Fullerene C60 Protects Against Intestinal Injury from Deoxynivalenol Toxicity by Improving Antioxidant Capacity
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reactions
2.2. Cell Culture
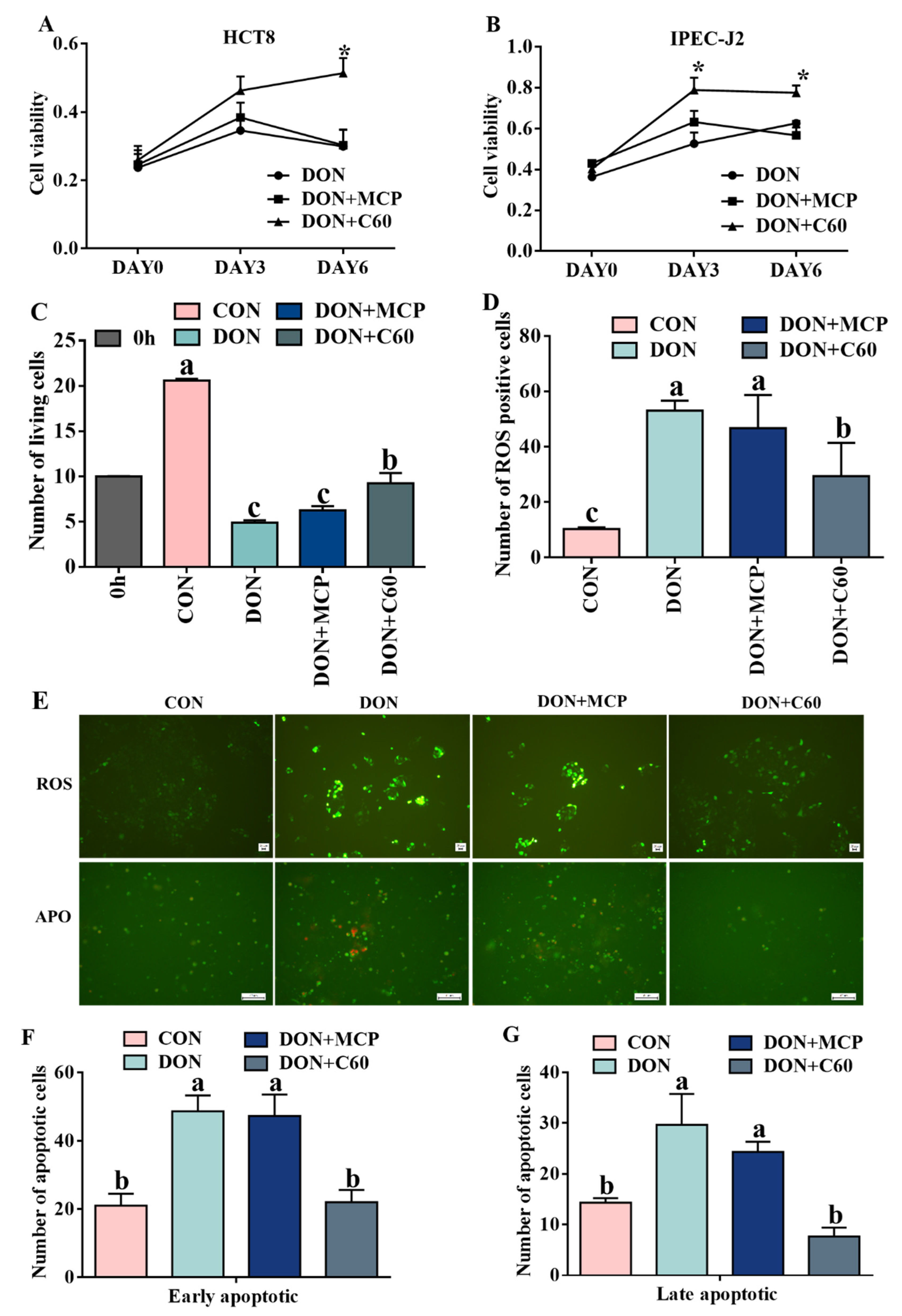
2.3. Cell Proliferation Measurement
2.4. ROS and Apoptosis Measurement
2.5. Transcriptome Sequencing (RNA-Seq)
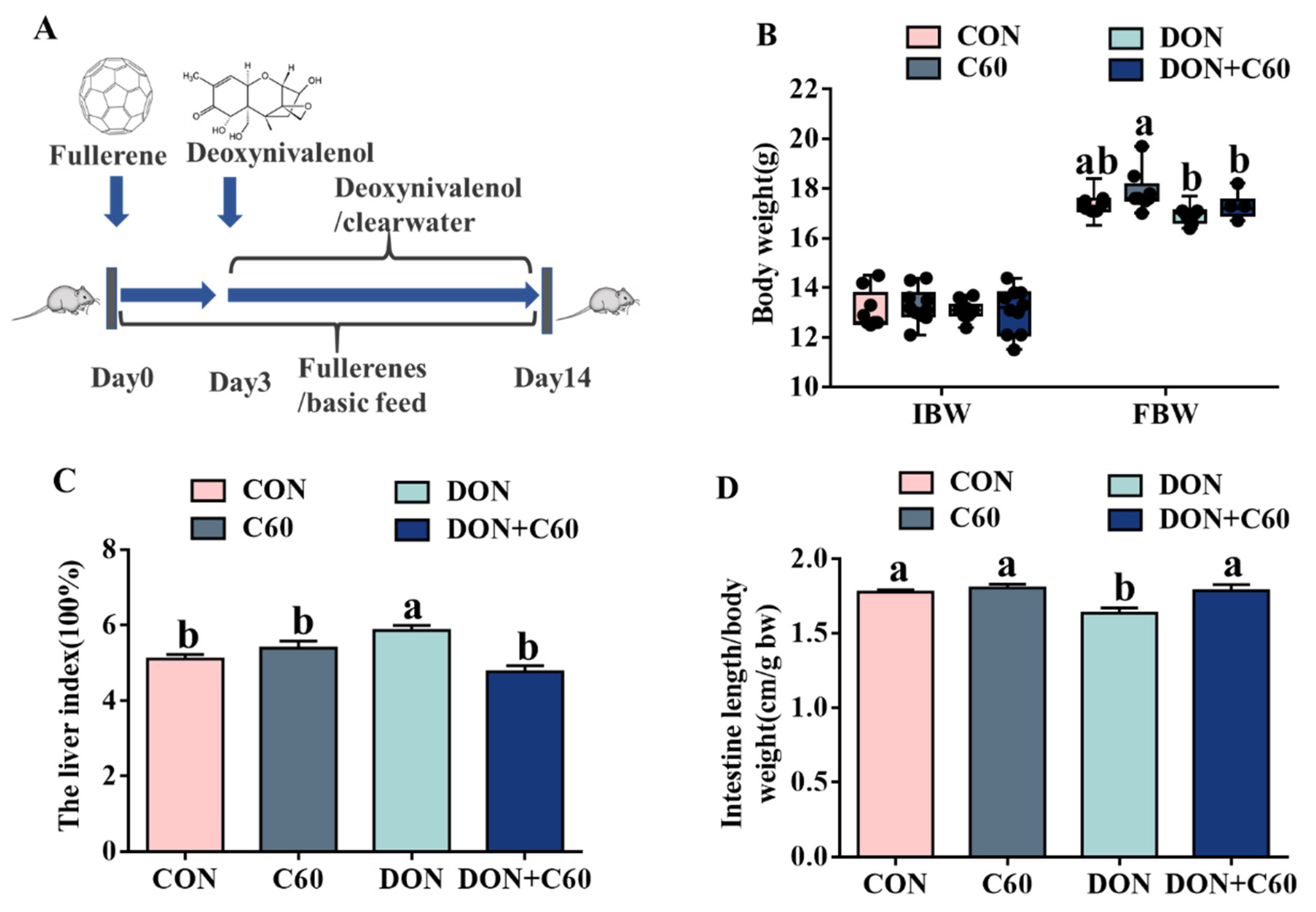
2.6. Animals and Experimental Design
2.7. Sample Collection and Preparation
2.8. Detection of Inflammatory Cytokines, Immune Factors, and LPS Marker
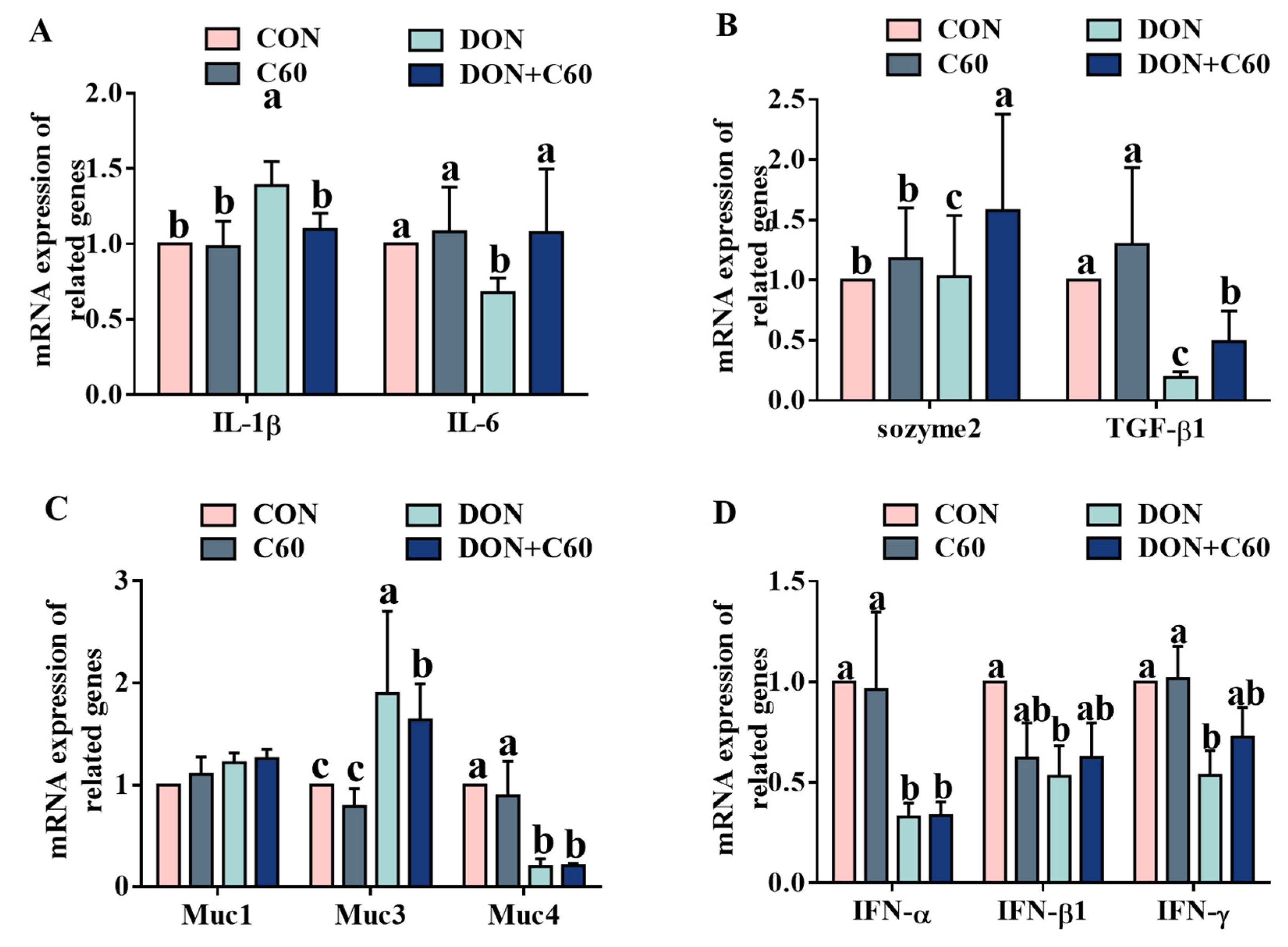
2.9. Real-Time Quantitative Reverse Transcriptase PCR
