Unique Endomembrane Systems and Virulence in Pathogenic Protozoa
Abstract
1. Introduction
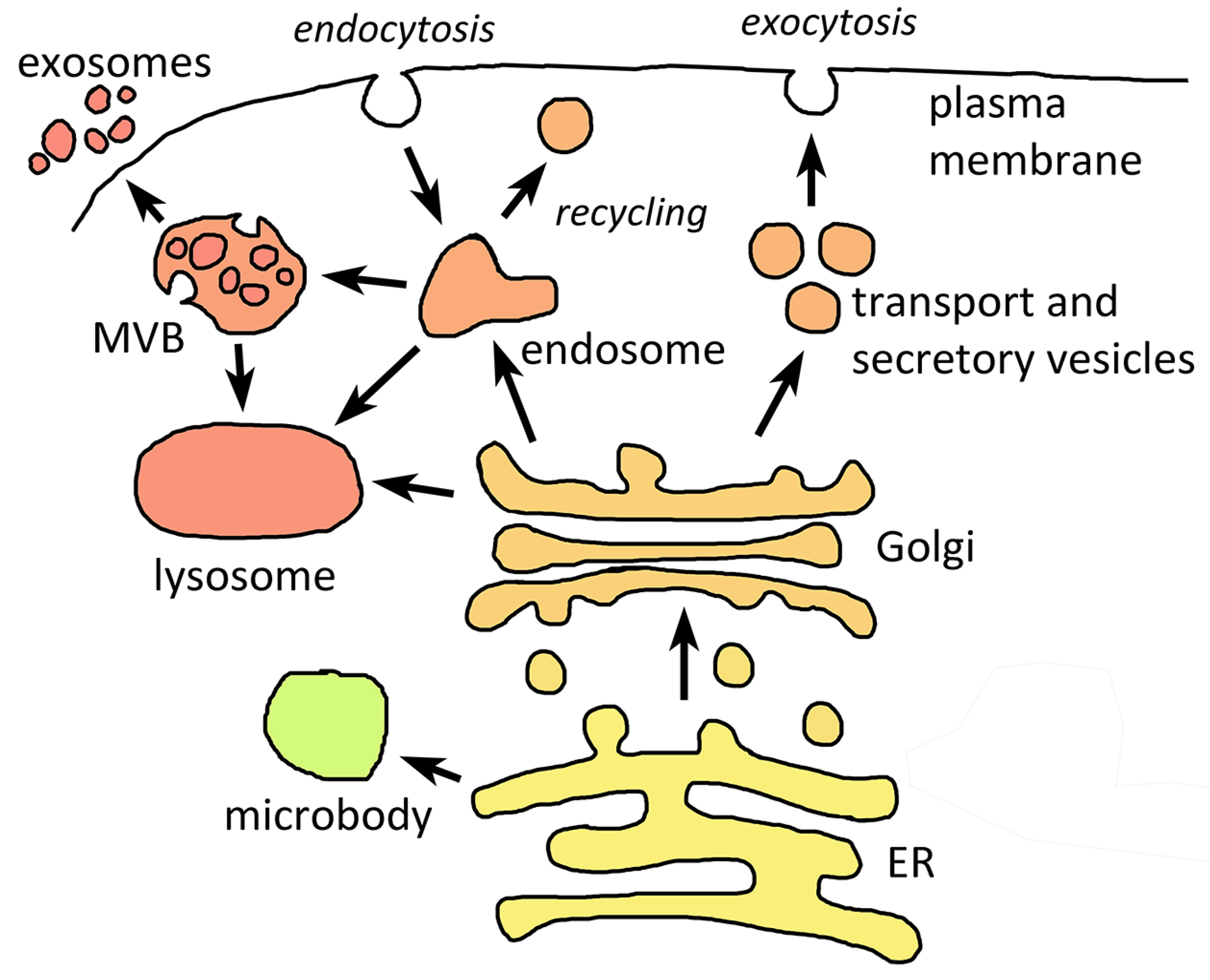
2. Basic Endomembrane Systems
3. Extracellular Vesicles
Extracellular Vesicles Are Virulence Factors in Pathogenic Protozoa
4. Anaerobic Luminal Pathogens
4.1. Pathogenesis Associated with Luminal Pathogens Is Associated with Cytoadherence and Secreted Proteases
4.2. Giardia Appears to Lack a Golgi and a Conventional Lysosome
5. Kinetoplastids
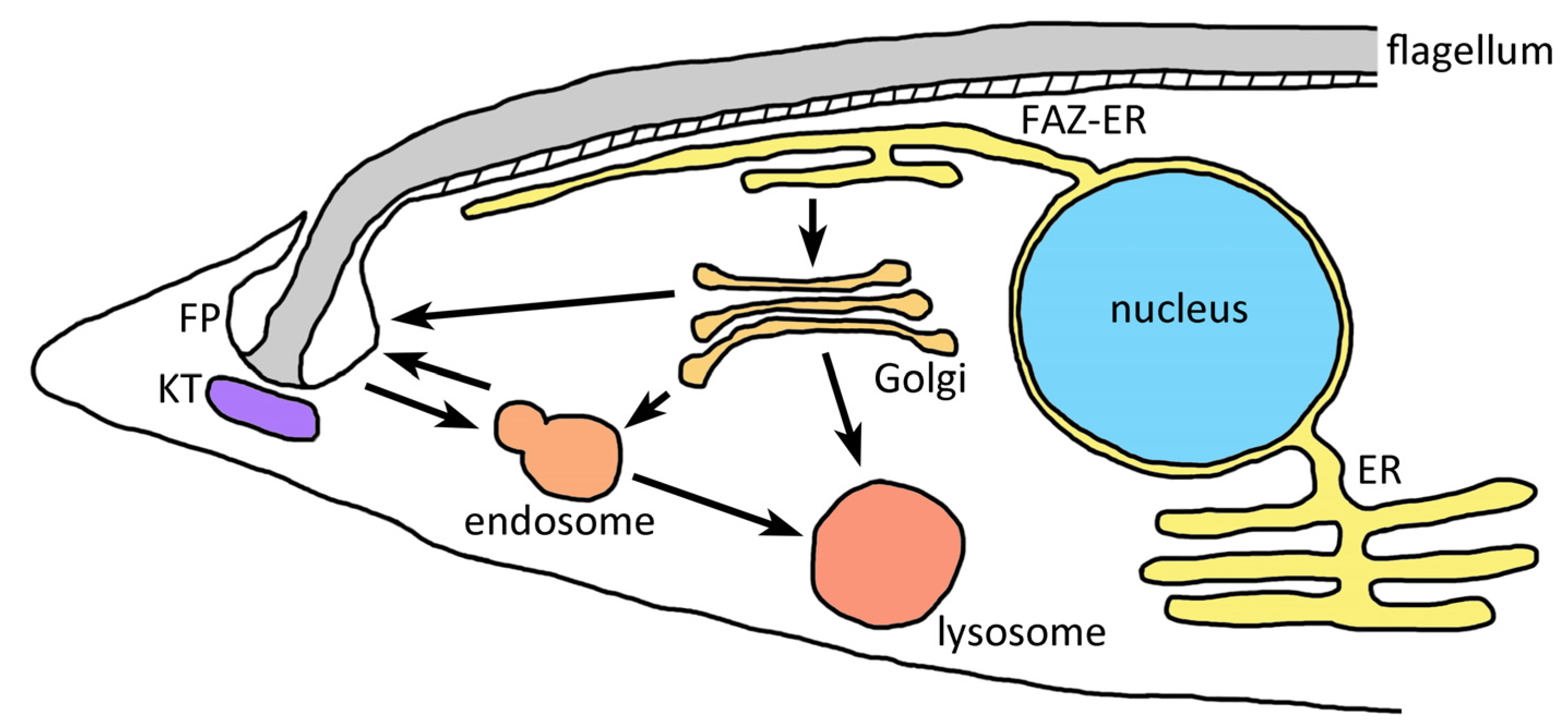
5.1. The Flagellar Pocket Is the Primary Site of Endocytosis and Exocytosis in Kinetoplastids
5.2. Glycosylphosphatidylinositol Anchors Are Abundant on the Plasma Membranes of Kinetoplastids
6. Apicomplexa and Apical Organelles
6.1. Micronemes and Rhoptries Facilitate Host Cell Entry, Exit and Modification
6.2. Dense Granules Participate in the Modification of Host Cells by Apicomplexa
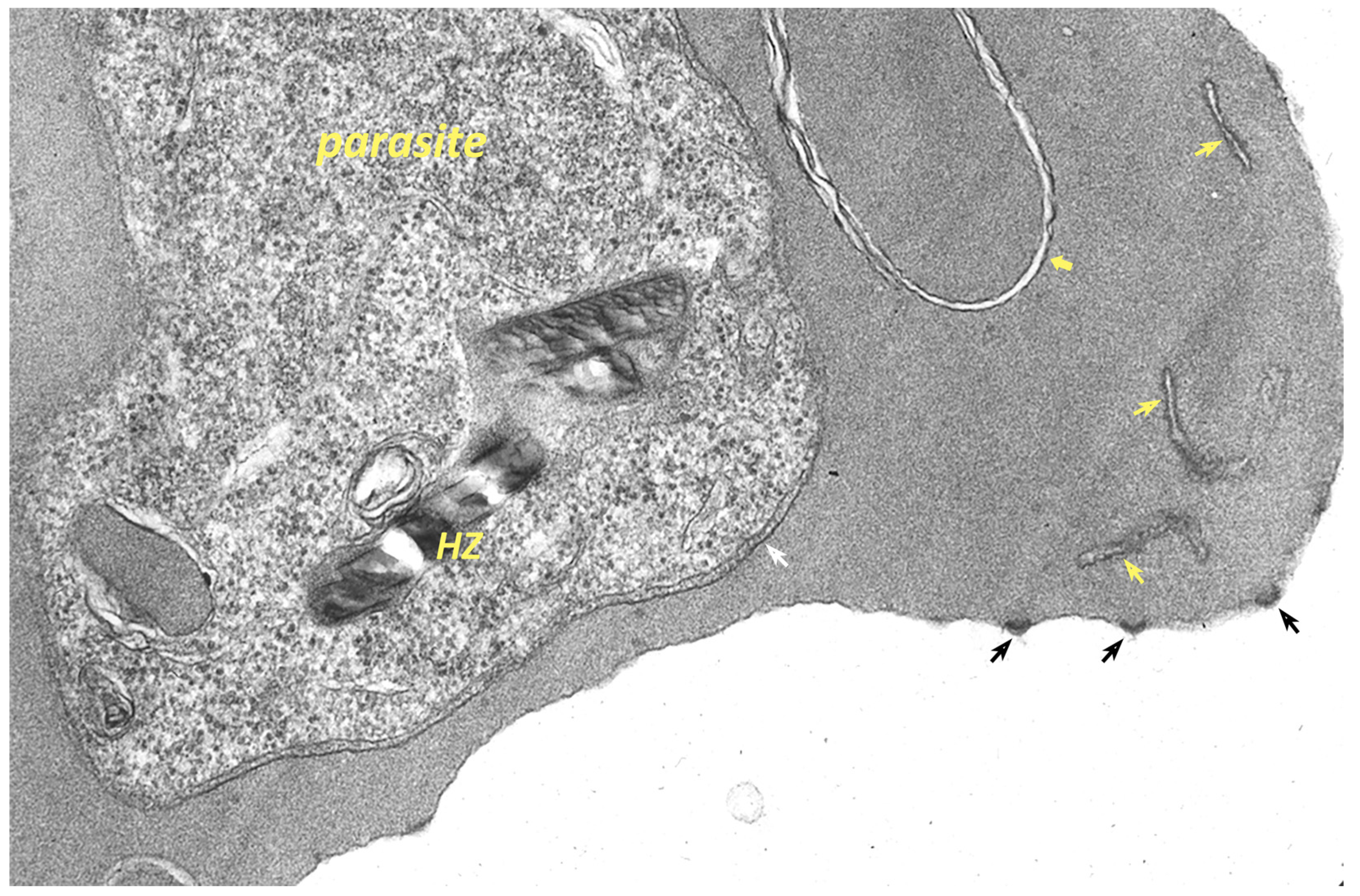
6.3. The PVM Is an Interface between the Parasite and the Host Cell
6.4. Endosomal Pathways May Function in the Formation of Micronemes and Rhoptries
6.5. Myzocytosis and the Apical Organelles
7. Alveolates and the Inner Membrane Complex
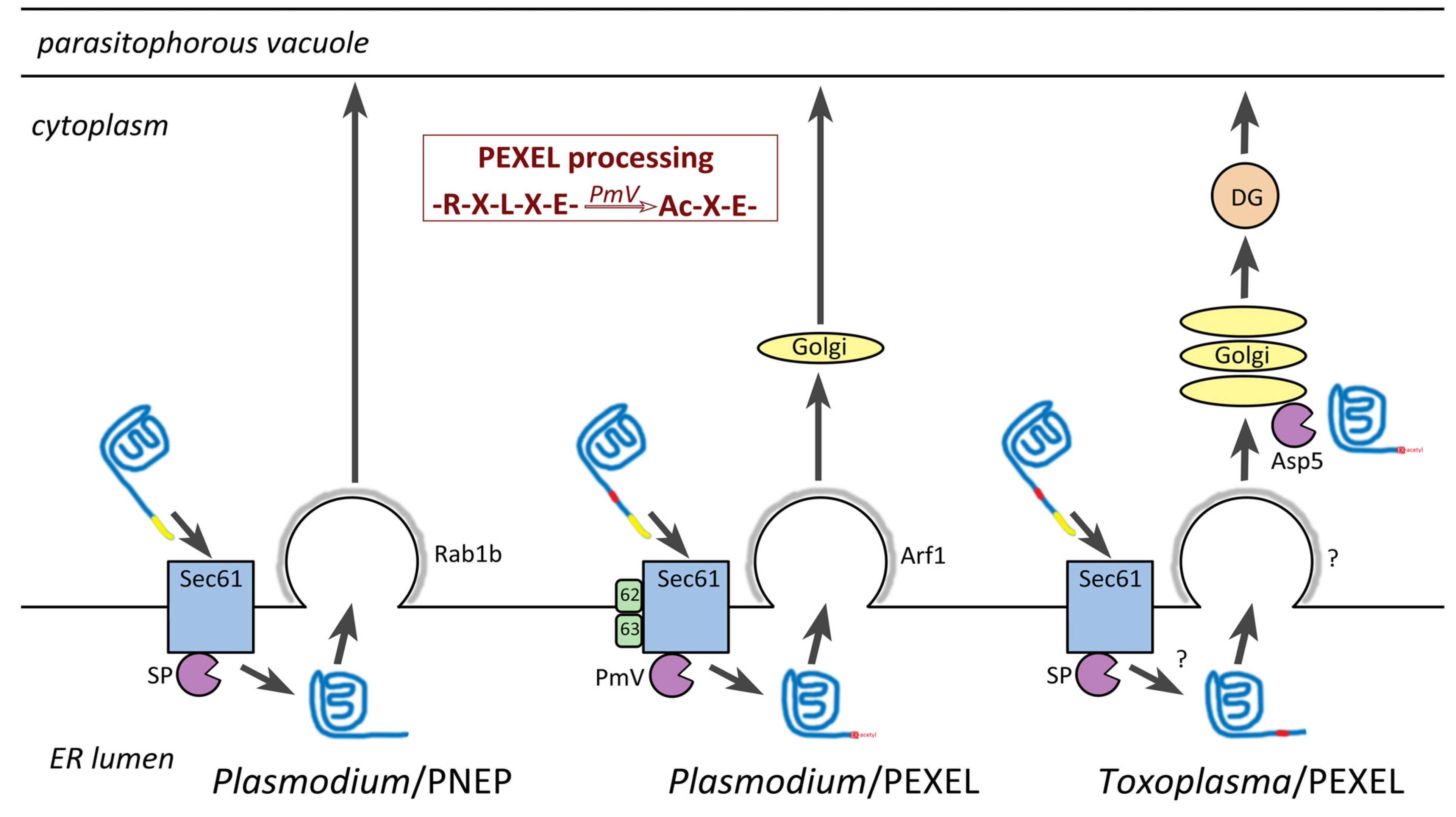
8. Host Targeting Sequences in Apicomplexa
8.1. PEXEL-Like Motifs in other Apicomplexans
8.2. Possible Ancient Origin of the PEXEL-Like Motif
9. Remodeling the Host Erythrocyte by the Malaria Parasite
9.1. A Possible Subdomain of the ER in Plasmodium for Host-Targeted Proteins
9.2. Plasmodium Has a Unique Translocon for Exporting Proteins from the Parasitophorous Vacuole
9.3. Extraparasite Trafficking within the Erythrocyte Cytoplasm
10. Summary
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Green, E.R.; Mecsas, J. Bacterial secretion systems: An overview. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef] [PubMed]
- More, K.; Klinger, C.M.; Barlow, L.D.; Dacks, J.B. Evolution and natural history of membrane trafficking in eukaryotes. Curr. Biol. 2020, 30, R553–R564. [Google Scholar] [CrossRef]
- Cavalier-Smith, T. Protist phylogeny and the high-level classification of protozoa. Eur. J. Protistol. 2003, 39, 338–348. [Google Scholar] [CrossRef]
- Burki, F.; Roger, A.J.; Brown, M.W.; Simpson, A.G.B. The new tree of eukaryotes. Trends Ecol. Evol. 2020, 35, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Wiser, M.F. Protozoa and Human Disease; Garland Science: New York, NY, USA, 2010; ISBN 9780429258282. [Google Scholar]
- Wiser, M.F. Nutrition and protozoan pathogens of humans: A primer. In Nutrition and Infectious Diseases: Shifting the Clinical Paradigm; Humphries, D.L., Scott, M.E., Vermund, S.H., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 165–187. ISBN 978-3-030-56913-6. [Google Scholar]
- Cavalier-Smith, T. The phagotrophic origin of eukaryotes and phylogenetic classification of protozoa. Int. J. Syst. Evol. Microbiol. 2002, 52, 297–354. [Google Scholar] [CrossRef] [PubMed]
- Dacks, J.B.; Peden, A.A.; Field, M.C. Evolution of specificity in the eukaryotic endomembrane system. Int. J. Biochem. Cell Biol. 2009, 41, 330–340. [Google Scholar] [CrossRef]
- Rothman, J.E. Mechanisms of intracellular protein transport. Nature 1994, 372, 55–63. [Google Scholar] [CrossRef]
- Balmer, E.A.; Faso, C. The road less traveled? Unconventional protein secretion at parasite-host interfaces. Front. Cell Dev. Biol. 2021, 9, 1070. [Google Scholar] [CrossRef]
- Peng, M.; Chen, F.; Wu, Z.; Shen, J. Endoplasmic reticulum stress, a target for drug design and drug resistance in parasitosis. Front. Microbiol. 2021, 12, 670874. [Google Scholar] [CrossRef]
- Mowbrey, K.; Dacks, J.B. Evolution and diversity of the Golgi body. FEBS Lett. 2009, 583, 3738–3745. [Google Scholar] [CrossRef]
- Touz, M.C.; Zamponi, N. Sorting without a Golgi complex. Traffic 2017, 18, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Virgen, K.; Chávez-Munguía, B.; Talamás-Lara, D.; Lagunes-Guillén, A.; Martínez-Higuera, A.; Lazcano, A.; Martínez-Palomo, A.; Espinosa-Cantellano, M. Giardia lamblia: Identification of peroxisomal-like proteins. Exp. Parasitol. 2018, 191, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Touz, M.C.; Rivero, M.R.; Miras, S.L.; Bonifacino, J.S. Lysosomal protein trafficking in Giardia lamblia: Common and distinct features. Front. Biosci. (Elite Ed.) 2012, 4, 1898–1909. [Google Scholar] [CrossRef]
- Müller, M.; Mentel, M.; van Hellemond, J.J.; Henze, K.; Woehle, C.; Gould, S.B.; Yu, R.-Y.; van der Giezen, M.; Tielens, A.G.M.; Martin, W.F. Biochemistry and evolution of anaerobic energy metabolism in eukaryotes. Microbiol. Mol. Biol. Rev. 2012, 76, 444–495. [Google Scholar] [CrossRef]
- Benchimol, M. Trichomonads under microscopy. Microsc. Microanal. 2004, 10, 528–550. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.K.; Field, J.; Frisardi, M.; Rosenthal, B.; Mai, Z.; Rogers, R.; Samuelson, J. Chitinase secretion by encysting Entamoeba invadens and transfected Entamoeba histolytica trophozoites: Localization of secretory vesicles, endoplasmic reticulum, and Golgi apparatus. Infect. Immun. 1999, 67, 3073–3081. [Google Scholar] [CrossRef]
- Field, M.C.; Natesan, S.K.A.; Gabernet-Castello, C.; Lila Koumandou, V. Intracellular trafficking in the trypanosomatids. Traffic 2007, 8, 629–639. [Google Scholar] [CrossRef]
- Szöör, B.; Haanstra, J.R.; Gualdrón-López, M.; Michels, P.A.M. Evolution, dynamics and specialized functions of glycosomes in metabolism and development of trypanosomatids. Curr. Opin. Microbiol. 2014, 22, 79–87. [Google Scholar] [CrossRef]
