Extant Earthly Microbial Mats and Microbialites as Models for Exploration of Life in Extraterrestrial Mat Worlds
Abstract
1. Introduction and Background
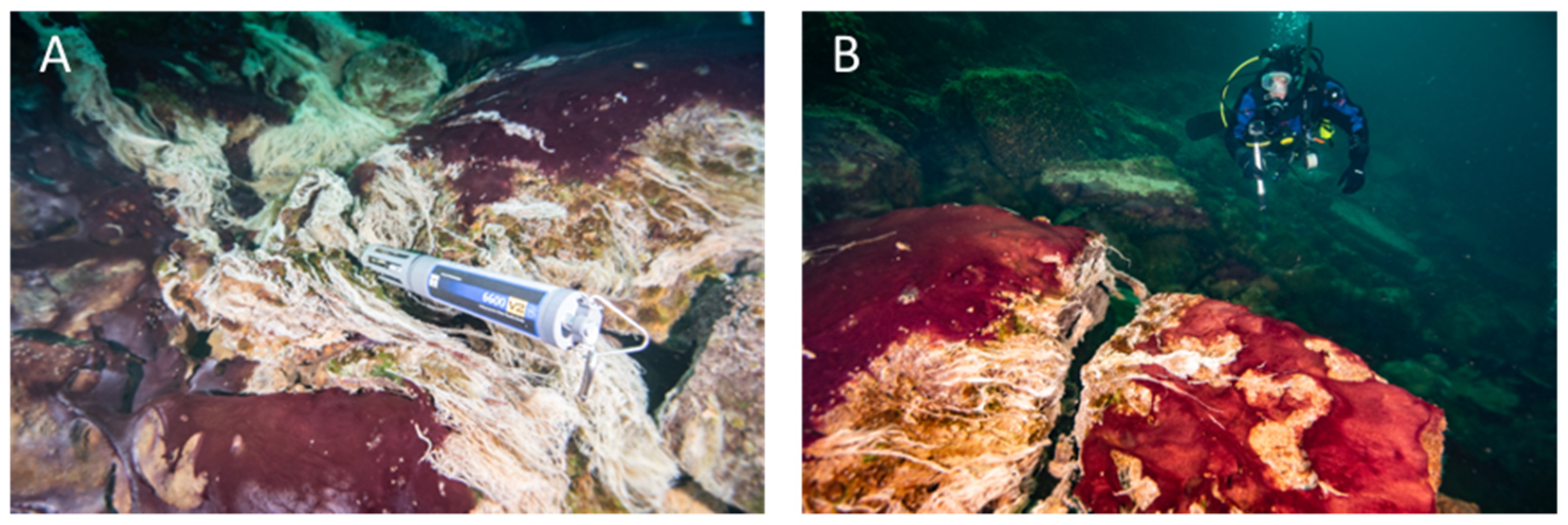
- Extensive soft and colorful microbial mats on the sediment surface of submerged sinkholes (~1–200 m) in Lake Huron, a freshwater Laurentian Great Lake (USA) (45.198790°, −83.327769°) (Figures S1 and S2).
- Semi-hard giant microbialite reefs in the shallow coastal waters (~0–5 m) of Laguna Bacalar, Quintana Roo, Mexico (18.644518°, −88.405611°) (Figures S3 and S4).
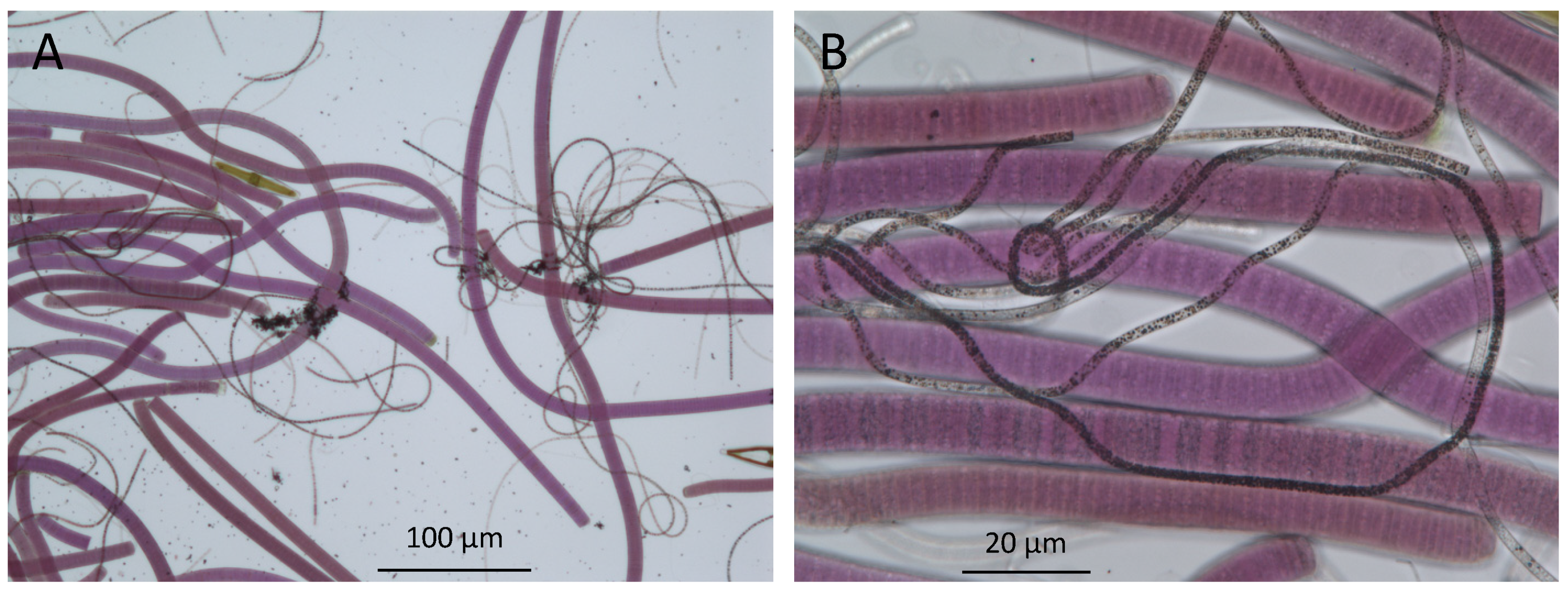
2. Microbial Mats in Lake Huron’s Submerged Sinkholes
3. Massive Microbialites of Laguna Bacalar
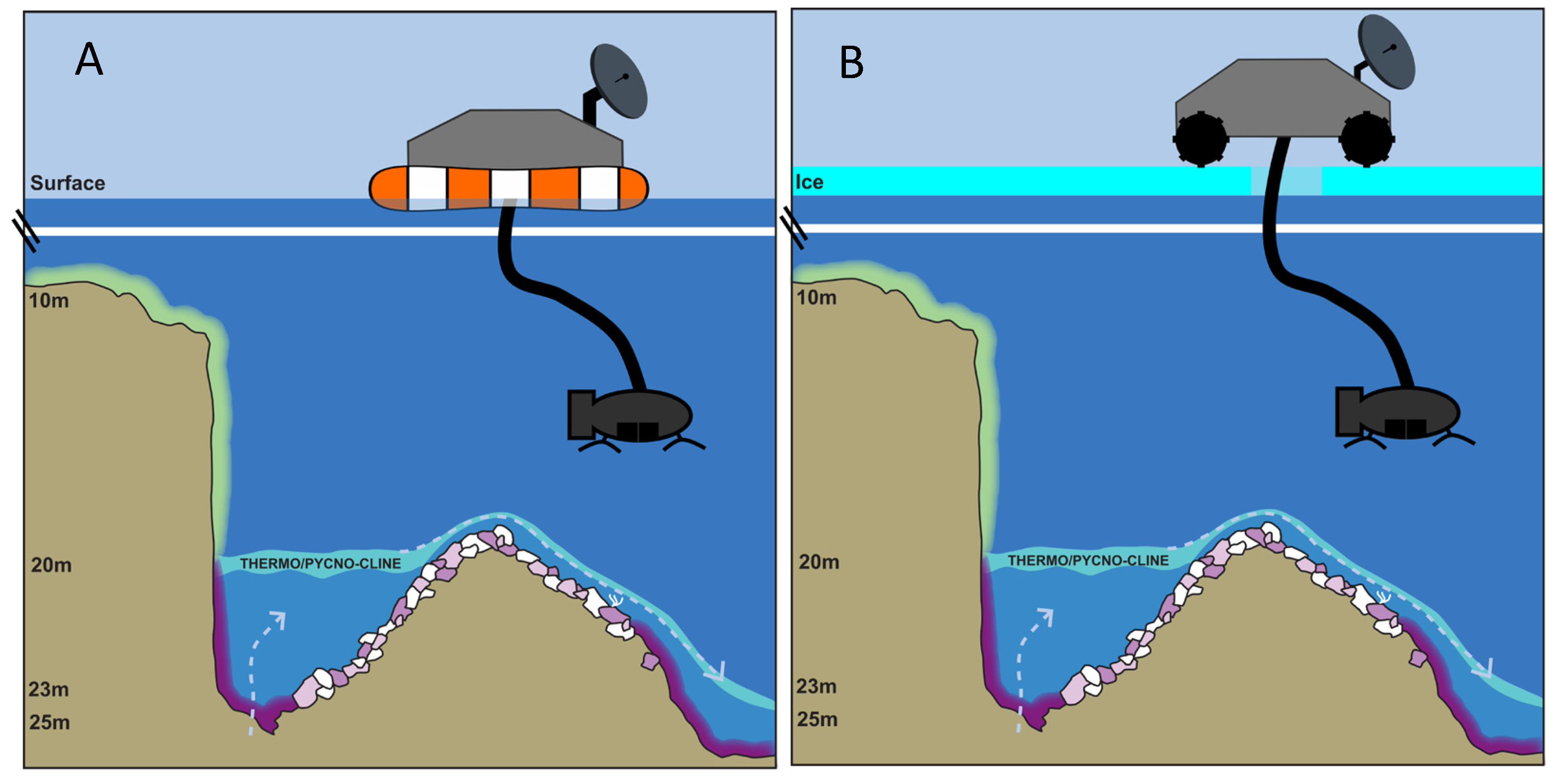
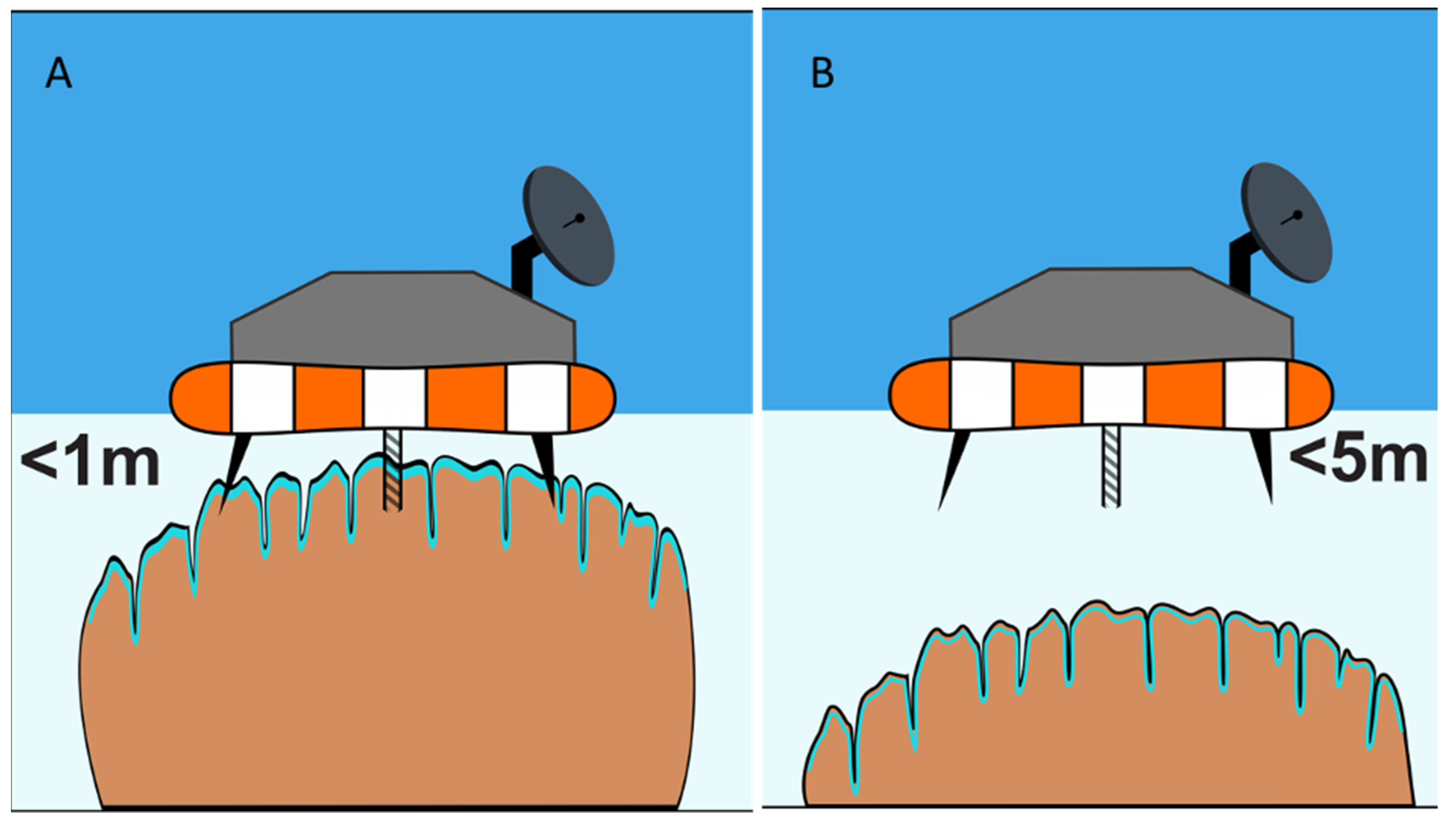
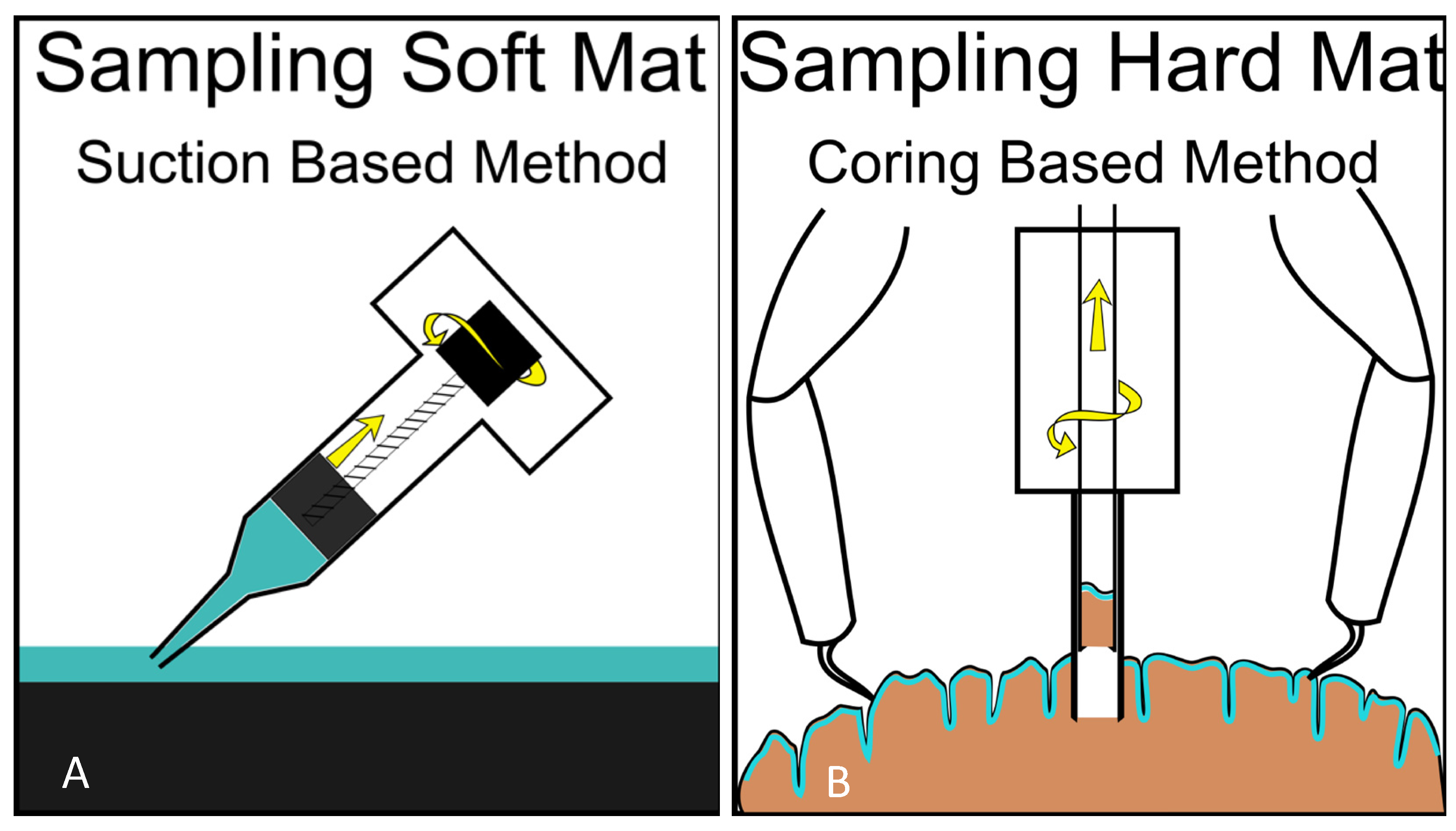
4. How to Study Mat Worlds of Extraterrestrial Hydrospheres When We Get There
5. Extant Mat Worlds in Ice and Rock Habitats—Examples from Polar Lakes and Earth’s Deep Biosphere
6. Aphotic Mat Worlds—Example of Deep-Water Sinkholes of Lake Huron
7. Extant Mats and Microbialites as Testbeds for Study of Extraterrestrial Life
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Whitman, W.B.; Coleman, D.C.; Wiebe, W.J. Prokaryotes: The unseen majority. Proc. Natl. Acad. Sci. USA 1998, 95, 6578–6583. [Google Scholar] [CrossRef]
- Cavicchioli, R.; Ripple, W.; Timmis, K.; Azam, F.; Bakken, L.R.; Baylis, M.; Behrenfeld, M.J.; Boetius, A.; Boyd, P.W.; Classen, A.T.; et al. Scientist’s warning to humanity: Microorganisms and climate change. Nat. Rev. Microbiol. 2019, 17, 569–586. [Google Scholar] [CrossRef]
- Biddanda, B.A.; Dila, D.; Weinke, A.; Mancuso, J.; Villar-Argaiz, M.; Medina-Sánchez, J.M.; González-Olalla, J.M.; Carrillo, P. Housekeeping in the hydrosphere: Microbial cooking, cleaning, and control under stress. Life 2021, 11, 152. [Google Scholar] [CrossRef] [PubMed]
- Stal, L.J. Physiological ecology of cyanobacteria in microbial mats and others communities. New Phytol. 1995, 131, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Schopf, J.W. Precambrian micro-organisms and evolutionary events prior to the origin of vascular plants. Biol. Rev. 1970, 45, 319–352. [Google Scholar] [CrossRef]
- Sanchez-Baracaldo, P. Origin of marine planktonic cyanobacteria. Sci. Rep. 2015, 5, 17418. [Google Scholar] [CrossRef] [PubMed]
