Experimental Setting for Applying Mechanical Stimuli to Study the Endothelial Response of Ex Vivo Vessels under Realistic Pathophysiological Environments
Abstract
:1. Introduction
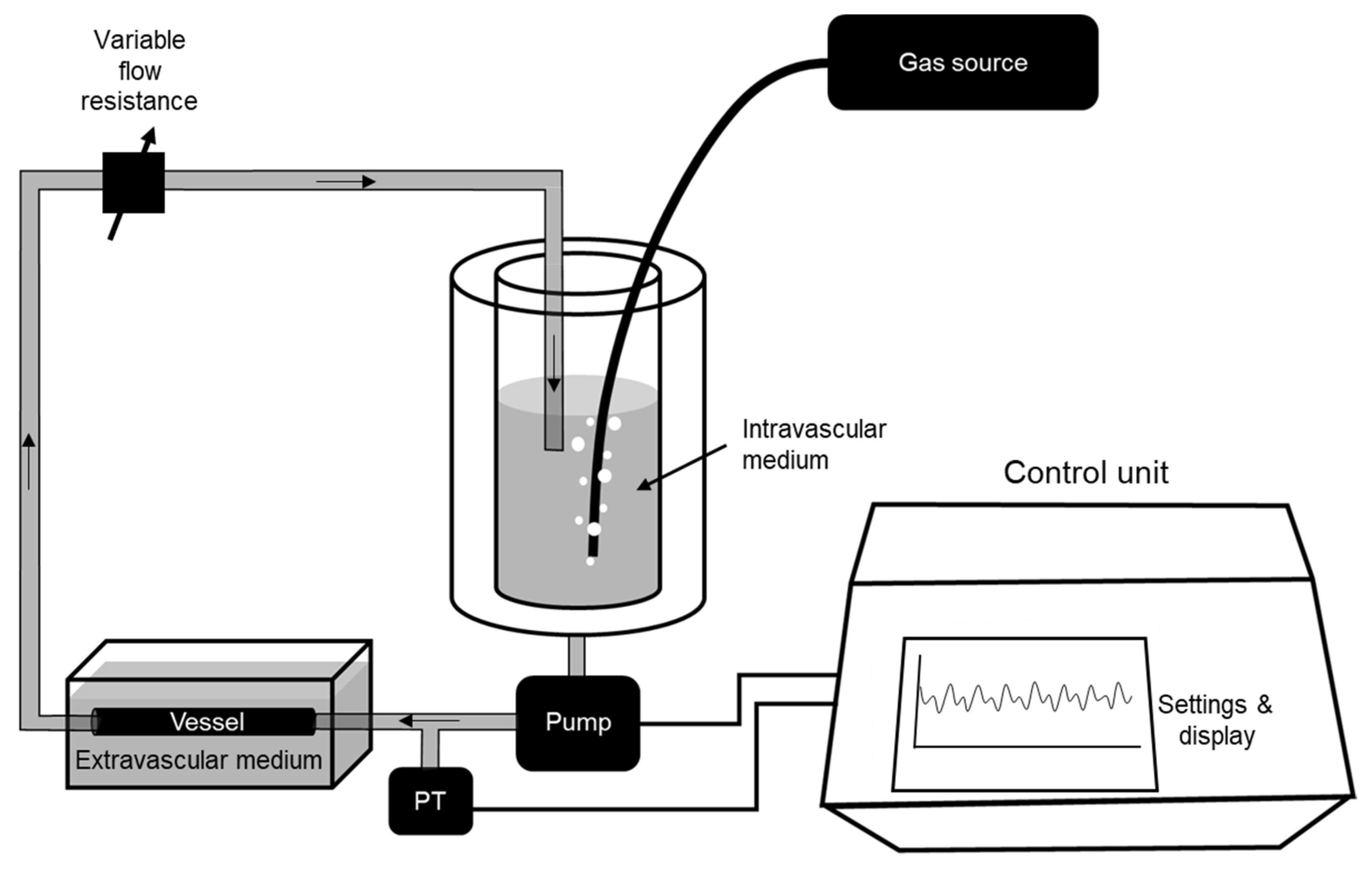
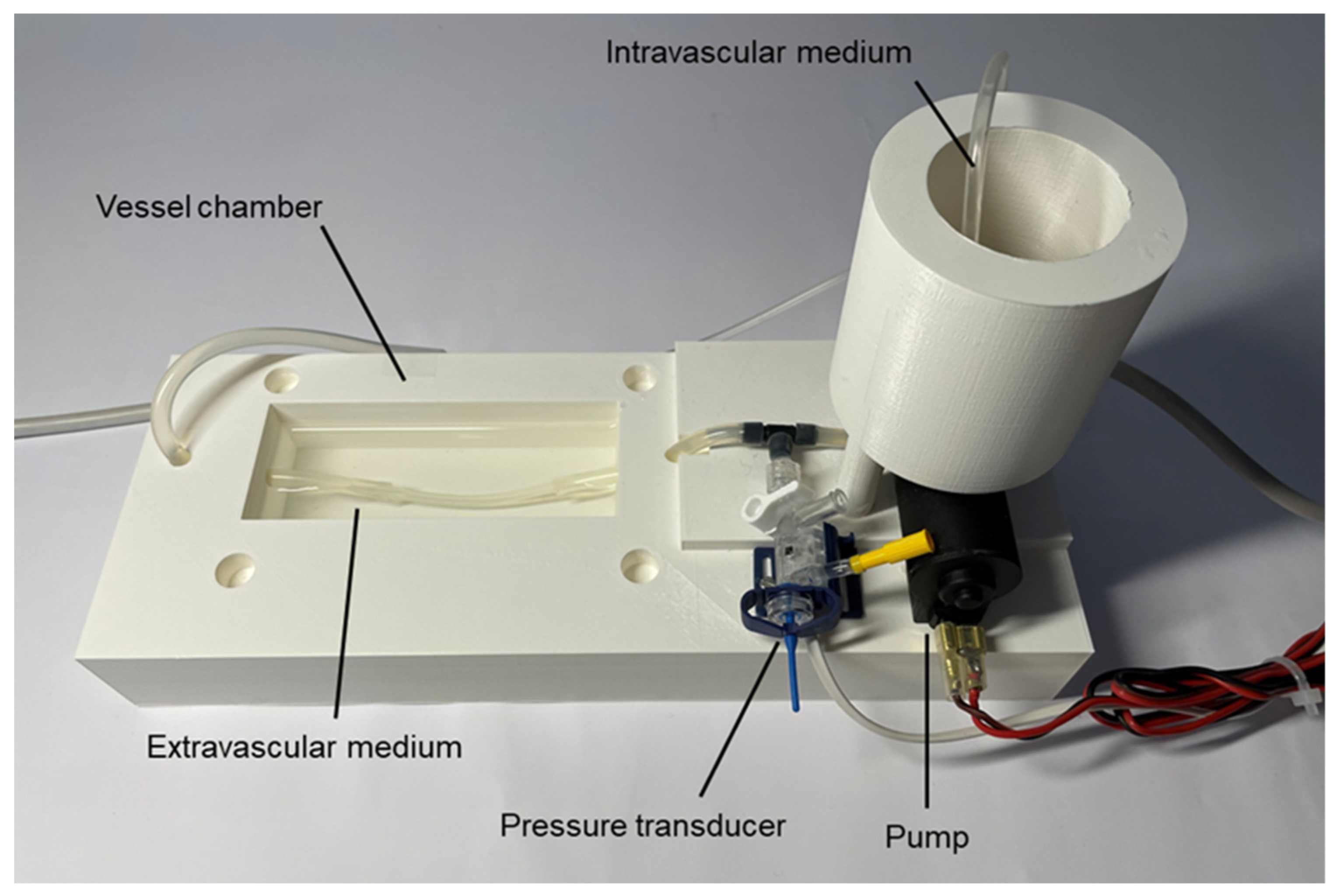
2. Materials and Methods
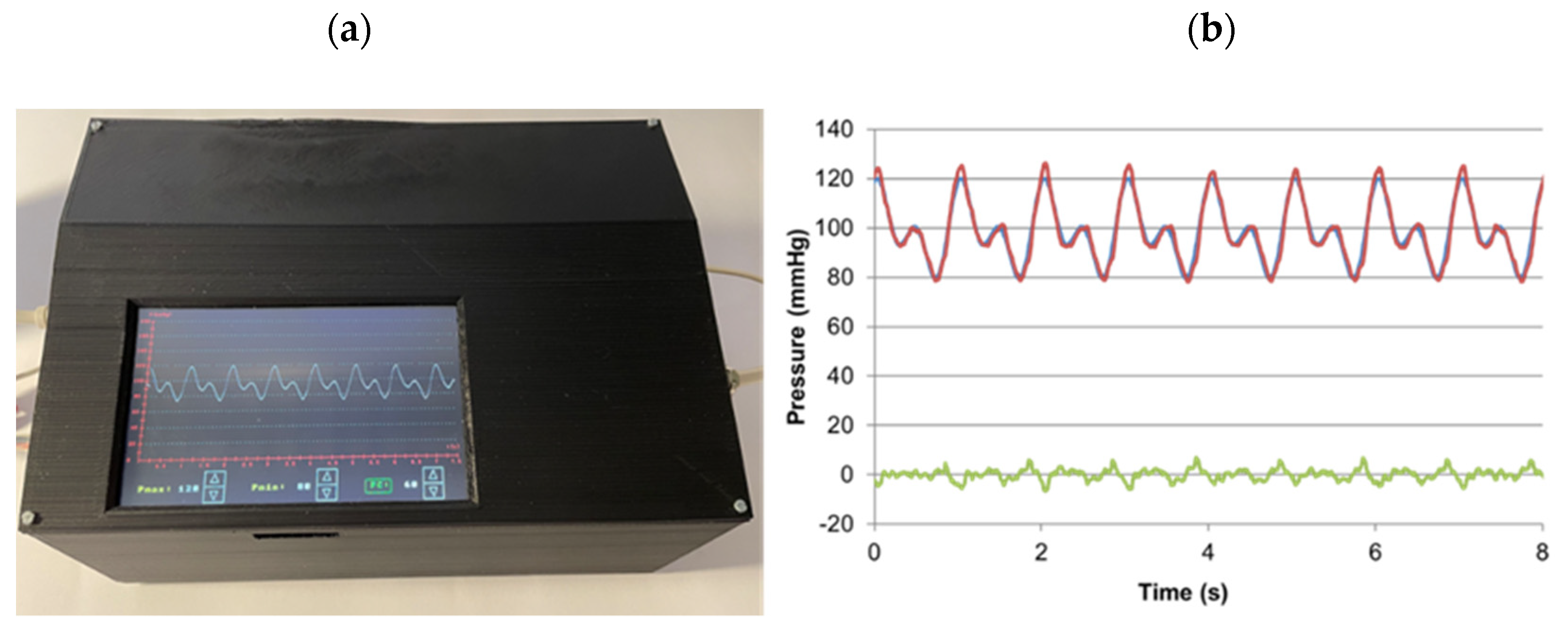
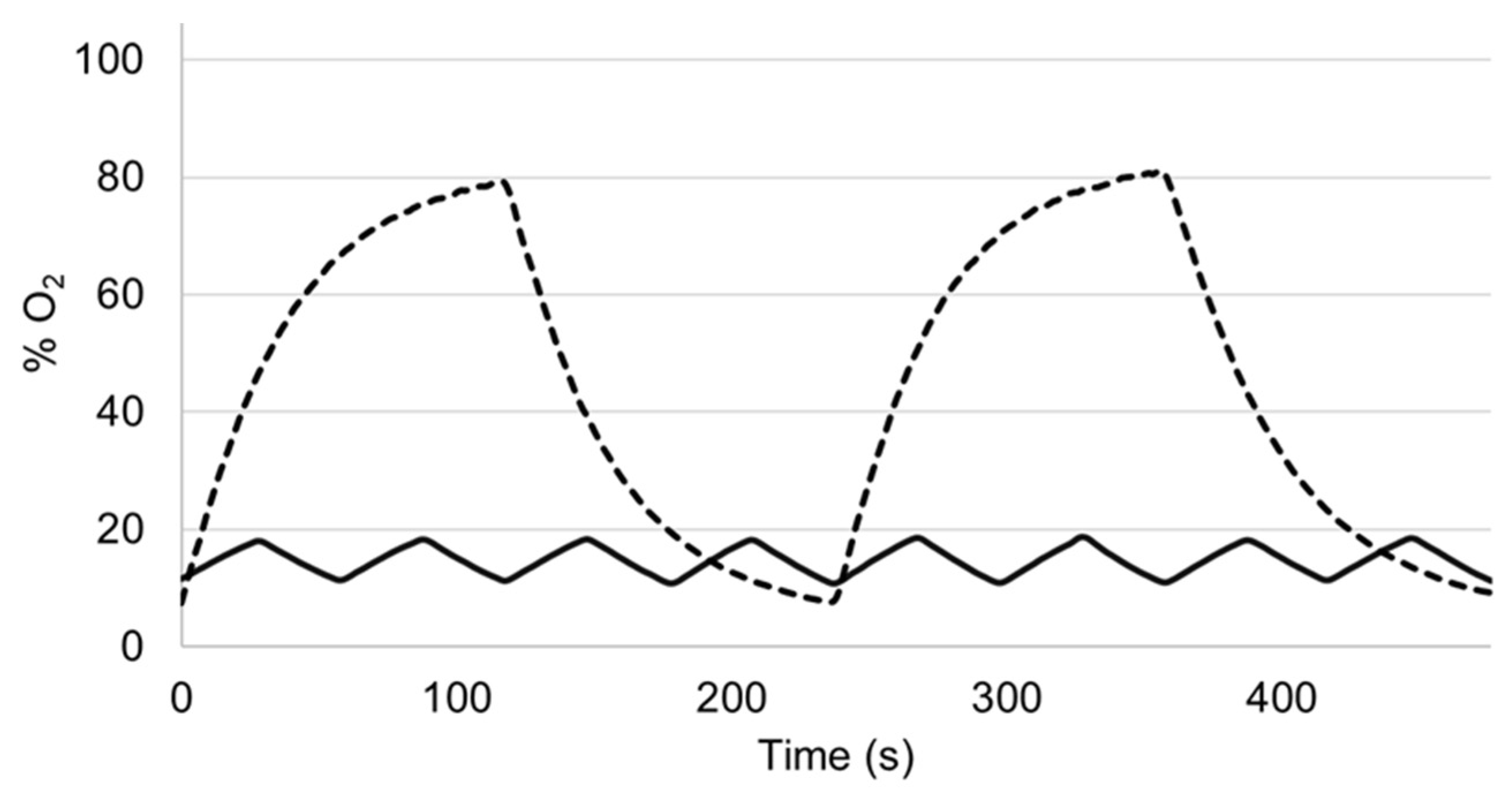
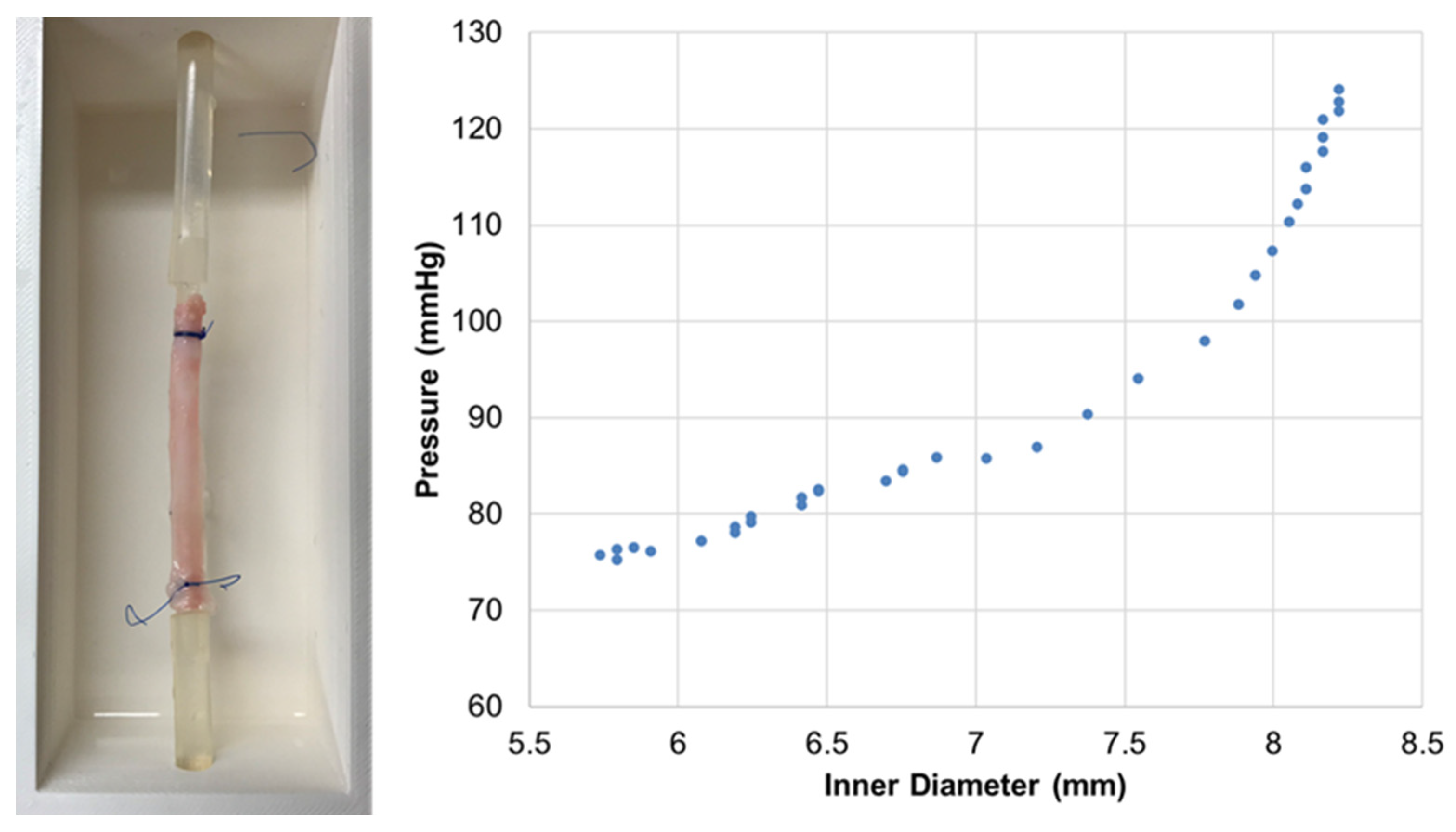
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Almendros, I.; Wang, Y.; Gozal, D. The polymorphic and contradictory aspects of intermittent hypoxia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2014, 307, L129–L140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-Plata, R.; Thirion-Romero, I.; Nava-Quiroz, K.J.; Pérez-Rubio, G.; Rodríguez-Llamazares, S.; Pérez-Kawabe, M.; Rodríguez-Reyes, Y.; Guerrero-Zuñiga, S.; Orea-Tejeda, A.; Falfán-Valencia, R.; et al. Clinical Markers of Chronic Hypoxemia in Respiratory Patients Residing at Moderate Altitude. Life 2021, 11, 428. [Google Scholar] [CrossRef]
- Shetty, S.; Parthasarathy, S. Obesity Hypoventilation Syndrome. Curr. Pulmonol. Rep. 2015, 4, 42–55. [Google Scholar] [CrossRef]
- Lacasse, Y.; Sériès, F.; Corbeil, F.; Baltzan, M.; Paradis, B.; Simão, P.; Abad Fernández, A.; Esteban, C.; Guimarães, M.; Bourbeau, J.; et al. Randomized Trial of Nocturnal Oxygen in Chronic Obstructive Pulmonary Disease. N. Engl. J. Med. 2020, 383, 1129–1138. [Google Scholar] [CrossRef] [PubMed]