- Halliday, C.; de Castro-Neto, A.; Alcantara, C.L.; Cunha-E-Silva, N.L.; Vaughan, S.; Sunter, J.D. Trypanosomatid flagellar pocket from structure to function. Trends Parasitol. 2021, 37, 317–329. [Google Scholar] [CrossRef]
- Cowman, A.F.; Crabb, B.S. Invasion of red blood cells by malaria parasites. Cell 2006, 124, 755–766. [Google Scholar] [CrossRef]
- Kono, M.; Prusty, D.; Parkinson, J.; Gilberger, T.W. The apicomplexan inner membrane complex. Front. Biosci. 2013, 18, 982–992. [Google Scholar] [CrossRef]
- Pelletier, L.; Stern, C.A.; Pypaert, M.; Sheff, D.; Ngô, H.M.; Roper, N.; He, C.Y.; Hu, K.; Toomre, D.; Coppens, I.; et al. Golgi biogenesis in Toxoplasma gondii. Nature 2002, 418, 548–552. [Google Scholar] [CrossRef]
- Gabaldón, T.; Ginger, M.L.; Michels, P.A.M. Peroxisomes in parasitic protists. Mol. Biochem. Parasitol. 2016, 209, 35–45. [Google Scholar] [CrossRef] [PubMed]
- McFadden, G.I.; Yeh, E. The apicoplast: Now you see it, now you don’t. Int. J. Parasitol. 2017, 47, 137–144. [Google Scholar] [CrossRef]
- Struck, N.S.; de Souza Dias, S.; Langer, C.; Marti, M.; Pearce, J.A.; Cowman, A.F.; Gilberger, T.W. Re-defining the Golgi complex in Plasmodium falciparum using the novel Golgi marker PfGRASP. J. Cell Sci. 2005, 118, 5603–5613. [Google Scholar] [CrossRef]
- Schrevel, J.; Asfaux-Foucher, G.; Hopkins, J.M.; Robert, V.; Bourgouin, C.; Prensier, G.; Bannister, L.H. Vesicle trafficking during sporozoite development in Plasmodium berghei: Ultrastructural evidence for a novel trafficking mechanism. Parasitology 2008, 135, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, D.E. Hemoglobin degradation. Curr. Top. Microbiol. Immunol. 2005, 295, 275–291. [Google Scholar] [CrossRef]
- Huotari, J.; Helenius, A. Endosome maturation. EMBO J. 2011, 30, 3481–3500. [Google Scholar] [CrossRef]
- Schliebs, W.; Girzalsky, W.; Erdmann, R. Peroxisomal protein import and ERAD: Variations on a common theme. Nat. Rev. Mol. Cell Biol. 2010, 11, 885–890. [Google Scholar] [CrossRef]
- Gill, S.; Catchpole, R.; Forterre, P. Extracellular membrane vesicles in the three domains of life and beyond. FEMS Microbiol. Rev. 2019, 43, 273–303. [Google Scholar] [CrossRef]
- Kastelowitz, N.; Yin, H. Exosomes and microvesicles: Identification and targeting by particle size and lipid chemical probes. Chembiochem 2014, 15, 923–928. [Google Scholar] [CrossRef]
- Evans-Osses, I.; Reichembach, L.H.; Ramirez, M.I. Exosomes or microvesicles? Two kinds of extracellular vesicles with different routes to modify protozoan-host cell interaction. Parasitol. Res. 2015, 114, 3567–3575. [Google Scholar] [CrossRef] [PubMed]
- de Souza, W.; Barrias, E.S. Membrane-bound extracellular vesicles secreted by parasitic protozoa: Cellular structures involved in the communication between cells. Parasitol. Res. 2020, 119, 2005–2023. [Google Scholar] [CrossRef]
- Sabatke, B.; Gavinho, B.; Coceres, V.; de Miguel, N.; Ramirez, M.I. Unveiling the role of EVs in anaerobic parasitic protozoa. Mol. Immunol. 2021, 133, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Gioseffi, A.; Edelmann, M.J.; Kima, P.E. Intravacuolar pathogens hijack host extracellular vesicle biogenesis to secrete virulence factors. Front. Immunol. 2021, 12, 662944. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, N.G.; Cheng, L.; Eriksson, E.M. The role of extracellular vesicles in malaria biology and pathogenesis. Malar. J. 2017, 16, 245. [Google Scholar] [CrossRef] [PubMed]
- Szempruch, A.J.; Sykes, S.E.; Kieft, R.; Dennison, L.; Becker, A.C.; Gartrell, A.; Martin, W.J.; Nakayasu, E.S.; Almeida, I.C.; Hajduk, S.L.; et al. Extracellular vesicles from Trypanosoma brucei mediate virulence factor transfer and cause host anemia. Cell 2016, 164, 246–257. [Google Scholar] [CrossRef]
- Regev-Rudzki, N.; Wilson, D.W.; Carvalho, T.G.; Sisquella, X.; Coleman, B.M.; Rug, M.; Bursac, D.; Angrisano, F.; Gee, M.; Hill, A.F.; et al. Cell-cell communication between malaria-infected red blood cells via exosome-like vesicles. Cell 2013, 153, 1120–1133. [Google Scholar] [CrossRef] [PubMed]
- Varikuti, S.; Jha, B.K.; Holcomb, E.A.; McDaniel, J.C.; Karpurapu, M.; Srivastava, N.; McGwire, B.S.; Satoskar, A.R.; Parinandi, N.L. The role of vascular endothelium and exosomes in human protozoan parasitic diseases. Vessel Plus 2020, 4, 28. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.-M.; Huang, Y.-H.; Aryal, S.; Liu, H.-W.; Chen, C.; Chen, S.-H.; Chu, C.-H.; Tai, J.-H. Endomembrane Protein Trafficking Regulated by a TvCyP2 Cyclophilin in the Protozoan Parasite, Trichomonas vaginalis. Sci. Rep. 2020, 10, 1275. [Google Scholar] [CrossRef]
- Müller, N.; von Allmen, N. Recent insights into the mucosal reactions associated with Giardia lamblia infections. Int. J. Parasitol. 2005, 35, 1339–1347. [Google Scholar] [CrossRef] [PubMed]
- Aguirre García, M.; Gutiérrez-Kobeh, L.; López Vancell, R. Entamoeba histolytica: Adhesins and lectins in the trophozoite surface. Molecules 2015, 20, 2802–2815. [Google Scholar] [CrossRef] [PubMed]
- Frederick, J.R.; Petri Jr., W. A. Roles for the galactose-/N-acetylgalactosamine-binding lectin of Entamoeba in parasite virulence and differentiation. Glycobiology 2005, 15, 53R–59R. [Google Scholar] [CrossRef]
- Ryan, C.M.; de Miguel, N.; Johnson, P.J. Trichomonas vaginalis: Current understanding of host-parasite interactions. Essays Biochem. 2011, 51, 161–175. [Google Scholar] [CrossRef]
- Allain, T.; Fekete, E.; Buret, A.G. Giardia cysteine proteases: The teeth behind the smile. Trends Parasitol. 2019, 35, 636–648. [Google Scholar] [CrossRef]