- Dick, G.J.; Grim, S.L.; Klatt, J.M. Controls on O2 production in cyanobacterial mats and implications for Earth’s oxygenation. Annu. Rev. Earth Planet. Sci. 2018, 46, 123–147. [Google Scholar] [CrossRef]
- Perez-Ortega, R. Improbable oasis. Science 2020, 369, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Cohen, Y.; Padan, E.; Shilo, M. Facultative anoxygenic photosynthesis in the cyanobacterium Oscillatoria limneticai. J. Bacteriol. 1975, 123, 855–861. [Google Scholar] [CrossRef]
- Lugomela, C.; Sderback, E.; Bjork, M. Photosynthesis rates in cyanobacteria-dominated sub-tidal biofilms near Zanzibar, Tanzania. Estuar. Coast. Shelf Sci. 2005, 63, 439–446. [Google Scholar] [CrossRef]
- Jungblut, A.D.; Lovejoy, C.; Vincent, W.F. Global distribution of cyanobacterial ecotypes in the cold biosphere. ISME J. 2010, 4, 191–202. [Google Scholar] [CrossRef]
- Yang, T.; Lyons, S.; Aguilar, C.; Cuhel, R.; Teske, A. Microbial community sand chemosynthesis in Yellowstone Lake sublacustrine hydrothermal vent waters. Front. Microbiol. 2011, 2, 130. [Google Scholar] [CrossRef]
- Biddanda, B.A.; Nold, S.C.; Dick, G.J.; Kendall, S.T.; Vail, J.H.; Ruberg, S.A.; Green, C.M. Rock, Water, Microbes: Underwater Sinkholes in Lake Huron are Habitats for Ancient Microbial Life. Nat. Educ. Knowl. 2012, 3, 13. [Google Scholar]
- Prieto-Barajas, C.M.; Valencia-Cantero, E.; Santoyo, G. Microbial mat ecosystems: Structure types, functional diversity, and biotechnological application. Electron. J. Biotechnol. 2018, 31, 48–56. [Google Scholar] [CrossRef]
- Rosen, M.R.; Arehart, G.B.; Lico, M.S. Exceptionally fast growth rates of <100-yr-old tufa, Big Soda Lake, Nevada: Implications for using tufa as a paleoclimate proxy. Geology 2004, 32, 409–412. [Google Scholar] [CrossRef]
- de Beer, D.; Weber, M.; Chennu, A.; Hamilton, T.; Lott, C.; Macalady, J.; Klatt, J.M. Oxygenic and anoxygenic photosynthesis in a microbial mat from an anoxic and sulfidic spring. Environ. Microbiol. 2017, 19, 1251–1265. [Google Scholar] [CrossRef] [PubMed]
- Zammuto, V.; Rizzo, M.G.; De Plano, L.M.; Franco, D.; Guglielmino, S.; Caccamo, M.T.; Magzaù, S.; Fujimori, A.; Lo Giudice, A.; Guglielmin, M. Effects of heavy ion particle irradiation on spore germination of Bacillus spp. from extremely hot and cold environments. Life 2020, 10, 264. [Google Scholar] [CrossRef] [PubMed]