- Allardet-Servent, J.; Sicard, G.; Metz, V.; Chiche, L. Benefits and risks of oxygen therapy during acute medical illness: Just a matter of dose! La Rev. Med. Interne 2019, 40, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Damiani, E.; Donati, A.; Girardis, M. Oxygen in the critically ill: Friend or foe? Curr. Opin. Anaesthesiol. 2018, 31, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Campillo, N.; Falcones, B.; Gozal, D.; Obeso, A.; Gallego-martin, T.; Navajas, X.D.; Almendros, I.; Farré, R. Frequency and magnitude of intermittent hypoxia modulate endothelial wound healing in a cell culture model of sleep apnea. J. Appl. Physiol. 2017, 123, 1047–1054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nonaka, P.N.; Falcones, B.; Farre, R.; Artigas, A.; Almendros, I.; Navajas, D. Biophysically Preconditioning Mesenchymal Stem Cells Improves Treatment of Ventilator-Induced Lung Injury. Arch. Bronconeumol. 2020, 56, 176–189. [Google Scholar] [CrossRef]
- Campillo, N.; Jorba, I.; Schaedel, L.; Casals, B.; Gozal, D.; Farré, R.; Almendros, I.; Navajas, D. A Novel Chip for Cyclic Stretch and Intermittent Hypoxia Cell Exposures Mimicking Obstructive Sleep Apnea. Front. Physiol. 2016, 7, 319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marhuenda, E.; Campillo, N.; Gabasa, M.; Martínez-García, M.A.; Campos-Rodríguez, F.; Gozal, D.; Navajas, D.; Alcaraz, J.; Farré, R.; Almendros, I. Effects of Sustained and Intermittent Hypoxia on Human Lung Cancer Cells. Am. J. Respir. Cell Mol. Biol. 2019, 61, 540–544. [Google Scholar] [CrossRef] [PubMed]