- Ortega-Pierres, M.G.; Argüello-García, R. Giardia duodenalis: Role of secreted molecules as virulent factors in the cytotoxic effect on epithelial cells. Adv. Parasitol. 2019, 106, 129–169. [Google Scholar] [CrossRef]
- Hernández, H.M.; Marcet, R.; Sarracent, J. Biological roles of cysteine proteinases in the pathogenesis of Trichomonas vaginalis. Parasite 2014, 21, 54. [Google Scholar] [CrossRef]
- Betanzos, A.; Bañuelos, C.; Orozco, E. Host invasion by pathogenic amoebae: Epithelial disruption by parasite proteins. Genes 2019, 10, 618. [Google Scholar] [CrossRef] [PubMed]
- Perdomo, D.; Manich, M.; Syan, S.; Olivo-Marin, J.-C.; Dufour, A.C.; Guillén, N. Intracellular traffic of the lysine and glutamic acid rich protein KERP1 reveals features of endomembrane organization in Entamoeba histolytica. Cell. Microbiol. 2016, 18, 1134–1152. [Google Scholar] [CrossRef] [PubMed]
- Bredeston, L.M.; Caffaro, C.E.; Samuelson, J.; Hirschberg, C.B. Golgi and endoplasmic reticulum functions take place in different subcellular compartments of Entamoeba histolytica. J. Biol. Chem. 2005, 280, 32168–32176. [Google Scholar] [CrossRef]
- Faso, C.; Hehl, A.B. Membrane trafficking and organelle biogenesis in Giardia lamblia: Use it or lose it. Int. J. Parasitol. 2011, 41, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Faso, C.; Konrad, C.; Schraner, E.M.; Hehl, A.B. Export of cyst wall material and Golgi organelle neogenesis in Giardia lamblia depend on endoplasmic reticulum exit sites. Cell. Microbiol. 2013, 15, 537–553. [Google Scholar] [CrossRef] [PubMed]
- Krtková, J.; Thomas, E.B.; Alas, G.C.M.; Schraner, E.M.; Behjatnia, H.R.; Hehl, A.B.; Paredez, A.R. Rac regulates Giardia lamblia encystation by coordinating cyst wall protein trafficking and secretion. MBio 2016, 7. [Google Scholar] [CrossRef]
- Long, M.; Simpson, J.C. Rho GTPases operating at the Golgi complex: Implications for membrane traffic and cancer biology. Tissue Cell 2017, 49, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Lanfredi-Rangel, A.; Attias, M.; de Carvalho, T.M.; Kattenbach, W.M.; De Souza, W. The peripheral vesicles of trophozoites of the primitive protozoan Giardia lamblia may correspond to early and late endosomes and to lysosomes. J. Struct. Biol. 1998, 123, 225–235. [Google Scholar] [CrossRef]
- Povelones, M.L. Beyond replication: Division and segregation of mitochondrial DNA in kinetoplastids. Mol. Biochem. Parasitol. 2014, 196, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Watanabe Costa, R.; da Silveira, J.F.; Bahia, D. Interactions between Trypanosoma cruzi secreted proteins and host cell signaling pathways. Front. Microbiol. 2016, 7, 388. [Google Scholar] [CrossRef] [PubMed]
- Arango Duque, G.; Descoteaux, A. Leishmania survival in the macrophage: Where the ends justify the means. Curr. Opin. Microbiol. 2015, 26, 32–40. [Google Scholar] [CrossRef]
- Rodríguez-Bejarano, O.H.; Avendaño, C.; Patarroyo, M.A. Mechanisms associated with Trypanosoma cruzi host target cell adhesion, recognition and internalization. Life 2021, 11, 534. [Google Scholar] [CrossRef]
- Fernandes, M.C.; Andrews, N.W. Host cell invasion by Trypanosoma cruzi: A unique strategy that promotes persistence. FEMS Microbiol. Rev. 2012, 36, 734–747. [Google Scholar] [CrossRef]
- Ferri, G.; Edreira, M.M. All roads lead to cytosol: Trypanosoma cruzi multi-strategic approach to invasion. Front. Cell. Infect. Microbiol. 2021, 11, 89. [Google Scholar] [CrossRef]
- Silverman, J.S.; Bangs, J.D. Form and function in the trypanosomal secretory pathway. Curr. Opin. Microbiol. 2012, 15, 463–468. [Google Scholar] [CrossRef][Green Version]
- Field, M.C.; Carrington, M. Intracellular membrane transport systems in Trypanosoma brucei. Traffic 2004, 5, 905–913. [Google Scholar] [CrossRef]
- Lacomble, S.; Vaughan, S.; Deghelt, M.; Moreira-Leite, F.F.; Gull, K. A Trypanosoma brucei protein required for maintenance of the flagellum attachment zone and flagellar pocket ER domains. Protist 2012, 163, 602–615. [Google Scholar] [CrossRef]
- Morotti, A.L.M.; Martins-Teixeira, M.B.; Carvalho, I. Protozoan parasites glycosylphosphatidylinositol anchors: Structures, functions and trends for drug discovery. Curr. Med. Chem. 2019, 26, 4301–4322. [Google Scholar] [CrossRef]
- Giorgi, M.E.; de Lederkremer, R.M. Trans-sialidase and mucins of Trypanosoma cruzi: An important interplay for the parasite. Carbohydr. Res. 2011, 346, 1389–1393. [Google Scholar] [CrossRef]
- Arango Duque, G.; Jardim, A.; Gagnon, É.; Fukuda, M.; Descoteaux, A. The host cell secretory pathway mediates the export of Leishmania virulence factors out of the parasitophorous vacuole. PLoS Pathog. 2019, 15, e1007982. [Google Scholar] [CrossRef] [PubMed]
- Debierre-Grockiego, F.; Schwarz, R.T. Immunological reactions in response to apicomplexan glycosylphosphatidylinositols. Glycobiology 2010, 20, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Horta, M.F.; Andrade, L.O.; Martins-Duarte, É.S.; Castro-Gomes, T. Cell invasion by intracellular parasites—the many roads to infection. J. Cell Sci. 2020, 133, jcs232488. [Google Scholar] [CrossRef] [PubMed]
- Sibley, L.D. Invasion and intracellular survival by protozoan parasites. Immunol. Rev. 2011, 240, 72–91. [Google Scholar] [CrossRef]
- Cowman, A.F.; Tonkin, C.J.; Tham, W.H.; Duraisingh, M.T. The molecular basis of erythrocyte invasion by malaria parasites. Cell Host Microbe 2017, 22, 232–245. [Google Scholar] [CrossRef] [PubMed]
- Dubois, D.J.; Soldati-Favre, D. Biogenesis and secretion of micronemes in Toxoplasma gondii. Cell. Microbiol. 2019, 21, e13018. [Google Scholar] [CrossRef]
