Review of the Literature. J. Sex. Med. 2015, 12, 2178–2189. [Google Scholar] [CrossRef] [PubMed]
- Stecco, C.; Macchi, V.; Porzionato, A.; Tiengo, C.; Parenti, A.; Gardi, M.; Artibani, W.; De Caro, R. Histotopographic study of the rectovaginal septum. Ital. J. Anat. Embryol. Arch. Ital. Anat. ed embriologia 2006, 110, 247–254. [Google Scholar]
- Wei, J.T.; De Lancey, J.O.L. Functional Anatomy of the Pelvic Floor and Lower Urinary Tract. Clin. Obstet. Gynecol. 2004, 47, 3–17. [Google Scholar] [CrossRef] [PubMed]
- DeLancey, J.O. Structural support of the urethra as it relates to stress urinary incontinence: The hammock hypothesis. Am. J. Obstet. Gynecol. 1994, 170, 1713–1723. [Google Scholar] [CrossRef]
- DeLancey, J. Structural aspects of the extrinsic continence mechanism. Obstet. Gynecol. 1988, 72, 296–301. [Google Scholar]
- DeLancey, J.O. Anatomie aspects of vaginal eversion after hysterectomy. Am. J. Obstet. Gynecol. 1992, 166, 1717–1728. [Google Scholar] [CrossRef]
- Li, J.-R.; Lei, L.; Luo, N.; Chen, N.; Xu, H.-T.; Hu, X.; Song, Y.; Wu, Y. Architecture of female urethral supporting structures based on undeformed high-resolution sectional anatomical images. Anat. Sci. Int. 2020, 96, 30–41. [Google Scholar] [CrossRef]
- El Sayed, R.F.; Morsy, M.M.; El Mashed, S.M.; Abdel-Azim, M.S. Anatomy of the Urethral Supporting Ligaments Defined by Dissection, Histology, and MRI of Female Cadavers and MRI of Healthy Nulliparous Women. Am. J. Roentgenol. 2007, 189, 1145–1157. [Google Scholar] [CrossRef]
- DeLancey, J.O.L. Pubovesical ligament: A separate structure from the urethral supports (“pubo-urethral ligaments”). Neurourol. Urodyn. 1989, 8, 53–61. [Google Scholar] [CrossRef]
- Nagata, I.; Murakami, G.; Suzuki, D.; Furuya, K.; Koyama, M.; Ohtsuka, A. Histological features of the rectovaginal septum in elderly women and a proposal for posterior vaginal defect repair. Int. Urogynecol. J. 2007, 18, 863–868. [Google Scholar] [CrossRef]
- Kraima, A.C.; West, N.; Treanor, D.; Magee, D.R.; Rutten, H.J.; Quirke, P.; DeRuiter, M.C.; Van De Velde, C.J.H. Whole mount microscopic sections reveal that Denonvilliers’ fascia is one entity and adherent to the mesorectal fascia; implications for the anterior plane in total mesorectal excision? . Eur. J. Surg. Oncol. (EJSO) 2015, 41, 738–745. [Google Scholar] [CrossRef] [PubMed]
- Hamner, J.J.; Carrick, K.S.; Ramirez, D.; Corton, M.M. Gross and histologic relationships of the retropubic urethra to lateral pelvic sidewall and anterior vaginal wall in female cadavers: Clinical applications to retropubic surgery. Am. J. Obstet. Gynecol. 2018, 219, 597.e1–597.e8. [Google Scholar] [CrossRef]
- Ersoy, M.; Sagsoz, N.; Bozkurt, M.; Apaydin, N.; Elhan, A.; Tekdemir, I. Important anatomical structures used in paravaginal defect repair: Cadaveric study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2004, 112, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Vazzoler, N.; Soulié, M.; Escourrou, G.; Seguin, P.; Pontonnier, F.; Bécue, J.; Plante, P. Pubourethral ligaments in women: Anatomical and clinical aspects. . Surg. Radiol. Anat. 2002, 24, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Stecco, A.; Meneghini, A.; Stern, R.; Stecco, C.; Imamura, M. Ultrasonography in myofascial neck pain: Randomized clinical trial for diagnosis and follow-up. Surg. Radiol. Anat. 2013, 36, 243–253. [Google Scholar] [CrossRef]
- Langevin, H.M.; Stevens-Tuttle, D.; Fox, J.R.; Badger, G.J.; A Bouffard, N.; Krag, M.H.; Wu, J.; Henry, S.M. Ultrasound evidence of altered lumbar connective tissue structure in human subjects with chronic low back pain. BMC Musculoskelet. Disord. 2009, 10, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Mense, S. Innervation of the thoracolumbar fascia. Eur. J. Transl. Myol. 2019, 29, 8297. [Google Scholar] [CrossRef]
- Schilder, A.; Hoheisel, U.; Magerl, W.; Benrath, J.; Klein, T.; Treede, R.-D. Sensory findings after stimulation of the thoracolumbar fascia with hypertonic saline suggest its contribution to low back pain. Pain 2014, 155, 222–231. [Google Scholar] [CrossRef]
- Taguchi, T.; Yasui, M.; Kubo, A.; Abe, M.; Kiyama, H.; Yamanaka, A.; Mizumura, K. Nociception originating from the crural fascia in rats. Pain 2013, 154, 1103–1114. [Google Scholar] [CrossRef]
- Tesarz, J.; Hoheisel, U.; Wiedenhöfer, B.; Mense, S. Sensory innervation of the thoracolumbar fascia in rats and humans. Neuroscience 2011, 194, 302–308. [Google Scholar] [CrossRef]