- Farré, R.; Almendros, I.; Montserrat, J.M.; Gozal, D.; Navajas, D. Gas Partial Pressure in Cultured Cells: Patho-Physiological Importance and Methodological Approaches. Front. Physiol. 2018, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Campillo, N.; Falcones, B.; Otero, J.; Colina, R.; Gozal, D.; Navajas, D.; Farré, R.; Almendros, I. Differential Oxygenation in Tumor Microenvironment Modulates Macrophage and Cancer Cell Crosstalk: Novel Experimental Setting and Proof of Concept. Front. Oncol. 2019, 9, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shoajei, S.; Tafazzoli-Shahdpour, M.; Shokrgozar, M.A.; Haghighipour, N. Alteration of human umbilical vein endothelial cell gene expression in different biomechanical environments. Cell Biol. Int. 2014, 38, 577–581. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.-X.; Han, Y.; Jiang, Z.-L. Mechanobiology and Vascular Remodeling: From Membrane to Nucleus. Adv. Exp. Med. Biol. 2018, 1097, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, P.; Rengarajan, T.; Thangavel, J.; Nishigaki, Y.; Sakthisekaran, D.; Sethi, G.; Nishigaki, I. The vascular endothelium and human diseases. Int. J. Biol. Sci. 2013, 9, 1057–1069. [Google Scholar] [CrossRef] [Green Version]