- Carruthers, V.B.; Tomley, F.M. Microneme proteins in apicomplexans. Subcell. Biochem. 2008, 47, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Frénal, K.; Dubremetz, J.-F.; Lebrun, M.; Soldati-Favre, D. Gliding motility powers invasion and egress in Apicomplexa. Nat. Rev. Microbiol. 2017, 15, 645–660. [Google Scholar] [CrossRef] [PubMed]
- Ben Chaabene, R.; Lentini, G.; Soldati-Favre, D. Biogenesis and discharge of the rhoptries: Key organelles for entry and hijack of host cells by the Apicomplexa. Mol. Microbiol. 2021, 115, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Counihan, N.A.; Kalanon, M.; Coppel, R.L.; de Koning-Ward, T.F. Plasmodium rhoptry proteins: Why order is important. Trends Parasitol. 2013, 29, 228–236. [Google Scholar] [CrossRef]
- Ghosh, S.; Kennedy, K.; Sanders, P.; Matthews, K.; Ralph, S.A.; Counihan, N.A.; de Koning-Ward, T.F. The Plasmodium rhoptry associated protein complex is important for parasitophorous vacuole membrane structure and intraerythrocytic parasite growth. Cell. Microbiol. 2017, 19, e12733. [Google Scholar] [CrossRef]
- Mercier, C.; Adjogble, K.D.Z.; Däubener, W.; Delauw, M.-F.-C. Dense granules: Are they key organelles to help understand the parasitophorous vacuole of all apicomplexa parasites? Int. J. Parasitol. 2005, 35, 829–849. [Google Scholar] [CrossRef]
- Mercier, C.; Cesbron-Delauw, M.-F. Toxoplasma secretory granules: One population or more? Trends Parasitol. 2015, 31, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Asada, M.; Goto, Y.; Yahata, K.; Yokoyama, N.; Kawai, S.; Inoue, N.; Kaneko, O.; Kawazu, S. Gliding motility of Babesia bovis merozoites visualized by time-lapse video microscopy. PLoS ONE 2012, 7, e35227. [Google Scholar] [CrossRef]
- Goldberg, D.E.; Zimmerberg, J. Hardly vacuous: The parasitophorous vacuolar membrane of malaria parasites. Trends Parasitol. 2020, 36, 138–146. [Google Scholar] [CrossRef]
- Matz, J.M.; Beck, J.R.; Blackman, M.J. The parasitophorous vacuole of the blood-stage malaria parasite. Nat. Rev. Microbiol. 2020, 18, 379–391. [Google Scholar] [CrossRef]
- Bisio, H.; Chaabene, R.B.; Sabitzki, R.; Maco, B.; Marq, J.B.; Gilberger, T.-W.; Spielmann, T.; Soldati-Favre, D. The ZIP code of vesicle trafficking in Apicomplexa: SEC1/Munc18 and SNARE proteins. MBio 2020, 11, e02092-20. [Google Scholar] [CrossRef]
- Jimenez-Ruiz, E.; Morlon-Guyot, J.; Daher, W.; Meissner, M. Vacuolar protein sorting mechanisms in apicomplexan parasites. Mol. Biochem. Parasitol. 2016, 209, 18–25. [Google Scholar] [CrossRef]
- Venugopal, K.; Marion, S. Secretory organelle trafficking in Toxoplasma gondii: A long story for a short travel. Int. J. Med. Microbiol. 2018, 308, 751–760. [Google Scholar] [CrossRef]
- Sloves, P.-J.; Delhaye, S.; Mouveaux, T.; Werkmeister, E.; Slomianny, C.; Hovasse, A.; Dilezitoko Alayi, T.; Callebaut, I.; Gaji, R.Y.; Schaeffer-Reiss, C.; et al. Toxoplasma sortilin-like receptor regulates protein transport and is essential for apical secretory organelle biogenesis and host infection. Cell Host Microbe 2012, 11, 515–527. [Google Scholar] [CrossRef]
- Hallée, S.; Counihan, N.A.; Matthews, K.; de Koning-Ward, T.F.; Richard, D. The malaria parasite Plasmodium falciparum sortilin is essential for merozoite formation and apical complex biogenesis. Cell. Microbiol. 2018, 20, e12844. [Google Scholar] [CrossRef]
- Hallée, S.; Boddey, J.A.; Cowman, A.F.; Richard, D. Evidence that the Plasmodium falciparum protein sortilin potentially acts as an escorter for the trafficking of the rhoptry-associated membrane antigen to the rhoptries. Msphere 2018, 3, e00551-17. [Google Scholar] [CrossRef] [PubMed]
- Carmeille, R.; Schiano Lomoriello, P.; Devarakonda, P.M.; Kellermeier, J.A.; Heaslip, A.T. Actin and an unconventional myosin motor, TgMyoF, control the organization and dynamics of the endomembrane network in Toxoplasma gondii. PLoS Pathog. 2021, 17, e1008787. [Google Scholar] [CrossRef]
- Leander, B.S. Marine gregarines: Evolutionary prelude to the apicomplexan radiation? Trends Parasitol. 2008, 24, 60–67. [Google Scholar] [CrossRef]
- Gubbels, M.-J.; Duraisingh, M.T. Evolution of apicomplexan secretory organelles. Int. J. Parasitol. 2012, 42, 1071–1081. [Google Scholar] [CrossRef]
- Sam-Yellowe, T.Y.; Yadavalli, R.; Fujioka, H.; Peterson, J.W.; Drazba, J.A. RhopH3, rhoptry gene conserved in the free-living alveolate flagellate Colpodella sp. (Apicomplexa). Eur. J. Protistol. 2019, 71, 125637. [Google Scholar] [CrossRef]
- Lendner, M.; Daugschies, A. Cryptosporidium infections: Molecular advances. Parasitology 2014, 141, 1511–1532. [Google Scholar] [CrossRef]
- Borowski, H.; Clode, P.L.; Thompson, R.C.A. Active invasion and/or encapsulation? A reappraisal of host-cell parasitism by Cryptosporidium. Trends Parasitol. 2008, 24, 509–516. [Google Scholar] [CrossRef]
- Perkins, M.E.; Riojas, Y.A.; Wu, T.W.; Le Blancq, S.M. CpABC, a Cryptosporidium parvum ATP-binding cassette protein at the host–parasite boundary in intracellular stages. Proc. Natl. Acad. Sci. USA 1999, 96, 5734–5739. [Google Scholar] [CrossRef]
- O’Hara, S.P.; Small, A.J.; Chen, X.-M.; LaRusso, N.F. Host cell actin remodeling in response to Cryptosporidium. Subcell. Biochem. 2008, 47, 92–100. [Google Scholar] [CrossRef]
- Burki, F.; Shalchian-Tabrizi, K.; Minge, M.; Skjæveland, Å.; Nikolaev, S.I.; Jakobsen, K.S.; Pawlowski, J. Phylogenomics reshuffles the eukaryotic supergroups. PLoS ONE 2007, 2, e790. [Google Scholar] [CrossRef]