- Laimi, K.; Mäkilä, A.; Bärlund, E.; Katajapuu, N.; Oksanen, A.; Seikkula, V.; Karppinen, J.; Saltychev, M. Effectiveness of myofascial release in treatment of chronic musculoskeletal pain: A systematic review. Clin. Rehabilitation 2017, 32, 440–450. [Google Scholar] [CrossRef]
- Jeppson, P.C.; Balgobin, S.; Washington, B.B.; Hill, A.J.; Lewicky-Gaupp, C.; Wheeler, T.; Ridgeway, B.; Mazloomdoost, D.; Balk, E.M.; Corton, M.M.; et al. Recommended standardized terminology of the anterior female pelvis based on a structured medical literature review. Am. J. Obstet. Gynecol. 2018, 219, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Balgobin, S.; Jeppson, P.C.; Wheeler, T.; Hill, A.J.; Mishra, K.; Mazloomdoost, D.; Dunivan, G.C.; Anand, M.; Mama, S.T.; Bochenska, K.; et al. Standardized terminology of apical structures in the female pelvis based on a structured medical literature review. Am. J. Obstet. Gynecol. 2020, 222, 204–218. [Google Scholar] [CrossRef] [PubMed]
- Ercoli, A.; Delmas, V.; Fanfani, F.; Gadonneix, P.; Ceccaroni, M.; Fagotti, A.; Mancuso, S.; Scambia, G. Terminologia Anatomica versus unofficial descriptions and nomenclature of the fasciae and ligaments of the female pelvis: A dissection-based comparative study. Am. J. Obstet. Gynecol. 2005, 193, 1565–1573. [Google Scholar] [CrossRef] [PubMed]
- Ramin, A.; Macchi, V.; Porzionato, A.; De Caro, R.; Stecco, C. Fascial continuity of the pelvic floor with the abdominal and lumbar region. Pelviperineology 2016, 35, 3–6. [Google Scholar]
- Loukas, M.; Benninger, B.; Tubbs, R.S. Gray’s Clinical Photographic Dissector of the Human Body, 2nd ed.; Elsevier: Philadelphia, PA, USA, 2019. [Google Scholar]
- Stecco, C.; Hammer, W.I. Functional Atlas of the Human Fascial System; Churchill Livingstone: Edinburgh, Scotland, 2015. [Google Scholar]
- Moore, K.L.; Dalley, A.F.; Agur, A.M.R. Clinically Oriented Anatomy; Wolters Kluwer Health: Philadelphia, PA, USA, 2018. [Google Scholar]
- Netter, F.H. Atlas of Human Anatomy, 6th ed.; Saunders/Elsevier: Philadelphia, PA, USA, 2014. [Google Scholar]
- Grant, M.J.; Booth, A. A typology of reviews: An analysis of 14 review types and associated methodologies. Heal. Inf. Libr. J. 2009, 26, 91–108. [Google Scholar] [CrossRef]
- Yammine, K. Evidence-based anatomy. Clin. Anat. 2014, 27, 847–852. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; A Ioannidis, J.P.; Clarke, M.; Devereaux, P.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef]
- Baggish, M.S.; Karram, M.M.; Buster, J.E. Atlas of Pelvic Anatomy and Gynecologic Surgery; Saunders Elsevier: Philadelphia, PA, USA, 2006; p. 1171. [Google Scholar]
- Walters, M.D.; Karram, M.M. Urogynecology and Reconstructive Pelvic Surgery; Elsevier Saunders: Philadelphia, PA, USA, 2015. [Google Scholar]
- Partin, A.W.; Dmochowski, R.R.; Kavoussi, L.R.; Peters, C.A. Campbell-Walsh-Wein Urology; Elsevier Health Sciences: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Detton, A.J.; Tank, P.W. Grant’s Dissector; Wolters Kluwer: Philadelphia, PA, USA, 2017. [Google Scholar]
- Terminologies, F.I.P.o.A. Terminologia Anatomica: International Anatomical Terminology; Georg Thieme Verlag: Stuttgart, Germany; New York, NY, USA, 2011. [Google Scholar]
- Bo, K.; Frawley, H.C.; Haylen, B.T.; Abramov, Y.; Almeida, F.G.; Berghmans, B.; Bortolini, M.; Dumoulin, C.; Gomes, M.; McClurg, D.; et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for the conservative and nonpharmacological management of female pelvic floor dysfunction. Neurourol. Urodynamics 2016, 36, 221–244. [Google Scholar] [CrossRef]
- Cicchetti, D.V. Methodological Commentary The Precision of Reliability and Validity Estimates Re-Visited: Distinguishing Between Clinical and Statistical Significance of Sample Size Requirements. J. Clin. Exp. Neuropsychol. 2001, 23, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Henry, B.M.; Tomaszewski, K.; Ramakrishnan, P.K.; Roy, J.; Vikse, J.; Loukas, M.; Tubbs, R.S.; Walocha, J.A. Development of the Anatomical Quality Assessment (AQUA) Tool for the quality assessment of anatomical studies included in meta-analyses and systematic reviews. Clin. Anat. 2016, 30, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Savović, J.; Page, M.J.; Elbers, R.G.; Jac, S. Cochrane Handbook for Systematic Reviews of Interventions Version 6.2. Available online: www.training.cochrane.org/handbook (accessed on 1 March 2021).