- Konukoglu, D.; Uzun, H. Endothelial Dysfunction and Hypertension. Adv. Exp. Med. Biol. 2017, 2, 511–540. [Google Scholar]
- Huertas, A.; Guignabert, C.; Barberà, J.A.; Bärtsch, P.; Bhattacharya, J.; Bhattacharya, S.; Bonsignore, M.R.; Dewachter, L.; Dinh-Xuan, A.T.; Dorfmüller, P.; et al. Pulmonary vascular endothelium: The orchestra conductor in respiratory diseases. Eur. Respir. J. 2018, 51. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, J.C. Through thick and thin: The interdependence of blood viscosity, shear stress and endothelial function. Exp. Physiol. 2020, 105, 232–233. [Google Scholar] [CrossRef] [PubMed]
- West, J.B. Causes of and compensations for hypoxemia and hypercapnia. Compr. Physiol. 2011, 1, 1541–1553. [Google Scholar] [CrossRef] [PubMed]
- Miñana, G.; Núñez, J.; Bañuls, P.; Sanchis, J.; Núñez, E.; Robles, R.; Mascarell, B.; Palau, P.; Chorro, F.J.; Llàcer, A. Prognostic implications of arterial blood gases in acute decompensated heart failure. Eur. J. Intern. Med. 2011, 22, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Jaffal, K.; Six, S.; Zerimech, F.; Nseir, S. Relationship between hyperoxemia and ventilator associated pneumonia. Ann. Transl. Med. 2017, 5, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Thomas, R.J.; Yee, B.J.; Grunstein, R.R. Hypercapnia is more important than hypoxia in the neuro-outcomes of sleep-disordered breathing. J. Appl. Physiol. 2016, 120, 1484–1486. [Google Scholar] [CrossRef] [Green Version]