- Aquilini, E.; Cova, M.M.; Mageswaran, S.K.; Dos Santos Pacheco, N.; Sparvoli, D.; Penarete-Vargas, D.M.; Najm, R.; Graindorge, A.; Suarez, C.; Maynadier, M.; et al. An Alveolata secretory machinery adapted to parasite host cell invasion. Nat. Microbiol. 2021, 6, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Plattner, H. Trichocysts-Paramecium’s projectile-like secretory organelles: Reappraisal of their biogenesis, composition, intracellular transport, and possible functions. J. Eukaryot. Microbiol. 2017, 64, 106–133. [Google Scholar] [CrossRef]
- Fast, N.M.; Xue, L.; Bingham, S.; Keeling, P.J. Re-examining alveolate evolution using multiple protein molecular phylogenies. J. Eukaryot. Microbiol. 2002, 49, 30–37. [Google Scholar] [CrossRef]
- Kono, M.; Herrmann, S.; Loughran, N.B.; Cabrera, A.; Engelberg, K.; Lehmann, C.; Sinha, D.; Prinz, B.; Ruch, U.; Heussler, V.; et al. Evolution and architecture of the inner membrane complex in asexual and sexual stages of the malaria parasite. Mol. Biol. Evol. 2012, 29, 2113–2132. [Google Scholar] [CrossRef]
- Länge, S.; Klauke, N.; Plattner, H. Subplasmalemmal Ca2+ stores of probable relevance for exocytosis in Paramecium. Alveolar sacs share some but not all characteristics with sarcoplasmic reticulum. Cell Calcium 1995, 17, 335–344. [Google Scholar] [CrossRef]
- Pozdnyakov, I.; Skarlato, S. Dinoflagellate amphiesma at different stages of the life cycle. Protistology 2012, 7, 108–115. [Google Scholar]
- Ferreira, J.L.; Heincke, D.; Wichers, J.S.; Liffner, B.; Wilson, D.W.; Gilberger, T.-W. The dynamic roles of the inner membrane complex in the multiple stages of the malaria parasite. Front. Cell. Infect. Microbiol. 2021, 10, 611801. [Google Scholar] [CrossRef]
- Kono, M.; Heincke, D.; Wilcke, L.; Wong, T.W.Y.; Bruns, C.; Herrmann, S.; Spielmann, T.; Gilberger, T.W. Pellicle formation in the malaria parasite. J. Cell Sci. 2016, 129, 673–680. [Google Scholar] [CrossRef]
- Yeoman, J.A.; Hanssen, E.; Maier, A.G.; Klonis, N.; Maco, B.; Baum, J.; Turnbull, L.; Whitchurch, C.B.; Dixon, M.W.A.; Tilley, L. Tracking glideosome-associated protein 50 reveals the development and organization of the inner membrane complex of Plasmodium falciparum. Eukaryot. Cell 2011, 10, 556–564. [Google Scholar] [CrossRef]
- Wetzel, J.; Herrmann, S.; Swapna, L.S.; Prusty, D.; John Peter, A.T.; Kono, M.; Saini, S.; Nellimarla, S.; Wong, T.W.Y.; Wilcke, L.; et al. The role of palmitoylation for protein recruitment to the inner membrane complex of the malaria parasite. J. Biol. Chem. 2015, 290, 1712–1728. [Google Scholar] [CrossRef]
- Ouologuem, D.T.; Roos, D.S. Dynamics of the Toxoplasma gondii inner membrane complex. J. Cell Sci. 2014, 127, 3320–3330. [Google Scholar] [CrossRef]
- Hu, K.; Mann, T.; Striepen, B.; Beckers, C.J.M.; Roos, D.S.; Murray, J.M. Daughter cell assembly in the protozoan parasite Toxoplasma gondii. Mol. Biol. Cell 2002, 13, 593–606. [Google Scholar] [CrossRef] [PubMed]
- Bullen, H.E.; Crabb, B.S.; Gilson, P.R. Recent insights into the export of PEXEL/HTS-motif containing proteins in Plasmodium parasites. Curr. Opin. Microbiol. 2012, 15, 699–704. [Google Scholar] [CrossRef]
- Marapana, D.S.; Dagley, L.F.; Sandow, J.J.; Nebl, T.; Triglia, T.; Pasternak, M.; Dickerman, B.K.; Crabb, B.S.; Gilson, P.R.; Webb, A.I.; et al. Plasmepsin V cleaves malaria effector proteins in a distinct endoplasmic reticulum translocation interactome for export to the erythrocyte. Nat. Microbiol. 2018, 3, 1010–1022. [Google Scholar] [CrossRef]
- Coffey, M.J.; Jennison, C.; Tonkin, C.J.; Boddey, J.A. Role of the ER and Golgi in protein export by Apicomplexa. Curr. Opin. Cell Biol. 2016, 41, 18–24. [Google Scholar] [CrossRef]
- Heiber, A.; Kruse, F.; Pick, C.; Grüring, C.; Flemming, S.; Oberli, A.; Schoeler, H.; Retzlaff, S.; Mesén-Ramírez, P.; Hiss, J.A.; et al. Identification of new PNEPs indicates a substantial non-PEXEL exportome and underpins common features in Plasmodium falciparum protein export. PLoS Pathog. 2013, 9, e1003546. [Google Scholar] [CrossRef]
- Taku, I.; Hirai, T.; Makiuchi, T.; Shinzawa, N.; Iwanaga, S.; Annoura, T.; Nagamune, K.; Nozaki, T.; Saito-Nakano, Y. Rab5b-associated Arf1 GTPase regulates export of N-myristoylated adenylate kinase 2 from the endoplasmic reticulum in Plasmodium falciparum. Front. Cell. Infect. Microbiol. 2020, 10, 908. [Google Scholar] [CrossRef]
- Pellé, K.G.; Jiang, R.H.Y.; Mantel, P.-Y.; Xiao, Y.-P.; Hjelmqvist, D.; Gallego-Lopez, G.M.; Lau, A.O.T.; Kang, B.-H.; Allred, D.R.; Marti, M. Shared elements of host-targeting pathways among apicomplexan parasites of differing lifestyles. Cell. Microbiol. 2015, 17, 1618–1639. [Google Scholar] [CrossRef]
- Lack, J.B.; Reichard, M.V.; Van Den Bussche, R.A. Phylogeny and evolution of the Piroplasmida as inferred from 18S rRNA sequences. Int. J. Parasitol. 2012, 42, 353–363. [Google Scholar] [CrossRef]
- Hsiao, C.-H.C.; Luisa Hiller, N.; Haldar, K.; Knoll, L.J. A HT/PEXEL motif in Toxoplasma dense granule proteins is a signal for protein cleavage but not export into the host cell. Traffic 2013, 14, 519–531. [Google Scholar] [CrossRef]
- Coffey, M.J.; Sleebs, B.E.; Uboldi, A.D.; Garnham, A.; Franco, M.; Marino, N.D.; Panas, M.W.; Ferguson, D.J.; Enciso, M.; O’Neill, M.T.; et al. An aspartyl protease defines a novel pathway for export of Toxoplasma proteins into the host cell. Elife 2015, 4. [Google Scholar] [CrossRef]
- Boddey, J.A.; Carvalho, T.G.; Hodder, A.N.; Sargeant, T.J.; Sleebs, B.E.; Marapana, D.; Lopaticki, S.; Nebl, T.; Cowman, A.F. Role of plasmepsin V in export of diverse protein families from the Plasmodium falciparum exportome. Traffic 2013, 14, 532–550. [Google Scholar] [CrossRef]