- Milley, P.S.; Nichols, D.H. The relationship between the pubo-urethral ligaments and the urogenital diaphragm in the human female. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 1971, 170, 281–283. [Google Scholar] [CrossRef]
- DeLancey, J.; A Starr, R. Histology of the connection between the vagina and levator ani muscles. Implications for urinary tract function. J. Reprod. Med. 1990, 35, 765–771. [Google Scholar] [PubMed]
- Aronson, M.P.; Bates, S.M.; Jacoby, A.F.; Chelmow, D.; Sant, G.R. Periurethral and paravaginal anatomy: An endovaginal magnetic resonance imaging study. Am. J. Obstet. Gynecol. 1995, 173, 1702–1710. [Google Scholar] [CrossRef]
- DeLancey, J.O. Structural anatomy of the posterior pelvic compartment as it relates to rectocele. Am. J. Obstet. Gynecol. 1999, 180, 815–823. [Google Scholar] [CrossRef]
- Mauroy, B.; Goullet, E.; Stefaniak, X.; Bonnal, J.L.; Amara, N. Tendinous arch of the pelvic fascia application to the technique of paravaginal colposuspension. Surg. Radiol. Anat. 2000, 22, 73–79. [Google Scholar] [CrossRef]
- Leffler, K.S.; Thompson, J.R.; Cundiff, G.; Buller, J.L.; Burrows, L.J.; Ybarra, M.A.S. Attachment of the rectovaginal septum to the pelvic sidewall. Am. J. Obstet. Gynecol. 2001, 185, 41–43. [Google Scholar] [CrossRef]
- Occelli, B.; Narducci, F.; Hautefeuille, J.; Francke, J.; Querleu, D.; Crépin, G.; Cosson, M. Anatomic study of arcus tendineus fasciae pelvis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2001, 97, 213–219. [Google Scholar] [CrossRef]
- Pit, M.J.; De Ruiter, M.C.; Nijeholt, A.A.L.; Marani, E.; Zwartendijk, J. Anatomy of the arcus tendineus fasciae pelvis in females. Clin. Anat. 2003, 16, 131–137. [Google Scholar] [CrossRef]
- Albright, T.S.; Gehrich, A.P.; Davis, G.D.; Sabi, F.L.; Buller, J.L. Arcus tendineus fascia pelvis: A further understanding. Am. J. Obstet. Gynecol. 2005, 193, 677–681. [Google Scholar] [CrossRef]
- Fritsch, H.; Pinggera, G.-M.; Lienemann, A.; Mitterberger, M.; Bartsch, G.; Strasser, H. What are the supportive structures of the female urethra? Neurourol. Urodyn. 2005, 25, 128–134. [Google Scholar] [CrossRef]
- Soga, H.; Nagata, I.; Murakami, G.; Yajima, T.; Takenaka, A.; Fujisawa, M.; Koyama, M. A histotopographic study of the perineal body in elderly women: The surgical applicability of novel histological findings. Int. Urogynecol. J. 2007, 18, 1423–1430. [Google Scholar] [CrossRef]
- Betschart, C.; Scheiner, D.; Maake, C.; Vich, M.; Slomianka, L.; Fink, D.; Perucchini, D. Histomorphological analysis of the urogenital diaphragm in elderly women: A cadaver study. Int. Urogynecol. J. 2008, 19, 1477–1481. [Google Scholar] [CrossRef]
- García-Armengol, J.; García-Botello, S.; Martinez-Soriano, F.; Roig, J.V.; Lledó, S. Review of the anatomic concepts in relation to the retrorectal space and endopelvic fascia: Waldeyer’s fascia and the rectosacral fascia. Color. Dis. 2008, 10, 298–302. [Google Scholar] [CrossRef]
- Kato, M.; Matsubara, A.; Murakami, G.; Abe, S.-I.; Ide, Y.; Sato, I.; Usui, T. Female perineal membrane: A study using pelvic floor semiserial sections from elderly nulliparous and multiparous women. Int. Urogynecol. J. 2008, 19, 1663–1670. [Google Scholar] [CrossRef] [PubMed]
- Stein, T.A.; DeLancey, J.O.L. Structure of the Perineal Membrane in Females. Obstet. Gynecol. 2008, 111, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Brandon, C.J.; Lewicky-Gaupp, C.; Larson, K.A.; DeLancey, J.O. Anatomy of the perineal membrane as seen in magnetic resonance images of nulliparous women. Am. J. Obstet. Gynecol. 2009, 200, 583.e1–583.e6. [Google Scholar] [CrossRef] [PubMed]
- Larson, K.A.; Yousuf, A.; Lewicky-Gaupp, C.; Fenner, D.E.; DeLancey, J.O. Perineal body anatomy in living women: 3-dimensional analysis using thin-slice magnetic resonance imaging. Am. J. Obstet. Gynecol. 2010, 203, 494.e15–494.e21. [Google Scholar] [CrossRef]
- Hirata, E.; Fujiwara, H.; Hayashi, S.; Ohtsuka, A.; Abe, S.; Murakami, G.; Kudo, Y. Intergender differences in histological architecture of the fascia pelvis parietalis: A cadaveric study. Clin. Anat. 2010, 24, 469–477. [Google Scholar] [CrossRef]
- Hirata, E.; Koyama, M.; Murakami, G.; Ohtsuka, A.; Abe, S.-I.; Ide, Y.; Fujiwara, H.; Kudo, Y. Comparative histological study of levels 1-3 supportive tissues using pelvic floor semiserial sections from elderly nulliparous and multiparous women. J. Obstet. Gynaecol. Res. 2010, 37, 13–23. [Google Scholar] [CrossRef]
- Tsai, P.-J.S.; Oyama, I.A.; Hiraoka, M.; Minaglia, S.; Thomas, J.; Kaneshiro, B. Perineal Body Length Among Different Racial Groups in the First Stage of Labor. Female Pelvic Med. Reconstr. Surg. 2012, 18, 165–167. [Google Scholar] [CrossRef]