- Bergh, N.; Ekman, M.; Ulfhammer, E.; Andersson, M.; Karlsson, L.; Jern, S. A new biomechanical perfusion system for ex vivo study of small biological intact vessels. Ann. Biomed. Eng. 2005, 33, 1808–1818. [Google Scholar] [CrossRef] [PubMed]
- Santelices, L.C.; Calano, S.J.; Erhart, J.C.; Prantil, R.L.; Haney, J.L.; Vorp, D.A.; Ahearn, J.M. Experimental system for ex vivo measurement of murine aortic stiffness. Physiol. Meas. 2007, 28, 39–49. [Google Scholar] [CrossRef]
- Pearce, J.M. Materials science. Building research equipment with free, open-source hardware. Science 2012, 337, 1303–1304. [Google Scholar] [CrossRef] [PubMed]
- Moritz, M.; Redlich, T.; Günyar, S.; Winter, L.; Wulfsberg, J.P. On the Economic Value of Open Source Hardware—Case Study of an Open Source Magnetic Resonance Imaging Scanner. J. Open Hardw. 2019, 3, 2. [Google Scholar] [CrossRef]
- Farré, R.; Trias, G.; Solana, G.; Ginovart, G.; Gozal, D.; Navajas, D. Novel Approach for Providing Pediatric Continuous Positive Airway Pressure Devices in Low-Income, Underresourced Regions. Am. J. Respir. Crit. Care Med. 2019, 199, 118–120. [Google Scholar] [CrossRef] [PubMed]
- Farré, R.; Montserrat, J.M.; Solana, G.; Gozal, D.; Navajas, D. Easy-to-build and affordable continuous positive airway pressure CPAP device for adult patients in low-income countries. Eur. Respir. J. 2019, 53. [Google Scholar] [CrossRef] [PubMed]