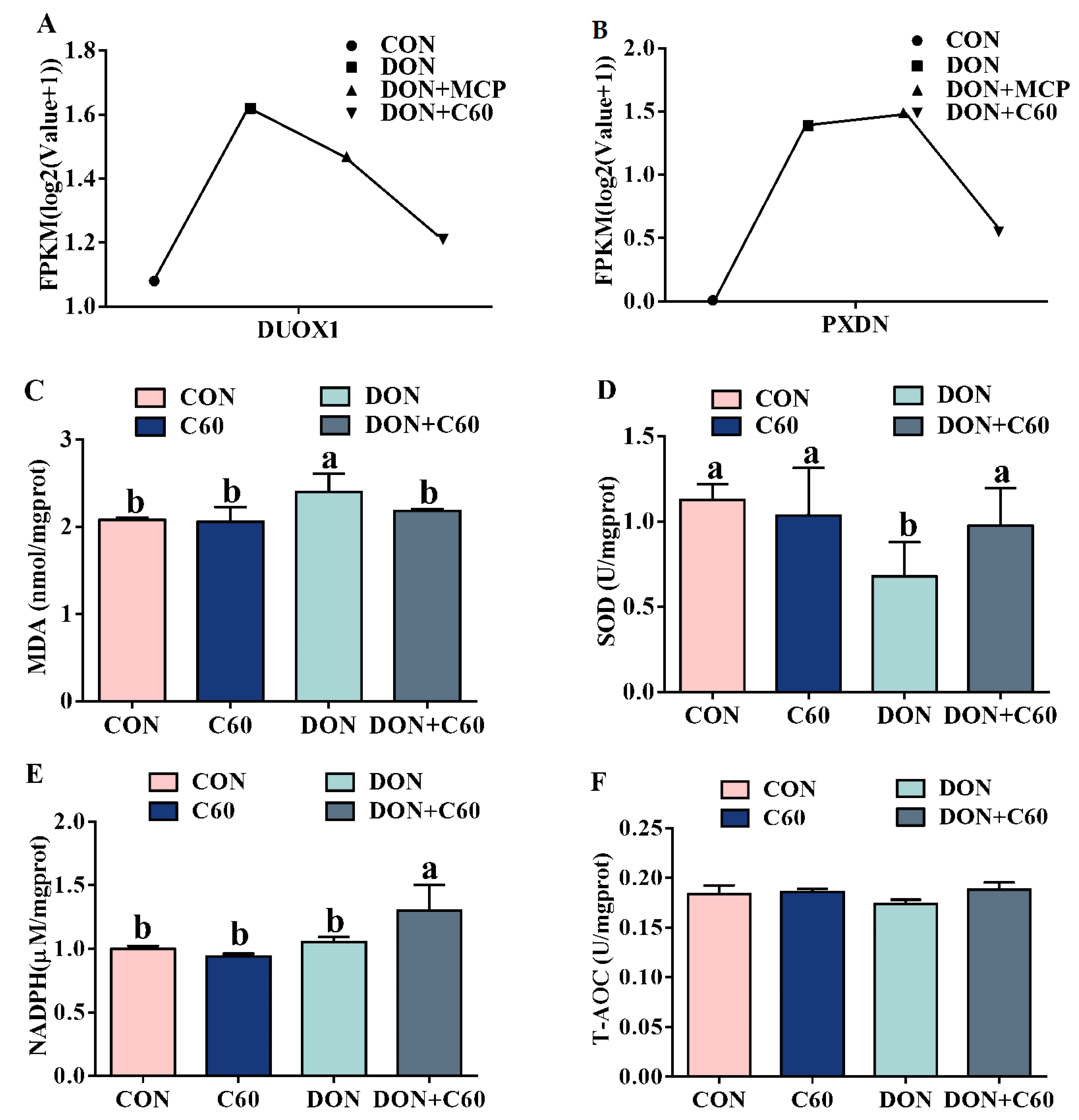
2.10. Tissues Antioxidative Capacity
2.11. Intestinal Morphology
2.12. Statistical Analysis
3. Results
3.1. Cell Proliferation, ROS Level, and Cell Apoptosis
3.2. Cell Antioxidant Related Genes and Antioxidant Enzymes
3.3. Growth Performance and Visceral Index
3.4. Serum Immunoglobulin and Inflammatory Factors
3.5. Expression of Inflammatory and Immune Genes
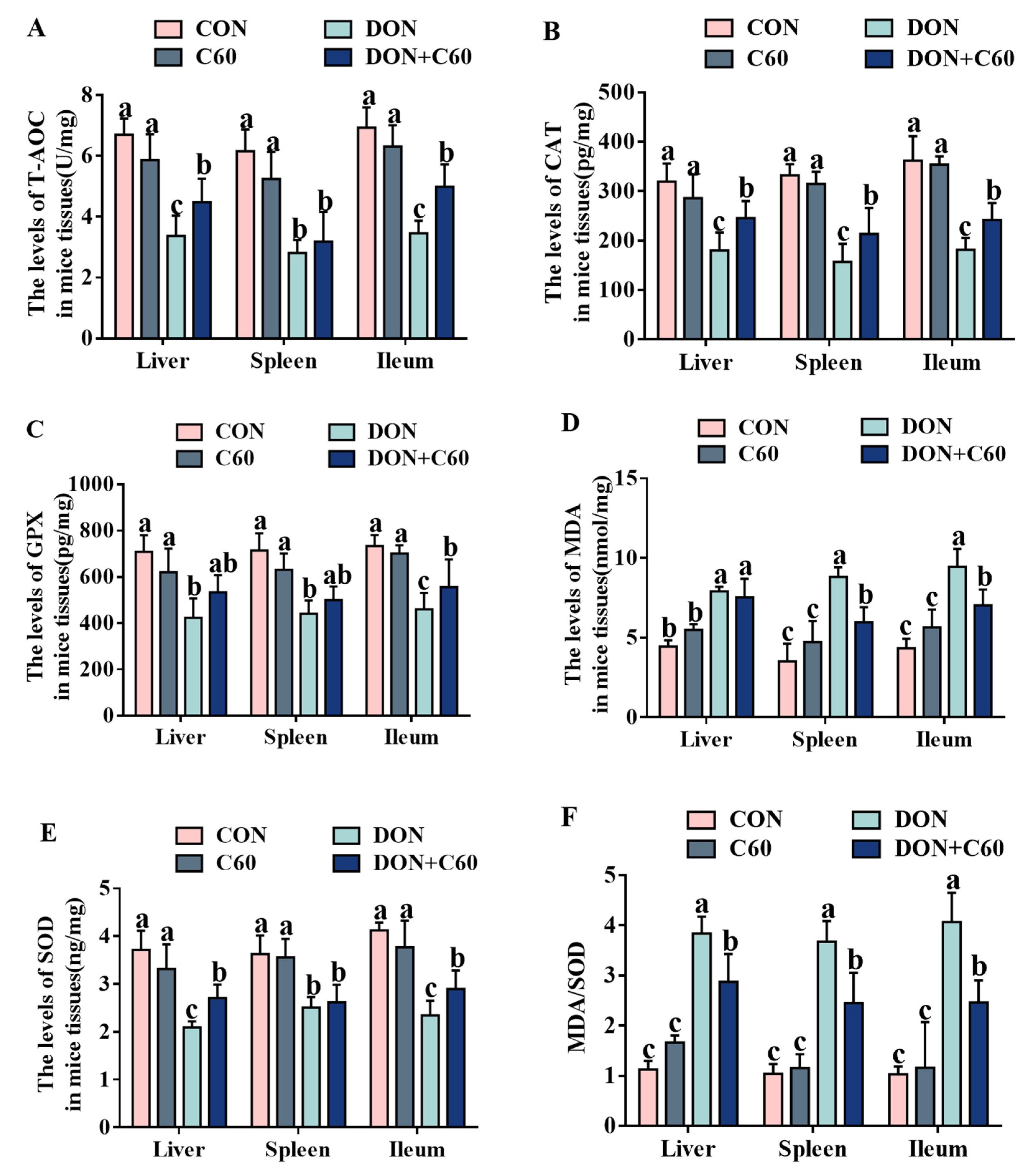
3.6. Antioxidant Index in Tissue
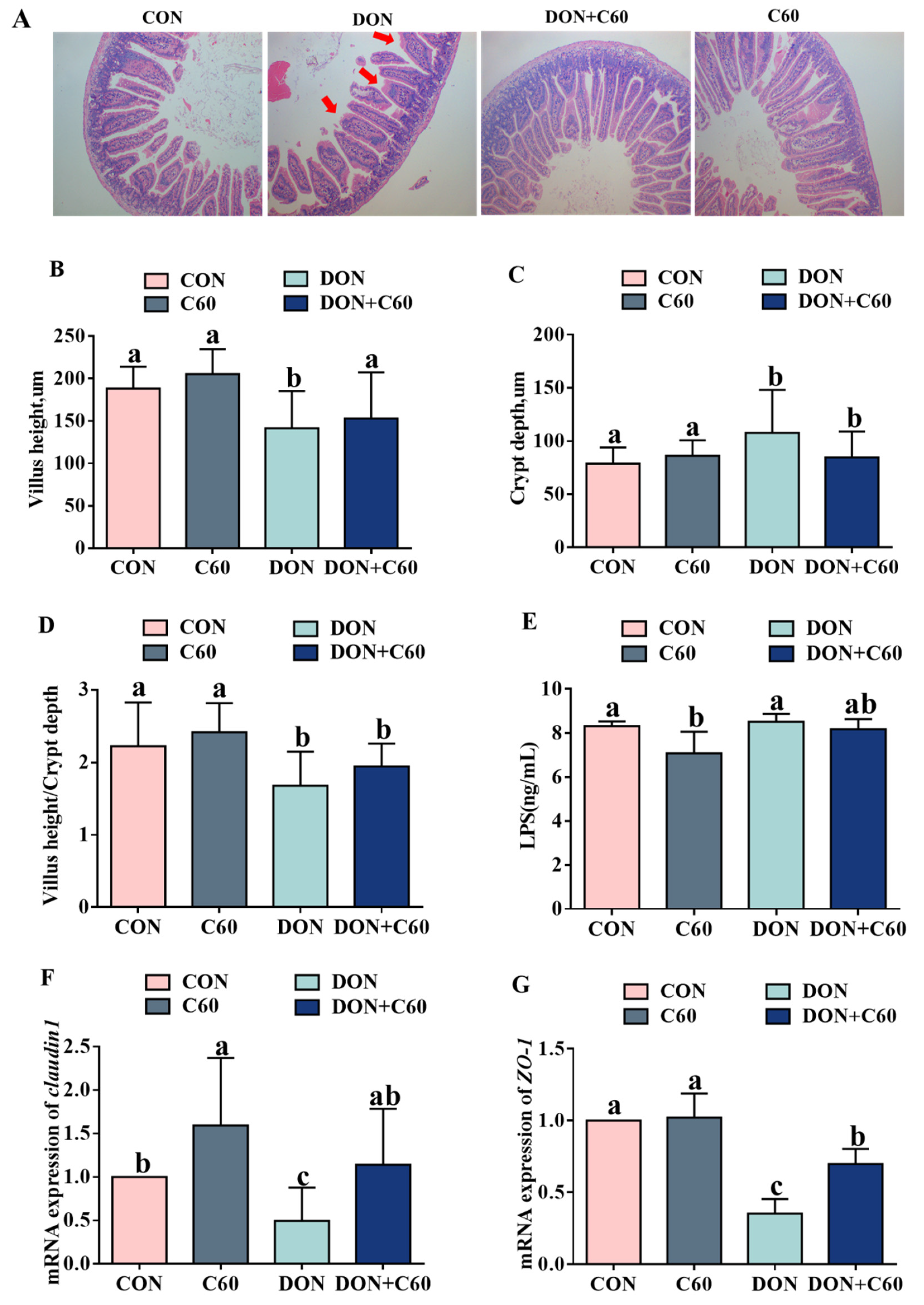
3.7. Intestinal Morphology and Permeability