- Cavicchioli, R. Extremophiles and the search for extraterrestrial life. Int. J. Astrobiol. 2002, 2, 281–292. [Google Scholar] [CrossRef]
- Ruberg, S.A.; Kendall, S.T.; Biddanda, B.A.; Black, T.; Lusardi, W.; Green, R.; Casserley, T.; Smith, E.; Nold, S.; Sanders, T.G.; et al. Observations of the Middle Island sinkhole in Lake Huron: A unique hydrologic and glacial creation of 400 million years. Mar. Technol. Soc. J. 2008, 42, 12–21. [Google Scholar] [CrossRef]
- Voorhies, A.A.; Biddanda, B.A.; Nold, S.; Kendall, S.; Kain, S.; Marcus, D.; Sheldon, N.; Dick, G. Cyanobacterial life at low O2: Community genomics and function reveal metabolic versatility & extremely low diversity in a Great Lakes sinkhole. Geobiology 2012, 10, 250–267. [Google Scholar] [CrossRef]
- Kinsman-Costello, L.E.; Sheik, C.S.; Sheldon, N.D.; Burton, G.A.; Costello, D.M.; Marcus, D.; Den Uyl, P.A.; Dick, G.J. Groundwater shapes sediment biogeochemistry and microbial diversity in a submerged Great Lake sinkhole. Geobiology 2017, 15, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Nold, S.C.; Pangborn, J.B.; Zajack, H.A.; Kendall, S.T.; Rediske, R.R.; Biddanda, B.A. Benthic bacterial diversity in submerged sinkhole ecosystems. Appl. Environ. Microbiol. 2009, 76, 347–351. [Google Scholar] [CrossRef]
- Castenholz, R.W.; Jørgensen, B.B.; D’Amelio, E.; Bauld, J. Photosynthetic and behavioral versatility of the cyanobacterium Oscillatoria boryana in a sulfide-rich microbial mat. FEMS Microbiol. Ecol. 1991, 9, 43–57. [Google Scholar] [CrossRef][Green Version]
- Andersen, D.T.; Sumner, D.Y.; Hawes, I.; Webster-Brown, J.; Mcay, C.P. Discovery of large conical stromatolites in Lake Untersee, Antarctica. Geobiology 2011, 9, 280–293. [Google Scholar] [CrossRef]
- Allwood, A.C.; Walter, M.R.; Kamber, B.S.; Marshall, C.P.; Burch, I.W. Stromatolite reef from the early Archaean era of Australia. Nature 2006, 441, 714–718. [Google Scholar] [CrossRef] [PubMed]
- Falkowski, P.G.; Fenchel, T.; DeLong, E.F. The microbial engines that drive Earth’s biogeochemical cycles. Science 2008, 320, 1034–1039. [Google Scholar] [CrossRef]
- Voorhies, A.A.; Eisenlord, S.D.; Marcus, D.M.; Duhaime, M.B.; Biddanda, B.A.; Cavalcoli, J.D.; Dick, G.J. Ecological and genetic interactions between cyanobacteria and viruses in a low-O2 mat community inferred through metagenomics and metatranscriptomics. Environ. Microbiol. 2016, 18, 358–371. [Google Scholar] [CrossRef] [PubMed]