- Gallup, N.; Bow, J.K.; Pearce, J.M. Economic Potential for Distributed Manufacturing of Adaptive Aids for Arthritis Patients in the U.S. Geriatrics 2018, 3, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fortune, B.C.; Pretty, C.G.; Chatfield, L.T.; McKenzie, L.R.; Hayes, M.P. Low-cost active electromyography. HardwareX 2019, 6, e00085. [Google Scholar] [CrossRef]
- Forouzanfar, M.; Balasingam, B.; Dajani, H.R.; Groza, V.Z.; Bolic, M.; Rajan, S.; Petriu, E.M. Mathematical modeling and parameter estimation of blood pressure oscillometric waveform. In Proceedings of the 2012 IEEE International Symposium on Medical Measurements and Applications Proceedings, Budapest, Hungary, 18–19 May 2012; pp. 1–6. [Google Scholar]
- Bi, Y.; Chen, H.; Li, Y.; Yu, Z.; Han, X.; Ren, J. Rabbit aortic aneurysm model with enlarging diameter capable of better mimicking human aortic aneurysm disease. PLoS ONE 2018, 13, e0198818. [Google Scholar] [CrossRef] [PubMed]
- Ai, L.; Yu, H.; Dai, W.; Hale, S.L.; Kloner, R.A.; Hsiai, T.K. Real-time intravascular shear stress in the rabbit abdominal aorta. IEEE Trans. Biomed. Eng. 2009, 56, 1755–1764. [Google Scholar] [CrossRef] [Green Version]