- Hinata, N.; Hieda, K.; Sasaki, H.; Kurokawa, T.; Miyake, H.; Fujisawa, M.; Murakami, G.; Fujimiya, M. Nerves and fasciae in and around the paracolpium or paravaginal tissue: An immunohistochemical study using elderly donated cadavers. Anat. Cell Biol. 2014, 47, 44–54. [Google Scholar] [CrossRef]
- Santoro, G.A.; Shobeiri, S.A.; Petros, P.; Zapater, P.; Wieczorek, A.P. Perineal body anatomy seen by three-dimensional endovaginal ultrasound of asymptomatic nulliparae. Color. Dis. 2016, 18, 400–409. [Google Scholar] [CrossRef]
- Lane, T.L.; Chung, C.P.; Yandell, P.M.; Kuehl, T.J.; Larsen, W.I. Perineal body length and perineal lacerations during delivery in primigravid patients. Bayl. Univ. Med Cent. Proc. 2017, 30, 151–153. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Dabhoiwala, N.F.; Hagoort, J.; Tan, L.; Zhang, S.; Lamers, W.H. Architectural differences in the anterior and middle compartments of the pelvic floor of young-adult and postmenopausal females. J. Anat. 2017, 230, 651–663. [Google Scholar] [CrossRef] [PubMed]
- Kochová, P.; Cimrman, R.; Jansová, M.; Michalová, K.; Kalis, V.; Kubíková, T.; Tonar, Z. The histological microstructure and in vitro mechanical properties of the human female postmenopausal perineal body. Menopause 2019, 26, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Ghareeb, W.M.; Wang, X.J.; Chi, P.; Wang, W. The ‘multilayer’ theory of Denonvilliers’ fascia: Anatomical dissection of cadavers with the aim to improve neurovascular bundle preservation during rectal mobilization. Color. Dis. 2019, 22, 195–202. [Google Scholar] [CrossRef]
- Rodríguez-Abarca, M.A.; Hernández-Grimaldo, E.G.; De la Fuente-Villarreal, D.; Jacobo-Baca, G.; Quiroga-Garza, A.; Pinales-Razo, R.; Elizondo-Omaña, R.E.; Guzman-Lopez, S. Gynecological influencing factors on the rectovaginal septum’s morphology. Int. Urogynecol. J. 2020, 32, 1427–1432. [Google Scholar] [CrossRef] [PubMed]
- Batoum, V.M.; Meka, E.N.U.; Essiben, F.; Robinson, M.E. Perineal body length and prevention of perineal lacerations during delivery in cameroonian primigravid patients. Int. J. Gynecol. Obstet. 2021. [Google Scholar] [CrossRef]
- Bornstein, J.; Goldstein, A.; Stockdale, C.; Bergeron, S.; Pukall, C.; Zolnoun, D.; Coady, D. 2015 ISSVD, ISSWSH and IPPS Consensus Terminology and Classification of Persistent Vulvar Pain and Vulvodynia. Obstet. Gynecol. 2016, 127, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Chapuis, P.H.; Kaw, A.; Zhang, M.; Sinclair, G.; Bokey, L. Rectal mobilization: The place of Denonvilliers’ fascia and inconsistencies in the literature. Color. Dis. 2016, 18, 939–948. [Google Scholar] [CrossRef]
- DeLancey, J.O.L. What’s new in the functional anatomy of pelvic organ prolapse? Curr. Opin. Obstet. Gynecol. 2016, 28, 420–429. [Google Scholar] [CrossRef]
- Zhu, X.-M.; Yu, G.-Y.; Zheng, N.-X.; Liu, H.-M.; Gong, H.-F.; Lou, Z.; Zhang, W. Review of Denonvilliers’ fascia: The controversies and consensuses. Gastroenterol. Rep. 2020, 8, 343–348. [Google Scholar] [CrossRef]
- Arenholt, L.T.S.; Pedersen, B.G.; Glavind, K.; Glavind-Kristensen, M.; DeLancey, J.O.L. Paravaginal defect: Anatomy, clinical findings, and imaging. Int. Urogynecol. J. 2016, 28, 661–673. [Google Scholar] [CrossRef] [PubMed]
- Dariane, C.; Moszkowicz, D.; Peschaud, F. Concepts of the rectovaginal septum: Implications for function and surgery. Int. Urogynecol. J. 2015, 27, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Oelrich, T.M. The striated urogenital sphincter muscle in the female. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 1983, 205, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Herschorn, S. Female Pelvic Floor Anatomy: The Pelvic Floor, Supporting Structures, and Pelvic Organs. Rev. Urol. 2004, 6, S2–S10. [Google Scholar] [PubMed]
- Mirilas, P.; E Skandalakis, J. Urogenital diaphragm: An erroneous concept casting its shadow over the sphincter urethrae and deep perineal space. J. Am. Coll. Surg. 2004, 198, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Fritsch, H.; Zwierzina, M.; Riss, P. Accuracy of concepts in female pelvic floor anatomy: Facts and myths! World J. Urol. 2011, 30, 429–435. [Google Scholar] [CrossRef]
- Carney, M.J.; Matatov, T.; Freeman, M.; Miller, J.; Vemula, R.; Schuster, J.; Dancisak, M.; Lindsey, J.; Rae, G. Clinical, Biomechanical, and Anatomic Investigation of Colles Fascia and Pubic Ramus Periosteum for Use During Medial Thighplasty. Ann. Plast. Surg. 2017, 78, S305–S310. [Google Scholar] [CrossRef]