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Gudkov, S.V.; Guryev, E.L.; Gapeyev, A.B.; Sharapov, M.G.; Bunkin, N.F.; Shkirin, A.V.; Zabelina, T.S.; Glinushkin, A.P.; Sevost’Yanov, M.A.; Belosludtsev, K.N.; et al. Unmodified hydrated C60 fullerene molecules exhibit antioxidant properties, prevent damage to DNA and proteins induced by reactive oxygen species and protect mice against injuries caused by radiation-induced oxidative stress. Nanomedicine 2019, 15, 37–46. [Google Scholar] [CrossRef]
- Rud, Y.; Buchatskyy, L.; Prylutskyy, Y.; Marchenko, O.; Senenko, A.; Schütze, C.; Ritter, U. Using C60 fullerenes for photodynamic inactivation of mosquito iridescent viruses. J. Enzym. Inhib. Med. Chem. 2011, 27, 614–617. [Google Scholar] [CrossRef][Green Version]
- Lim, C.S.; Porter, D.W.; Orandle, M.S.; Green, B.J.; Barnes, M.A.; Croston, T.L.; Wolfarth, M.G.; Battelli, L.A.; Andrew, M.E.; Beezhold, D.H.; et al. Resolution of Pulmonary Inflammation Induced by Carbon Nanotubes and Fullerenes in Mice: Role of Macrophage Polarization. Front. Immunol. 2020, 11, 1186. [Google Scholar] [CrossRef]
- Hsieh, F.-Y.; Zhilenkov, A.V.; Voronov, I.I.; Khakina, E.A.; Mischenko, D.V.; Troshin, P.A.; Hsu, S.-H. Water-Soluble Fullerene Derivatives as Brain Medicine: Surface Chemistry Determines If They Are Neuroprotective and