- Schmitter-Soto, J.J.; Comín, F.A.; Escobar-Briones, E.; Herrera-Silveira, J.; Alcocer, J.; Suárez-Morales, E.; Elías-Gutiérrez, M.; Díaz-Arce, V.; Steinich, B. Hydrogeochemical and biological characteristics of cenotes in the Yucatan Peninsula (SE Mexico). Hydrobiologia 2002, 467, 215–228. [Google Scholar] [CrossRef]
- Adame, M.F.; Santini, N.S.; Torres-Talamante, O.; Rogers, K. Mangrove sinkholes (cenotes) of the Yucatan Peninsula, a global hotspot of carbon sequestration. Biol. Lett. 2021, 17, 20210037. [Google Scholar] [CrossRef]
- Nold, S.C.; Bellecourt, M.J.; Kendall, S.T.; Ruberg, S.A.; Sanders, T.G.; Biddanda, B.A. Underwater sinkhole sediments sequester Lake Huron’s carbon. Biogeochemistry 2013, 115, 235–250. [Google Scholar] [CrossRef]
- Gishler, E.; Gibson, M.; Oschmann, W. Giant Holoce Microbialites, Laguna Bacalar, Quintana Roo, Mexico. Sedimentology 2008, 55, 1293–1309. [Google Scholar] [CrossRef]
- Gishler, E.; Golubic, S.; Gibson, M.; Oschmann, W.; Hudson, J. Microbial mats and microbialites of the freshwater Lacuna Bacalar, Yucatan Peninsula, Mexico. In Advances in Stromatolite Geobiology. Lecture Notes in Earth Sciences; Springer: Berlin/Heidelberg, Germany, 2011; Volume 131, pp. 187–205. ISBN 978-3-642-10415-2. [Google Scholar]
- Yanez-Montalvo, A.; Gomez-Acata, S.; Aguilla, B.; Hernadez-Arana, H.; Falcon, L.I. The microbiome of modern microbialites in Bacalar Lagoon, Mexico. PLoS ONE 2020, 15, e0230071. [Google Scholar] [CrossRef]
- Biddanda, B.A.; McMillan, A.C.; Long, S.A.; Snider, M.J.; Weinke, A.D. Seeking sunlight: Rapid phototactic motility of filamentous mat-forming cyanobacteria optimize photosynthesis and enhance carbon burial in Lake Huron’s submerged sinkholes. Front. Microbiol. 2015, 6, 930. [Google Scholar] [CrossRef] [PubMed]
- Biddanda, B.A.; Stone, I.P.; Weinke, A.D. Extant mat world microbes synchronize vertical migration to a diel tempo. Biogeochemistry 2021. under review. [Google Scholar]
- Lovelock, J.E. A Physical Basis for Life Detection Experiments. Nature 1965, 207, 568. [Google Scholar] [CrossRef] [PubMed]
- Hegde, S.; Paulino-Lima, I.G.; Kendt, R.; Keltenegger, L.; Rothschild, L. Surface biosignatures of exo-Earths: Remote detection of extraterrestrial life. Proc. Natl. Acad. Sci. USA 2015, 112, 3886–3891. [Google Scholar] [CrossRef] [PubMed]