- Lockwood, T.E. Superficial Fascial System (SFS) of the Trunk and Extremities: A New Concept. Plast. Reconstr. Surg. 1991, 87, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Al-Ali, S.; Blyth, P.; Beatty, S.; Duang, A.; Parry, B.; Bissett, I.P. Correlation between gross anatomical topography, sectional sheet plastination, microscopic anatomy and endoanal sonography of the anal sphincter complex in human males. J. Anat. 2009, 215, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Kinugasa, Y.; Arakawa, T.; Abe, S.; Ohtsuka, A.; Suzuki, D.; Murakami, G.; Fujimiya, M.; Sugihara, K. Anatomical Reevaluation of the Anococcygeal Ligament and Its Surgical Relevance. Dis. Colon Rectum 2011, 54, 232–237. [Google Scholar] [CrossRef]
- Bordoni, B.; Marelli, F.; Morabito, B.; Sacconi, B. The indeterminable resilience of the fascial system. J. Integr. Med. 2017, 15, 337–343. [Google Scholar] [CrossRef]
- Guimberteau, J.-C. Endoscopic anatomical approach of the muscle: Intramuscular fibrillar continuity. Ann. Chir. Plast. Esthet. 2012, 57, 482–483. [Google Scholar] [CrossRef]
- Bordoni, B.; Sugumar, K.; Leslie, S.W. Anatomy, Abdomen and Pelvis, Pelvic Floor. In StatPearls; StatPearls Publishing. Copyright © 2021, StatPearls Publishing LLC.: Treasure Island, FL, USA, 2021. [Google Scholar]
- Wendell-Smith, C.P. Fascia: An illustrative problem in international terminology. Surg. Radiol. Anat. 1997, 19, 273–277. [Google Scholar] [CrossRef]
- Gray, H.; Clemente, C.D.; Clemente, C.D. Anatomy of the Human Body, 30th ed.; Lea & Febiger: Philadelphia, PA, USA, 1985. [Google Scholar]
- Standring, S. Gray’s Anatomy, 39th ed.; The Anatomical Basis of Clinical Practice; Elsevier Health Sciences: Amsterdam, The Netherlands, 2005; Volume 26, pp. 2703–2704. [Google Scholar]
- Luschka, H.v. Die Anatomie des Menschen in R¸cksicht auf die Bed¸rfnisse der praktischen Heilkunde; 1862. [Google Scholar]
- Bordoni, B.; Simonelli, M.; Morabito, B. The Other Side of the Fascia: The Smooth Muscle Part 1. Cureus 2019, 11, e4651. [Google Scholar] [CrossRef]
- Purslow, P.P. Muscle fascia and force transmission. J. Bodyw. Mov. Ther. 2010, 14, 411–417. [Google Scholar] [CrossRef]
- Stecco, C.; Stern, R.; Porzionato, A.; Macchi, V.; Masiero, S.; Stecco, A.; De Caro, R. Hyaluronan within fascia in the etiology of myofascial pain. Surg. Radiol. Anat. 2011, 33, 891–896. [Google Scholar] [CrossRef] [PubMed]
- Langevin, H.M.; Fox, J.R.; Koptiuch, C.; Badger, G.J.; Naumann, A.C.G.; A Bouffard, N.; E Konofagou, E.; Lee, W.-N.; Triano, J.J.; Henry, S.M. Reduced thoracolumbar fascia shear strain in human chronic low back pain. BMC Musculoskelet. Disord. 2011, 12, 203. [Google Scholar] [CrossRef] [PubMed]
- Fede, C.; Albertin, G.; Petrelli, L.; Sfriso, M.; Biz, C.; De Caro, R.; Stecco, C. Expression of the endocannabinoid receptors in human fascial tissue. Eur. J. Histochem. 2016, 60, 2643. [Google Scholar] [CrossRef]
- Woodman, P.J.; Graney, D.O. Anatomy and physiology of the female perineal body with relevance to obstetrical injury and repair. Clin. Anat. 2002, 15, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Gray, H.; Ellis, H.; Berkovitz, B.K.B.; Standring, S. Gray’s Anatomy: The Anatomical Basis of Clinical Practice, 39th ed.; Susan, S., Harold, E., Barry, K., Berkovitz, B., Eds.; Elsevier Churchill Livingstone: Edinburgh, UK, 2005. [Google Scholar]
- Sato, K.; Sato, T. The vascular and neuronal composition of the lateral ligament of the rectum and the rectosacral fascia. Surg. Radiol. Anat. 1991, 13, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Perry, W.B.; Connaughton, J.C. Abdominoperineal Resection: How Is It Done and What Are the Results? Clin. Colon Rectal Surg. 2007, 20, 213–220. [Google Scholar] [CrossRef]
- Muallem, M.; Jöns, T.; Seidel, N.; Sehouli, J.; Diab, Y.; Querleu, D. A Concise Paradigm on Radical Hysterectomy: The Comprehensive Anatomy of Parametrium, Paracolpium and the Pelvic Autonomic Nerve System and Its Surgical Implication. Cancers 2020, 12, 1839. [Google Scholar] [CrossRef] [PubMed]
- Cuschieri, S. The STROBE guidelines. Saudi J. Anaesth. 2019, 13, S31–S34. [Google Scholar] [CrossRef] [PubMed]
| Authors | Study Design | Assessment Approach | Sample | Age, Years Mean (SD or Range) |
|---|---|---|---|---|
| Milley and Nichols [52] | Observational study (cohort) | Dissection | 14 embalmed female cadavers | N/A (range 22–91) |
| DeLancey [19] | Observational study (cohort) | Dissection + Histological | 14 embalmed and 14 fresh female cadavers | 53 (range 28–78) 50–60 (range 46–106) |