- O’Hara, S.P.; Huang, B.Q.; Chen, X.-M.; Nelson, J.; LaRusso, N.F. Distribution of Crytosporidium parvum sporozoite apical organelles during attachment to and internalization by cultured biliary epithelial cells. J. Parasitol. 2005, 91, 995–999. [Google Scholar] [CrossRef]
- Hammoudi, P.-M.; Jacot, D.; Mueller, C.; Di Cristina, M.; Dogga, S.K.; Marq, J.-B.; Romano, J.; Tosetti, N.; Dubrot, J.; Emre, Y.; et al. Fundamental roles of the Golgi-associated Toxoplasma aspartyl protease, ASP5, at the host-parasite interface. PLoS Pathog. 2015, 11, e1005211. [Google Scholar] [CrossRef] [PubMed]
- Whisson, S.C.; Boevink, P.C.; Moleleki, L.; Avrova, A.O.; Morales, J.G.; Gilroy, E.M.; Armstrong, M.R.; Grouffaud, S.; van West, P.; Chapman, S.; et al. A translocation signal for delivery of oomycete effector proteins into host plant cells. Nature 2007, 450, 115–118. [Google Scholar] [CrossRef]
- Stassen, J.H.M.; Van den Ackerveken, G. How do oomycete effectors interfere with plant life? Curr. Opin. Plant. Biol. 2011, 14, 407–414. [Google Scholar] [CrossRef]
- Chen, T.; Liu, R.; Dou, M.; Li, M.; Li, M.; Yin, X.; Liu, G.; Wang, Y.; Xu, Y. Insight into function and subcellular localization of Plasmopara viticola putative RxLR effectors. Front. Microbiol. 2020, 11, 692. [Google Scholar] [CrossRef] [PubMed]
- Strassert, J.F.H.; Irisarri, I.; Williams, T.A.; Burki, F. A molecular timescale for eukaryote evolution with implications for the origin of red algal-derived plastids. Nat. Commun. 2021, 12, 1879. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Hiller, N.L.; Liolios, K.; Win, J.; Kanneganti, T.-D.; Young, C.; Kamoun, S.; Haldar, K. The malarial host-targeting signal is conserved in the Irish potato famine pathogen. PLoS Pathog. 2006, 2, e50. [Google Scholar] [CrossRef] [PubMed]
- Grouffaud, S.; van West, P.; Avrova, A.O.; Birch, P.R.J.; Whisson, S.C. Plasmodium falciparum and Hyaloperonospora parasitica effector translocation motifs are functional in Phytophthora infestans. Microbiology 2008, 154, 3743–3751. [Google Scholar] [CrossRef][Green Version]
- Wawra, S.; Trusch, F.; Matena, A.; Apostolakis, K.; Linne, U.; Zhukov, I.; Stanek, J.; Koźmiński, W.; Davidson, I.; Secombes, C.J.; et al. The RxLR motif of the host targeting effector AVR3a of Phytophthora infestans is cleaved before secretion. Plant. Cell 2017, 29, 1184–1195. [Google Scholar] [CrossRef]
- Whisson, S.C.; Boevink, P.C.; Wang, S.; Birch, P.R.J. The cell biology of late blight disease. Curr. Opin. Microbiol. 2016, 34, 127–135. [Google Scholar] [CrossRef]
- de Koning-Ward, T.F.; Dixon, M.W.A.; Tilley, L.; Gilson, P.R. Plasmodium species: Master renovators of their host cells. Nat. Rev. Microbiol. 2016, 14, 494–507. [Google Scholar] [CrossRef]
- Warncke, J.D.; Beck, H.-P. Host cytoskeleton remodeling throughout the blood stages of Plasmodium falciparum. Microbiol. Mol. Biol. Rev. 2019, 83. [Google Scholar] [CrossRef]
- Counihan, N.A.; Modak, J.K.; de Koning-Ward, T.F. How malaria parasites acquire nutrients from their host. Front. Cell Dev. Biol. 2021, 9, 582. [Google Scholar] [CrossRef]
- Vincensini, L.; Fall, G.; Berry, L.; Blisnick, T.; Braun Breton, C. The RhopH complex is transferred to the host cell cytoplasm following red blood cell invasion by Plasmodium falciparum. Mol. Biochem. Parasitol. 2008, 160, 81–89. [Google Scholar] [CrossRef]
- Jonsdottir, T.K.; Counihan, N.A.; Modak, J.K.; Kouskousis, B.; Sanders, P.R.; Gabriela, M.; Bullen, H.E.; Crabb, B.S.; de Koning-Ward, T.F.; Gilson, P.R. Characterisation of complexes formed by parasite proteins exported into the host cell compartment of Plasmodium falciparum infected red blood cells. Cell. Microbiol. 2021, e13332. [Google Scholar] [CrossRef]
- Spielmann, T.; Gilberger, T.-W. Critical steps in protein export of Plasmodium falciparum blood stages. Trends Parasitol. 2015, 31, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Alampalli, S.V.; Grover, M.; Chandran, S.; Tatu, U.; Acharya, P. Proteome and structural organization of the knob complex on the surface of the Plasmodium infected red blood cell. Proteomics Clin. Appl. 2018, 12, 1600177. [Google Scholar] [CrossRef] [PubMed]
- Akinyi, S.; Hanssen, E.; Meyer, E.V.S.; Jiang, J.; Korir, C.C.; Singh, B.; Lapp, S.; Barnwell, J.W.; Tilley, L.; Galinski, M.R. A 95 kDa protein of Plasmodium vivax and P. cynomolgi visualized by three-dimensional tomography in the caveola-vesicle complexes (Schüffner’s dots) of infected erythrocytes is a member of the PHIST family. Mol. Microbiol. 2012, 84, 816–831. [Google Scholar] [CrossRef]
- Lanners, H.N.; Bafford, R.A.; Wiser, M.F. Characterization of the parasitophorous vacuole membrane from Plasmodium chabaudi and implications about its role in the export of parasite proteins. Parasitol. Res. 1999, 85, 349–355. [Google Scholar] [CrossRef]
- Charnaud, S.C.; Jonsdottir, T.K.; Sanders, P.R.; Bullen, H.E.; Dickerman, B.K.; Kouskousis, B.; Palmer, C.S.; Pietrzak, H.M.; Laumaea, A.E.; Erazo, A.-B.; et al. Spatial organization of protein export in malaria parasite blood stages. Traffic 2018, 19, 605–623. [Google Scholar] [CrossRef]
- Mundwiler-Pachlatko, E.; Beck, H.-P. Maurer’s clefts, the enigma of Plasmodium falciparum. Proc. Natl. Acad. Sci. USA 2013, 110, 19987–19994. [Google Scholar] [CrossRef]
- Cutts, E.E.; Laasch, N.; Reiter, D.M.; Trenker, R.; Slater, L.M.; Stansfeld, P.J.; Vakonakis, I. Structural analysis of P. falciparum KAHRP and PfEMP1 complexes with host erythrocyte spectrin suggests a model for cytoadherent knob protrusions. PLoS Pathog. 2017, 13, e1006552. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.D.; Rowe, J.A.; Higgins, M.K.; Lavstsen, T. Malaria’s deadly grip: Cytoadhesion of Plasmodium falciparum-infected erythrocytes. Cell. Microbiol. 2013, 15, 1976–1983. [Google Scholar] [CrossRef]