- Fox, S.; Strasdeit, H. Inhabited or uninhabited? Pitfalls in the interpretation of possible chemical signatures of extraterrestrial life. Front. Micobiol. 2017, 8, 1622. [Google Scholar] [CrossRef]
- Georgiou, C.G. Functional properties of amino acid side chains as biomarkers of extraterrestrial life. Int. J. Astrobiol. 2018, 18, 1479–1496. [Google Scholar] [CrossRef] [PubMed]
- Lindensmith, C.A.; Rider, S.; Bedrossian, M.; Wallace, J.K.; Serabyn, E.; Showalter, G.M.; Deming, J.W.; Nadeau, J.L. A Submersible, Off-Axis Holographic Microscope for Detection of Microbial Motility and Morphology in Aqueous and Icy Environments. PLoS ONE 2016, 11, e0147700. [Google Scholar] [CrossRef] [PubMed]
- Nadeau, J.; Bedrossian, M.; Lindensmith, C. Imaging technologies and strategies for detection of extant extraterrestrial microorganisms. Adv. Phys. X 2018, 3, 1424032. [Google Scholar] [CrossRef]
- Zammuto, V.; Fuchs, F.M.; Fiebrandt, M.; Stapelmann, K.; Ulrich, N.J.; Maugeri, T.L.; Pukall, R.; Gugliandolo, C.; Moeller, R. Comparing spore resistance of Bacillus strains isolated from hydrothermal vents and spacecraft assembly facilities to environmental stressors and decontamination treatments. Astrobiology 2018, 18, 1425–1434. [Google Scholar] [CrossRef] [PubMed]
- Thoesen, A.; Marvi, H. Planetary surface mobility and exploration: A review. Curr Robot. Rep. 2021, 1–11. [Google Scholar] [CrossRef]
- Backus, S.B.; Onishi, R.; Bocklund, A.; Berg, A.; Contreras, E.D.; Parness, A. Design and testing of the JPL-Nautilus gripper for deep-ocean geological sampling. J. Field Robot. 2020, 37, 972–986. [Google Scholar] [CrossRef]
- Bada, J.L. State-of-the-art instruments for detecting extraterrestrial life. Proc. Natl. Acad. Sci. USA 2001, 98, 797–800. [Google Scholar] [CrossRef]
- Scoles, S. Shamu Dreams of Europa: The Microscope that Could Look for Life on Jupitor’s Moon; Popular Science, Bonnier Corporation: New York, NY, USA, 2018; pp. 70–75, 125. [Google Scholar]
- Vopel, K.; Hawes, I. Photosynthetic performance of benthic microbial mats in Lake Hoare, Antarctica. Limnol. Oceaongr. 2006, 51, 1801–1812. [Google Scholar] [CrossRef]
- Taton, A.; Grubisic, S.; Brambilla, E.; De Wit, R.; Wilmotte, A. Cyanobacterial diversity in natural and artificial microbial mats of Lake Fryxell (McMurdo Dry Valleys, antarctica): A morphological and molecular approach. Appl. Environ. Microbiol. 2003, 69, 5157–5169. [Google Scholar] [CrossRef] [PubMed]