- Peiffer, V.; Sherwin, S.J.; Weinberg, P.D. Computation in the rabbit aorta of a new metric-the transverse wall shear stress-to quantify the multidirectional character of disturbed blood flow. J. Biomech. 2013, 46, 2651–2658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serebrovska, T.V.; Grib, O.N.; Portnichenko, V.I.; Serebrovska, Z.O.; Egorov, E.; Shatylo, V.B. Intermittent Hypoxia/Hyperoxia Versus Intermittent Hypoxia/Normoxia: Comparative Study in Prediabetes. High Alt. Med. Biol. 2019, 20, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Logan, S.; Tobin, K.E.; Fallon, S.C.; Deng, K.S.; McDonough, A.B.; Bavis, R.W. Chronic intermittent hyperoxia alters the development of the hypoxic ventilatory response in neonatal rats. Respir. Physiol. Neurobiol. 2016, 220, 69–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bavis, R.W.; Millström, A.H.; Kim, S.M.; MacDonald, C.A.; O’Toole, C.A.; Asklof, K.; McDonough, A.B. Combined effects of intermittent hyperoxia and intermittent hypercapnic hypoxia on respiratory control in neonatal rats Ryan. Respir. Physiol. Neurobiol. 2019, 260, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Koniari, I.; Mavrilas, D.; Papadaki, H.; Karanikolas, M.; Mandellou, M.; Papalois, A.; Koletsis, E.; Dougenis, D.; Apostolakis, E. Structural and biomechanical alterations in rabbit thoracic aortas are associated with the progression of atherosclerosis. Lipids Health Dis. 2011, 10, 125. [Google Scholar] [CrossRef] [Green Version]