- Wahlgren, M.; Goel, S.; Akhouri, R.R. Variant surface antigens of Plasmodium falciparum and their roles in severe malaria. Nat. Rev. Microbiol. 2017, 15, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Brejt, J.A.; Golightly, L.M. Severe malaria: Update on pathophysiology and treatment. Curr. Opin. Infect. Dis. 2019, 32, 413–418. [Google Scholar] [CrossRef]
- Wiser, M.F. Export and trafficking of Plasmodium proteins within the host erythrocyte. Acta Biológica Colomb. 2007, 12, 3–18. [Google Scholar] [CrossRef]
- Wiser, M.F.; Lanners, H.N.; Bafford, R.A.; Favaloro, J.M. A novel alternate secretory pathway for the export of Plasmodium proteins into the host erythrocyte. Proc. Natl. Acad. Sci. USA 1997, 94, 9108–9113. [Google Scholar] [CrossRef]
- Wiser, M.F.; Lanners, H.N.; Bafford, R.A. Export of Plasmodium proteins via a novel secretory pathway. Parasitol. Today 1999, 15, 194–198. [Google Scholar] [CrossRef]
- Wiser, M.F.; Grab, D.J.; Lanners, H.N. An alternative secretory pathway in Plasmodium: More questions than answers. In Transport and Trafficking in the Malaria-Infected Erythrocyte (Novaritis Foundation Symposium); John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1999; Volume 226, pp. 199–214. [Google Scholar]
- Cortés, G.T.; Winograd, E.; Wiser, M.F. Characterization of proteins localized to a subcellular compartment associated with an alternate secretory pathway of the malaria parasite. Mol. Biochem. Parasitol. 2003, 129, 127–135. [Google Scholar] [CrossRef]
- Jensen, D.; Schekman, R. COPII-mediated vesicle formation at a glance. J. Cell Sci. 2011, 124, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Cortés, G.T.; Wiser, M.F.; Gómez-Alegría, C.J. Identification of Plasmodium falciparum HSP70-2 as a resident of the Plasmodium export compartment. Heliyon 2020, 6, e04037. [Google Scholar] [CrossRef] [PubMed]
- Beck, J.R.; Ho, C.-M. Transport mechanisms at the malaria parasite-host cell interface. PLoS Pathog. 2021, 17, e1009394. [Google Scholar] [CrossRef] [PubMed]
- Egea, P.F. Crossing the vacuolar rubicon: Structural insights into effector protein trafficking in apicomplexan parasites. Microorganisms 2020, 8, 865. [Google Scholar] [CrossRef] [PubMed]
- Bullen, H.E.; Charnaud, S.C.; Kalanon, M.; Riglar, D.T.; Dekiwadia, C.; Kangwanrangsan, N.; Torii, M.; Tsuboi, T.; Baum, J.; Ralph, S.A.; et al. Biosynthesis, localization, and macromolecular arrangement of the Plasmodium falciparum translocon of exported proteins (PTEX). J. Biol. Chem. 2012, 287, 7871–7884. [Google Scholar] [CrossRef] [PubMed]
- Batinovic, S.; McHugh, E.; Chisholm, S.A.; Matthews, K.; Liu, B.; Dumont, L.; Charnaud, S.C.; Schneider, M.P.; Gilson, P.R.; de Koning-Ward, T.F.; et al. An exported protein-interacting complex involved in the trafficking of virulence determinants in Plasmodium-infected erythrocytes. Nat. Commun. 2017, 8, 16044. [Google Scholar] [CrossRef]
- Beck, J.R.; Muralidharan, V.; Oksman, A.; Goldberg, D.E. PTEX component HSP101 mediates export of diverse malaria effectors into host erythrocytes. Nature 2014, 511, 592–595. [Google Scholar] [CrossRef]
- Elsworth, B.; Matthews, K.; Nie, C.Q.; Kalanon, M.; Charnaud, S.C.; Sanders, P.R.; Chisholm, S.A.; Counihan, N.A.; Shaw, P.J.; Pino, P.; et al. PTEX is an essential nexus for protein export in malaria parasites. Nature 2014, 511, 587–591. [Google Scholar] [CrossRef]
- Garten, M.; Nasamu, A.S.; Niles, J.C.; Zimmerberg, J.; Goldberg, D.E.; Beck, J.R. EXP2 is a nutrient-permeable channel in the vacuolar membrane of Plasmodium and is essential for protein export via PTEX. Nat. Microbiol. 2018, 3, 1090–1098. [Google Scholar] [CrossRef]
- Gold, D.A.; Kaplan, A.D.; Lis, A.; Bett, G.C.L.; Rosowski, E.E.; Cirelli, K.M.; Bougdour, A.; Sidik, S.M.; Beck, J.R.; Lourido, S.; et al. The Toxoplasma dense granule proteins GRA17 and GRA23 mediate the movement of small molecules between the host and the parasitophorous vacuole. Cell Host Microbe 2015, 17, 642–652. [Google Scholar] [CrossRef]
- Franco, M.; Panas, M.W.; Marino, N.D.; Lee, M.-C.W.; Buchholz, K.R.; Kelly, F.D.; Bednarski, J.J.; Sleckman, B.P.; Pourmand, N.; Boothroyd, J.C. A novel secreted protein, MYR1, is central to Toxoplasma’s manipulation of host cells. MBio 2016, 7, e02231-15. [Google Scholar] [CrossRef] [PubMed]
- Florentin, A.; Cobb, D.W.; Kudyba, H.M.; Muralidharan, V. Directing traffic: Chaperone-mediated protein transport in malaria parasites. Cell. Microbiol. 2020, 22, e13215. [Google Scholar] [CrossRef]
- Zhang, Q.; Ma, C.; Oberli, A.; Zinz, A.; Engels, S.; Przyborski, J.M. Proteomic analysis of exported chaperone/co-chaperone complexes of P. falciparum reveals an array of complex protein-protein interactions. Sci. Rep. 2017, 7, 42188. [Google Scholar] [CrossRef]
- Külzer, S.; Rug, M.; Brinkmann, K.; Cannon, P.; Cowman, A.; Lingelbach, K.; Blatch, G.L.; Maier, A.G.; Przyborski, J.M. Parasite-encoded Hsp40 proteins define novel mobile structures in the cytosol of the P. falciparum-infected erythrocyte. Cell. Microbiol. 2010, 12, 1398–1420. [Google Scholar] [CrossRef]
- Külzer, S.; Charnaud, S.; Dagan, T.; Riedel, J.; Mandal, P.; Pesce, E.R.; Blatch, G.L.; Crabb, B.S.; Gilson, P.R.; Przyborski, J.M. Plasmodium falciparum-encoded exported HSP70/HSP40 chaperone/co-chaperone complexes within the host erythrocyte. Cell. Microbiol. 2012, 14, 1784–1795. [Google Scholar] [CrossRef] [PubMed]