- Bowman, J.S.; Vick-Majors, T.J.; Morgan-Kiss, R.; Takkacs-Vesbach, C.; Ducklow, H.W.; Priscu, J.C. Microbial community dynamics in tow polar extremes: The lakes of the McMurdo Dy Valleys and the West Antarctic Peninsula marine ecosystem. BioScience 2016, 66, 829–847. [Google Scholar] [CrossRef]
- Mikucki, M.; Priscu, J.C.; Alekhina, I.A.; Wadham, J.L.; Berry-Lyons, W. Subglacial lake Whilhans microbial biogeochemistry: A synthesis of current knowledge. Philos. Trans. R. Soc. A. 2015, 374, 20140466. [Google Scholar] [CrossRef]
- Achberger, A.M.; Christner, B.C.; Michaud, A.B.; Priscu, J.C.; Skidmore, M.L.; Vick-Majors, T.J.; WISSARD Science Team. Microbial community structure of subglacial Lake Whilhans, West Antarctica. Front. Microbiol. 2016, 7, 1457. [Google Scholar] [CrossRef]
- Foreman, C.M.; Dieser, M.; Greenwood, M.; Cory, R.M.; Laybourn-Parry, J.; Lisle, J.T.; Jaros, C.; Miller, P.L.; Chin, Y.-P.; McKnight, D.M. When habitat freezes solid: Microorganisms over-winter within the ice column of a coastal Antarctic lake. FEMS Microbiol. Ecol. 2011, 76, 401–412. [Google Scholar] [CrossRef]
- Dudeja, S.; Bhattacherjee, A.B.; Chela-Flores, J. Microbial mats in Antarctica as models for the search of life on the Jovian moon Europa. In Microbial Mats; COLE Series; Springer: Dordrecht, The Netherlands, 2010; pp. 543–561. [Google Scholar]
- Siegert, M.J.; Ellis-Evans, J.C.; Tranter, M.; Mayer, C.; Petit, J.R.; Salamatin, A.; Priscu, J.C. Physical, chemical and biological processes in Lake Vostok and other Antarctic subglacial lakes. Nature 2001, 414, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Priscu, J.C.; Bell, R.E.; Bulat, S.A.; Ellis-Evans, C.J.; Kennicutt, M.C.; Lukin, V.V.; Petit, J.-R.; Powell, R.D.; Siegert, M.J.; Tabacco, I. An international plan for Antarctica subglacial lake exploration. Polar Geogr. 2003, 27, 69–83. [Google Scholar] [CrossRef]
- Christner, B.C.; Roysto-Bishop, G.; Foreman, C.M.; Arnold, B.R.; Tranter, M.; Welh, K.A.; Lyons, W.B.; Tsapin, A.I.; Studinger, M.; Priscu, J.C. Limnological conditions in subglacial Lake Vostok, Antarctica. Limnol. Oceanogr. 2006, 51, 2485–2501. [Google Scholar] [CrossRef]