| DeLancey and Starr [53] | Observational study (cohort) | Dissection | 2 embalmed and 2 fresh female cadavers | N/A (range 27–74) |
| DeLancey [16] | Observational study (cohort) | Dissection | 19 embalmed and 42 fresh female cadavers | N/A (range 0–104) |
| Aronson, et al. [54] | Observational study (case-control) | Radiological (MRI) | 4 living females (continent) (and 4 incontinent living females) | 33 (range 29–38) |
| DeLancey [55] | Observational study (cohort) | Dissection | 22 embalmed and 42 fresh female cadavers | N/A (range 0–104) |
| Mauroy, et al. [56] | Observational study (cohort) | Dissection | 25 embalmed female cadavers | N/A |
| Leffler, et al. [57] | Observational study (cohort) | Dissection | 10 embalmed and 2 fresh female cadavers | N/A |
| Occelli, et al. [58] | Observational study (cohort) | Dissection | 2 fresh female cadavers | 66 and 88 |
| Vazzoler, et al. [24] | Observational study (cohort) | Dissection + Histological | 8 embalmed and 2 fresh female cadavers | N/A (range 60–102) |
| Pit, et al. [59] | Observational study (cohort) | Dissection | 10 embalmed female cadavers | N/A |
| Ersoy, et al. [23] | Observational study (cohort) | Dissection | 5 embalmed female cadavers | 61 (range 52–71) |
| Albright, et al. [60] | Observational study (cohort) | Dissection (including measures) | 23 embalmed and 7 fresh female cadavers | N/A (range 64–94) |
| Ercoli, et al. [34] | Observational study (cohort) | Dissection | 30 fresh female cadavers | 67 (SD 8) (range 48–92) |
| Stecco, et al. [12] | Observational study (cohort) | Dissection + Histological | 20 female cadavers (≥8 embalmed) | N/A (range 54–72) |
| Fritsch, et al. [61] | Observational study (cohort) | Dissection + Radiological (MRI) + Histological | 6 embalmed female cadavers 41 living females | Cadavers: N/A Living females (N/A): (range 19–43) |
| El-Sayed, et al. [18] | Observational study (cohort) | Dissection + Radiological (MRI) + Histological | 7 embalmed female cadavers and 17 living females | Cadavers: N/A (range 25–50) Living females: 26 (SD 4) (range 20–35) |
| Nagata, et al. [20] | Observational study (cohort) | Dissection + Histological | 20 embalmed female cadavers | 82 (range 71–95) |
| Soga, et al. [62] | Observational study (cohort) | Dissection + Histological | 15 embalmed female cadavers | 84 (range 66–99) |
| Betschart, et al. [63] | Observational study (cohort) | Histological | 22 embalmed female cadavers | 87 (range 74–101) |
| Garcia-Armengol, et al. [64] | Observational study (cohort) | Dissection | 5 embalmed female cadavers | N/A |
| Kato, et al. [65] | Observational study (cohort) | Dissection + Histological | 15 embalmed female cadavers | 75 (range 64–90) |
| Stein and DeLancey [66] | Observational study (cohort) | Dissection | 3 female cadavers | N/A (range 28–56) |
| Brandon, et al. [67] | Observational study (cohort) | Radiological (MRI) | 20 living females | N/A (range 23–55) |
| Larson, et al. [68] | Observational study (cohort) | Radiological (MRI) + Clinical morphometric measure | 11 living females | 61 (SD 10) |
| Hirata, et al. [69] | Observational study (case-control) | Histological | 17 embalmed female cadavers (and 10 embalmed male cadavers) | N/A (range 56–84) (females and males combined) |
| Hirata, et al. [70] | Observational study (cohort) | Histological | 17 embalmed female cadavers | 75 (range 56–91) |
| Tsai, et al. [71] | Observational study (cohort) | Clinical morphometric measure | 200 living females in the first stage of labor | 27 (SD 6) |
| Hinata, et al. [72] | Observational study (cohort) | Dissection + Histological | 10 embalmed female cadavers | 85 (range 73–100) |
| Kraima, et al. [21] | Observational study (case-control) | Dissection + Histological | 2 embalmed female cadavers (and 2 embalmed male cadavers) | N/A |
| Santoro, et al. [73] | Observational study (cohort) | Radiological (3D endovaginal ultrasound imaging) | 5 fresh female cadavers and 44 living females | Cadavers: N/A Living females: 28 (range 18–34) |
| Lane, et al. [74] | Observational study (cohort) | Clinical morphometric measure | 127 living females in the first stage of labor | 24 (SD 5) (range 15–38) |
| Wu, et al. [75] | Observational study (cohort) | Dissection | 5 female cadavers | 33 (range 22–59) |
| Hamner, et al. [22] | Observational study (cohort) | Dissection + Histological | 25 female cadavers (≥4 fresh) | 76 (range 33–95) |
| Kochová, et al. [76] | Observational study (cohort) | Histological (including software for quantification) | 15 fresh female cadavers | 74 (SD 10) |