- Behl, A.; Kumar, V.; Bisht, A.; Panda, J.J.; Hora, R.; Mishra, P.C. Cholesterol bound Plasmodium falciparum co-chaperone PFA0660w complexes with major virulence factor PfEMP1 via chaperone PfHsp70-x. Sci. Rep. 2019, 9, 1–17. [Google Scholar] [CrossRef] [PubMed]
- McMillan, P.J.; Millet, C.; Batinovic, S.; Maiorca, M.; Hanssen, E.; Kenny, S.; Muhle, R.A.; Melcher, M.; Fidock, D.A.; Smith, J.D.; et al. Spatial and temporal mapping of the PfEMP1 export pathway in Plasmodium falciparum. Cell. Microbiol. 2013, 15, 1401–1418. [Google Scholar] [CrossRef] [PubMed]
- Petersen, W.; Külzer, S.; Engels, S.; Zhang, Q.; Ingmundson, A.; Rug, M.; Maier, A.G.; Przyborski, J.M. J-dot targeting of an exported HSP40 in Plasmodium falciparum-infected erythrocytes. Int. J. Parasitol. 2016, 46, 519–525. [Google Scholar] [CrossRef] [PubMed]
- McHugh, E.; Carmo, O.M.S.; Blanch, A.; Looker, O.; Liu, B.; Tiash, S.; Andrew, D.; Batinovic, S.; Low, A.J.Y.; Cho, H.J.; et al. Role of Plasmodium falciparum protein GEXP07 in Maurer’s cleft morphology, knob architecture, and P. falciparum EMP1 trafficking. MBio 2020, 11, e03320-19. [Google Scholar] [CrossRef]
- Knuepfer, E.; Rug, M.; Klonis, N.; Tilley, L.; Cowman, A.F. Trafficking of the major virulence factor to the surface of transfected P. falciparum-infected erythrocytes. Blood 2005, 105, 4078–4087. [Google Scholar] [CrossRef]
- Sundararaman, S.A.; Plenderleith, L.J.; Liu, W.; Loy, D.E.; Learn, G.H.; Li, Y.; Shaw, K.S.; Ayouba, A.; Peeters, M.; Speede, S.; et al. Genomes of cryptic chimpanzee Plasmodium species reveal key evolutionary events leading to human malaria. Nat. Commun. 2016, 7, 11078. [Google Scholar] [CrossRef]
- Sargeant, T.J.; Marti, M.; Caler, E.; Carlton, J.M.; Simpson, K.; Speed, T.P.; Cowman, A.F. Lineage-specific expansion of proteins exported to erythrocytes in malaria parasites. Genome Biol. 2006, 7, R12. [Google Scholar] [CrossRef]
- De Niz, M.; Ullrich, A.-K.; Heiber, A.; Blancke Soares, A.; Pick, C.; Lyck, R.; Keller, D.; Kaiser, G.; Prado, M.; Flemming, S.; et al. The machinery underlying malaria parasite virulence is conserved between rodent and human malaria parasites. Nat. Commun. 2016, 7, 11659. [Google Scholar] [CrossRef] [PubMed]
- Iriko, H.; Ishino, T.; Tachibana, M.; Omoda, A.; Torii, M.; Tsuboi, T. Skeleton binding protein 1 (SBP1) of Plasmodium falciparum accumulates in electron-dense material before passing through the parasitophorous vacuole membrane. Parasitol. Int. 2020, 75, 102003. [Google Scholar] [CrossRef] [PubMed]

| Pathogen (Super Group) | Golgi | Microbodies | Unique Compartments |
|---|---|---|---|
| Giardia (Excavata) | No stacks and reduced function [13] | Peroxisomes [14] | |
| Trichomonas (Excavata) | Stacked [17] | lacking |
|
| Entamoeba (Amorphea) | Vesicles [18] | lacking |
|
| Kinetoplastids 1 (Excavata) | Usually a single stack [19] | Glycosomes [20] | |
| Cryptosporidium (TSAR) | Not yet identified | lacking | |
| Toxoplasma (TSAR) | Single stacked of 3-5 cisternae [24] | Peroxisomes (lipid metabolism) [25] | |
| Plasmodium (TSAR) | Single cisterna in blood stage [27]; single Golgi with 1–3 cisternae in mosquito stage [28] | lacking |
| Organelle | Description | Features |
|---|---|---|
| Microneme | Oval vesicles congregated at apical end | Contents include adhesins that are integrated into the microneme membrane. Secretion of microneme exposes the adhesin on surface of parasite at apical end. The adhesins bind to receptors on host cells to form a junction. |
| Rhoptry | Club-shaped organelles with duct at apical end. | Proteins found in the neck region of the rhoptry participate in the formation of the moving junction and glideosome. Material in bulbs of the rhoptry contributes to the formation of the parasitophorous vacuolar membrane. |
| Dense Granules | Secretory vesicles found at the apical end and throughout the parasite cytoplasm. | Material in the dense granules is released shortly after parasite invasion and modify the parasitophorous vacuole and host cell. Some species produce dense granules throughout the intracellular period. |
| Feature | Plasmodium | Toxoplasma |
|---|---|---|
| Replication | Schizogony (multiple rounds of nuclear replication followed by cytoplasmic segmentation) | Endodyogeny (internal formation of invasive stages) |
| IMC | De novo formation of IMC during each replication cycle | Extensive recycling of IMC material from mother cell to daughter cell |
| Dense Granules | Only found in invasive stages and contents released shortly after invasion | Found in invasive stages and continuously produced during intracellular period |
| PEXEL processing | Occurs in ER | Occurs in Golgi |
| PEXEL targeting | Primarily to the parasitophorous vacuole with possible exception of RESA to the dense granules | To the dense granules first and then to the parasitophorous vacuole |
| PVM translocon | PTEX | MYR1 [162] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiser, M.F. Unique Endomembrane Systems and Virulence in Pathogenic Protozoa. Life 2021, 11, 822. https://doi.org/10.3390/life11080822
Wiser MF. Unique Endomembrane Systems and Virulence in Pathogenic Protozoa. Life. 2021; 11(8):822. https://doi.org/10.3390/life11080822
Chicago/Turabian StyleWiser, Mark F. 2021. "Unique Endomembrane Systems and Virulence in Pathogenic Protozoa" Life 11, no. 8: 822. https://doi.org/10.3390/life11080822
APA StyleWiser, M. F. (2021). Unique Endomembrane Systems and Virulence in Pathogenic Protozoa. Life, 11(8), 822. https://doi.org/10.3390/life11080822