- Reusser, M.; Hunter, K.S.; Lammers, S.R.; Stenmark, K.R. Validation of a pressure diameter method for determining modulus and strain of collagen engagement for long branches of bovine pulmonary arteries. J. Biomech. Eng. 2012, 134, 3–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osuna, A.; Ulldemolins, A.; Sanz-Fraile, H.; Otero, J.; Farré, N.; Farré, R.; Almendros, I. Experimental Setting for Applying Mechanical Stimuli to Study the Endothelial Response of Ex Vivo Vessels under Realistic Pathophysiological Environments. Life 2021, 11, 671. https://doi.org/10.3390/life11070671
Osuna A, Ulldemolins A, Sanz-Fraile H, Otero J, Farré N, Farré R, Almendros I. Experimental Setting for Applying Mechanical Stimuli to Study the Endothelial Response of Ex Vivo Vessels under Realistic Pathophysiological Environments. Life. 2021; 11(7):671. https://doi.org/10.3390/life11070671
Chicago/Turabian StyleOsuna, Ana, Anna Ulldemolins, Hector Sanz-Fraile, Jorge Otero, Núria Farré, Ramon Farré, and Isaac Almendros. 2021. "Experimental Setting for Applying Mechanical Stimuli to Study the Endothelial Response of Ex Vivo Vessels under Realistic Pathophysiological Environments" Life 11, no. 7: 671. https://doi.org/10.3390/life11070671
APA StyleOsuna, A., Ulldemolins, A., Sanz-Fraile, H., Otero, J., Farré, N., Farré, R., & Almendros, I. (2021). Experimental Setting for Applying Mechanical Stimuli to Study the Endothelial Response of Ex Vivo Vessels under Realistic Pathophysiological Environments. Life, 11(7), 671. https://doi.org/10.3390/life11070671