| Ghareeb, et al. [77] | Observational study (case-control) | Dissection | 5 embalmed female cadavers (and 13 embalmed male cadavers) | 74 (SD 7) (range 65–83) |
| Rodríguez-Abarca, et al. [78] | Observational study (case-control) | Radiological (MRI) | 102 living females | 41 (SD 15) (range 15–77) |
| Li, et al. [17] | Observational study (cohort) | Radiological (MRI and 3D reconstruction) | 4 female cadavers and 10 living females | Cadavers: N/A (range 22–25) Living females: N/A |
| Mboua Batoum, et al. [79] | Observational study (cohort) | Clinical morphometric measures | 103 living females in the first stage of labor | 23 (range 16–40) |
| Structures and Authors | Description and Course | Length, Mean (SD or Range) | Width, Mean (SD or Range) | Thickness, Mean (SD or Range) | Histological Assessment |
| Perineal membrane (PM) | |||||
| Aronson, et al. [54] |
| ||||
| DeLancey [55] |
| ||||
| Betschart, et al. [63] |
| ||||
| Kato, et al. [65] |
| Anterior area (range 3–12 mm) |
| ||
| Stein, et al. [66] |
| ||||
| Brandon, et al. [67] |
| ||||
| Hirata, et al. [69] |
| ||||
| Perineal body (PB) | |||||
| DeLancey [55] |
| Thickest in its inferior part and progressively thinner toward its superior margin. | |||
| At the midsagittal section, the antero-posterior length of the PB range was 10–20 mm (dissection). |
| |||
| Kato, et al. [65] |
|
| |||
| Larson, et al. [68] | Using MRI:
| 3.2 cm (SD 1.3) (clinical measure) | |||
| Tsai, et al. [71] | 3.9 (95% CI 3.8–4.0) cm (range 2.2–6.0) (clinical measure) | ||||
| Santoro, et al. [73] |
| Antero-posterior diameter (depth) 14.49 mm (SD 1.48) as measured with US | Latero-lateral diameter (width) 13.26 mm (SD 1.19) as measured with US | Superior-inferior diameter (height) 7.57 mm (SD 0.45) as measured with US | |
| Lane, et al. [74] | 3.7 cm (SD 0.5) (range 2.3–5.0) (clinical measures) | ||||
| Wu, et al. [75] |
| Size depended on the fibrous tissue development. Biometry: 2.7 (SD 1.7) mL (n = 4 Asian) and 0.6 mL (n = 1 Caucasian) (dissection). | |||
| Kochova, et al. [76] |
|
| |||
| Mboua Batoum, et al. [79] |
| 3.21 cm (SD 0.75) (range 1.5–5.5) (clinical measures) | |||
| Endopelvic fascia | |||||
| DeLancey [16] |
| ||||
| Aronson, et al. [54] |
| ||||
| DeLancey [55] |
| ||||
| Ercoli, et al. [34] |
| ||||
| Hirata, et al. [69] |
|
| |||
| Pubourethral ligament/Pubovesical ligament | |||||
| Milley and Nichols [52] |
| ||||
| DeLancey [19] |
|
| |||
| DeLancey [19] |
|
| |||
| Aronson, et al. [54] |
| ||||
| Vazzoler, et al. [24] |
| 10 mm | 3 mm |
| |
| N/A (range 2–4) mm |
| |||
|
| ||||
| Pit, et al. [59] |
| ||||
| Ercoli, et al. [34] |
| ||||
| Fritsch, et al. [61] |
|
| |||
| El-Sayed, et al. [18] |
|
| |||
|
| ||||
| Tendinous arch of pelvic fascia (TAPF) | |||||
| Hamner, et al. [22] |
| ||||
| Li, et al. [17] |
| 12.3 mm (SD 5.0) | |||
| Occelli, et al. [58] |
| 10 mm | |||
| Pit, et al. [59] |
| ||||
| Ersoy, et al. [23] |
| 8.1 cm (range 7.5–9.0) | |||
| Albright, et al. [60] |
| 8.99 cm (SD 0.70) (range 7.0–10.5); variability between right and left TAPF (range 0–0.75 cm) | |||
| Ercoli, et al. [34] |
| ||||
| Brandon, et al. [67] |
| ||||
| Hirata, et al. [69] |
| ||||
| Li, et al. [17] |
| 81.1 mm (SD 2.4) and divided in 2 parts at its conjoining point with the TALA; anterior portion 53.8 mm (SD 20.6); posterior portion 31.6 mm (SD 18.6) | Anterior portion 4.0 mm (SD 0.6); posterior portion 10.8 mm (SD 3.1) | The posterior portion was much thicker than the anterior portion. | |
| Tendinous arch of levator ani (TALA) | |||||
| Pit, et al. [59] |
| ||||
| Ersoy, et al. [23] |
| ||||
| Ercoli, et al. [34] |
| ||||
| Li, et al. [17] |
| 48.0 mm (SD 17.4) | TALA thinner than the TAPF. | ||
| Pubocervical fascia | |||||
| DeLancey [16] |
| ||||
| Ercoli, et al. [34] |
| Became progressively thinner, from the perineum toward the vaginal apex. | |||
| Hinata, et al. [72] |
|
| |||
| Rectovaginal fascia (RVF) | |||||
| DeLancey [16] |
| ||||
| Leffler, et al. [57] |
| ||||
| Ercoli, et al. [34] |
| Progressively thinner, from the perineum toward the vaginal apex. | |||
| Stecco, et al. [12] |
| Superior part of the median third of the vagina 1.75 mm (SD 0.75). Superior part of the lower third in the midline 1.70 mm (SD 0.88). Lateral portions of RVF 2.67 mm (SD 1.08), 2.64 mm (SD 1.12). Inferior median third at the inferior part in the midline 0.20 mm (SD 0.11), and laterally 0.17 mm (SD 0.07). |
| ||
| Nagata, et al. [20] |
| 35–60 mm (as defined between the peritoneal reflection at the Douglas’s pouch and the superior end of the internal anal sphincter). | Ranged from 0.1 mm to 0.3 mm, depending on the sites or levels of the vagina. |