- Dudeja, S.; Bhattacharya, A.B.; Chela-Flores, J. Antarctica as model for the possible emergence of life on Europa. In Life on Earth and Other Planetary Bodies; Hanslmeier, A., Kempe, S., Seckbach, J., Eds.; Cellular Origin and Life in Extreme Habitats and Astrobiology; Springer: Dordrecht, The Netherlands, 2012; pp. 409–422. [Google Scholar] [CrossRef]
- Kallemeyer, J.; Pockalny, R.; Adhikari, R.R.; Smith, D.C.; D’Hondt, S. Global distribution of microbial abundance and biomass in subseafloor sediment. Proc. Natl. Acad. Sci. USA 2012, 109, 16213–16216. [Google Scholar] [CrossRef]
- Parkes, R.J.; Cragg, B.; Roussel, E.; Webster, G.; Weightman, A.; Sass, H. A review of prokaryotic populations and processes in sub-seafloor sediments, including biosphere: Geosphere interactions. Mar. Geol. 2014, 352, 409–425. [Google Scholar] [CrossRef]
- Heuer, V.B.; Inagaki, F.; Morono, Y.; Kubo, Y.; Spivack, A.J.; Viehweger, B.; Treude, T.; Beulig, F.; Schubotz, F.; Tonai, S.; et al. Temperature limits to deep subseafloor life in the Nankai Trough subduction zone. Science 2020, 370, 1230–1234. [Google Scholar] [CrossRef]
- Trembath-Reichert, E.; Sah Walter, S.R.; Fontanez Otitz, M.A.; Carter, P.D.; Girguls, P.R.; Huber, J.A. Multiple carbon incorporation strategies support microbial survival in cold subseafloor crustal fluids. Sci. Adv. 2021, 7, eabg0153. [Google Scholar] [CrossRef]
- Reid, I.N.; Sparks, W.B.; Lubow, S.; McGrath, M.; Livio, M.; Sowers, K.R.; Shukla, H.D.; MacAuley, S.; Miller, T.; Suvanasuthi, R. Terrestrial models for extraterrestrial life: Methanogens and halophiles at Martian temperatures. Int. J. Astrobiol. 2006, 5, 89–97. [Google Scholar] [CrossRef]
- Biddanda, B.A.; Coleman, D.F.; Johengen, T.H.; Ruberg, S.A.; Meadows, G.A.; VanSumeren, H.W.; Rediske, R.R.; Kendall, S.T. Exploration of a submerged sinkhole ecosystem in Lake Huron. Ecosystems 2006, 9, 828–842. [Google Scholar] [CrossRef]
- Sharrar, A.; Flood, B.; Bailey, J.; Jones, D.; Biddanda, B.A.; Ruberg, S.; Marcus, D.; Dick, G. Novel large sulfur bacteria in the metagenomes of groundwater-fed chemosynthetic microbial mats in the Lake Huron basin. Front. Microbiol. 2017, 8, 791. [Google Scholar] [CrossRef] [PubMed]
- Beatty, J.T.; Overmann, J.; Lince, M.T.; Manske, A.K.; Lang, A.S.; Blankenship, R.E.; Van Dover, C.L.; Martinson, T.A.; Plumley, F.G. An obligately photosynthetic bacterial anaerobe form a deep-sea hydrothermal vent. Proc. Natl. Acad. Sci. USA 2005, 102, 9306–9310. [Google Scholar] [CrossRef] [PubMed]