| |
| Hinata, et al. [72] |
|
| |||
| Kraima, et al. [21] |
|
| |||
| Ghareeb, et al. [77] |
| ||||
| Rodriguez-Abaraca, et al. [78] | 73.2 mm (SD 15.3), (mid-sagittal plane by using the posterior fornix and the end of the recto-uterine pouch to the PB) | Superior third 2.8 mm (SD 1.7), middle third 2.2 mm (SD 1.2), inferior third 2.5 mm (SD 1.3) | |||
| Tendinous arch of rectovaginal fascia | |||||
| Ercoli, et al. [34] |
| ||||
| Rectovaginal ligament | |||||
| Ercoli, et al. [34] |
| ||||
| Rectosacral fascia/Inferior fascia of the pelvic diaphragm | |||||
| Ercoli, et al. [34] |
| ||||
| Garcia-Armengol, et al. [64] |
| ||||
| |||||
| Paracolpium | |||||
| DeLancey [16] |
| ||||
| Hinata, et al. [72] |
| ||||
| Authors | Domain 1 Objectives and Subject Characteristics | Domain 2 Study Design | Domain 3 Characterization of Methods | Domain 4 Descriptive Anatomy | Domain 5 Results Reporting | Overall Risk of Bias |
|---|---|---|---|---|---|---|
| Milley and Nichol [52] | Unclear | High | High | Unclear | High | High |
| DeLancey [19] | Unclear | Unclear | High | High | High | High |
| DeLancey and Starr [53] | Unclear | Unclear | Unclear | Unclear | High | High |
| DeLancey [16] | Unclear | Unclear | Unclear | Unclear | High | High |
| Aronson, et al. [54] | Unclear | Low | Low | Unclear | Unclear | High |
| DeLancey [55] | Unclear | Unclear | Low | Low | Unclear | High |
| Mauroy, et al. [56] | High | Low | High | High | High | High |
| Leffler, et al. [57] | Unclear | Low | Unclear | Low | Low | High |
| Occelli, et al. [58] | High | Unclear | Unclear | Unclear | Unclear | High |
| Vazzoler, et al. [24] | Unclear | Unclear | Unclear | Low | Low | High |
| Pit, et al. [59] | Unclear | Low | Low | Low | Low | Unclear |
| Ersoy, et al. [23] | Unclear | Unclear | Low | Low | High | High |
| Albright, et al. [60] | Low | Low | Low | Low | Low | Low |
| Ercoli, et al. [34] | Low | Low | Low | Unclear | Unclear | High |
| Stecco, et al. [12] | Low | Low | Low | Unclear | Low | Unclear |
| Fritsch, et al. [61] | Unclear | Low | Unclear | Unclear | Unclear | High |
| El-Sayed, et al. [18] | Low | Low | Low | Low | Low | Low |
| Nagata, et al. [20] | Low | Low | Low | Low | Low | Low |
| Soga, et al. [62] | Low | Low | Low | Low | Low | Low |
| Betschart, et al. [63] | Low | Low | Low | Low | Low | Low |
| Garcia-Armengol, et al. [64] | Unclear | Low | Low | Low | Unclear | High |
| Kato, et al. [65] | Low | Low | Low | Low | Low | Low |
| Stein and DeLancey [66] | Unclear | Low | Unclear | Low | Low | High |
| Brandon, et al. [67] | Unclear | Low | Low | Unclear | High | High |
| Larson, et al. [68] | Low | Low | Low | Low | Low | Low |
| Hirata, et al. [69] | Low | Low | Low | Low | Low | Low |
| Hirata, et al. [70] | Low | Low | Low | Low | Low | Low |
| Tsai, et al. [71] | Low | Low | Low | Unclear | Unclear | High |
| Hinata, et al. [72] | Low | Low | Low | Low | Low | Low |
| Kraima, et al. [21] | Unclear | Low | Low | Low | Low | Unclear |
| Santoro, et al. [73] | Low | Low | Low | Unclear | Unclear | High |
| Lane, et al. [74] | Low | Low | Low | Low | Low | Low |
| Wu, et al. [75] | Unclear | Low | Unclear | Unclear | Unclear | High |
| Hamner, et al. [22] | Low | Low | Low | Low | Low | Low |
| Kochova, et al. [76] | Low | Low | Low | Low | Low | Low |
| Ghareeb, et al. [77] | Low | Low | Low | Low | Low | Low |
| Rodriguez-Abarca, et al. [78] | Low | Low | Low | Low | Low | Low |
| Li, et al. [17] | Unclear | Unclear | Unclear | Unclear | Unclear | High |
| Mboua Batoum, et al. [79] | Low | Low | Low | Low | Low | Low |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roch, M.; Gaudreault, N.; Cyr, M.-P.; Venne, G.; Bureau, N.J.; Morin, M. The Female Pelvic Floor Fascia Anatomy: A Systematic Search and Review. Life 2021, 11, 900. https://doi.org/10.3390/life11090900
Roch M, Gaudreault N, Cyr M-P, Venne G, Bureau NJ, Morin M. The Female Pelvic Floor Fascia Anatomy: A Systematic Search and Review. Life. 2021; 11(9):900. https://doi.org/10.3390/life11090900
Chicago/Turabian StyleRoch, Mélanie, Nathaly Gaudreault, Marie-Pierre Cyr, Gabriel Venne, Nathalie J. Bureau, and Mélanie Morin. 2021. "The Female Pelvic Floor Fascia Anatomy: A Systematic Search and Review" Life 11, no. 9: 900. https://doi.org/10.3390/life11090900
APA StyleRoch, M., Gaudreault, N., Cyr, M.-P., Venne, G., Bureau, N. J., & Morin, M. (2021). The Female Pelvic Floor Fascia Anatomy: A Systematic Search and Review. Life, 11(9), 900. https://doi.org/10.3390/life11090900