- Vick-Majors, T.J.; Michaud, A.B.; Skidmore, M.L.; Turetta, C.; Barbante, C.; Christner, B.C.; Dore, J.E.; Christianson, K.; Mitchell, A.C.; Achberger, A.M.; et al. Biogeochemical connectivity between freshwater ecosystems beneath the West Antarctic Ice Sheet and he sub-ice marine environment. Glob. Biogeochem. Cycles 2020, 34, e2019GB006446. [Google Scholar] [CrossRef]
- Klatt, J.M.; Chennu, A.; Arbic, B.K.; Biddanda, B.A.; Dick, G.J. Possible link between Earth’s rotation rate and oxygenation. Nat. Geosci. 2021, 14, 564–570. [Google Scholar] [CrossRef]
- Mack, K. The End of Everything: Astrophysically Speaking; Scribner: New York, NY, USA, 2020; p. 227. [Google Scholar]
- Gould, S.J. Leonardo’s Mountain of Clams and the Diet of Worms; Harmony Books: New York, NY, USA, 1998; p. 422. [Google Scholar]
- Sagan, C. The Search for Extraterrestrial Life; Scientific American, Springer Nature: New York, NY, USA, 1994; Volume 271, pp. 92–99. [Google Scholar]
- Pace, N.R. The universal nature of biochemistry. Proc. Natl. Acad. Sci. USA 2001, 98, 805–808. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biddanda, B.; Weinke, A.; Stone, I.; Kendall, S.; Hartmeyer, P.; Lusardi, W.; Gandulla, S.; Bright, J.; Ruberg, S. Extant Earthly Microbial Mats and Microbialites as Models for Exploration of Life in Extraterrestrial Mat Worlds. Life 2021, 11, 883. https://doi.org/10.3390/life11090883
Biddanda B, Weinke A, Stone I, Kendall S, Hartmeyer P, Lusardi W, Gandulla S, Bright J, Ruberg S. Extant Earthly Microbial Mats and Microbialites as Models for Exploration of Life in Extraterrestrial Mat Worlds. Life. 2021; 11(9):883. https://doi.org/10.3390/life11090883
Chicago/Turabian StyleBiddanda, Bopaiah, Anthony Weinke, Ian Stone, Scott Kendall, Phil Hartmeyer, Wayne Lusardi, Stephanie Gandulla, John Bright, and Steven Ruberg. 2021. "Extant Earthly Microbial Mats and Microbialites as Models for Exploration of Life in Extraterrestrial Mat Worlds" Life 11, no. 9: 883. https://doi.org/10.3390/life11090883
APA StyleBiddanda, B., Weinke, A., Stone, I., Kendall, S., Hartmeyer, P., Lusardi, W., Gandulla, S., Bright, J., & Ruberg, S. (2021). Extant Earthly Microbial Mats and Microbialites as Models for Exploration of Life in Extraterrestrial Mat Worlds. Life, 11(9), 883. https://doi.org/10.3390/life11090883