Antitumor. ACS Appl. Mater. Interfaces 2017, 9, 11482–11492. [Google Scholar] [CrossRef] [PubMed]
- Demir, E.; Nedzvetsky, V.S.; Ağca, C.A.; Kirici, M. Pristine C60 Fullerene Nanoparticles Ameliorate Hyperglycemia-Induced Disturbances via Modulation of Apoptosis and Autophagy Flux. Neurochem. Res. 2020, 45, 2385–2397. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, H.; Hu, Y.; Zhang, Y.S.; Wen, L.; Yin, F.; Wang, Z.; Zhang, Y.; Li, S.; Miao, Y.; et al. Inhibition of CaMKIIα Activity Enhances Antitumor Effect of Fullerene C60 Nanocrystals by Suppression of Autophagic Degradation. Adv. Sci. 2019, 6, 1801233. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-C.; Chen, Y.-L.; Wang, C.-C.; Huang, J.-H.; Cho, E.-C. Refluxed Esterification of Fullerene-Conjugated P25 TiO2 Promotes Free Radical Scavenging Capacity and Facilitates Antiaging Potentials in Human Cells. ACS Appl. Mater. Interfaces 2018, 11, 311–319. [Google Scholar] [CrossRef]
- Chen, L.; Miao, Y.; Chen, L.; Xu, J.; Wang, X.; Zhao, H.; Shen, Y.; Hu, Y.; Bian, Y.; Shen, Y.; et al. The role of low levels of fullerene C60 nanocrystals on enhanced learning and memory of rats through persistent CaMKII activation. Biomaterials 2014, 35, 9269–9279. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yang, W.; Man, N.; Zheng, F.; Shen, Y.; Sun, K.; Li, Y.; Wen, L.-P. Autophagy-mediated chemosensitization in cancer cells by fullerene C60 nanocrystal. Autophagy 2009, 5, 1107–1117. [Google Scholar] [CrossRef] [PubMed]
- Sforzini, S.; Oliveri, C.; Barranger, A.; Jha, A.N.; Banni, M.; Moore, M.N.; Viarengo, A. Effects of fullerene C60 in blue mussels: Role of mTOR in autophagy related cellular/tissue alterations. Chemosphere 2020, 246, 125707. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxidative Med. Cell. Longev. 2017. [Google Scholar] [CrossRef] [PubMed]
- De, R.; Mazumder, S.; Bandyopadhyay, U. Mediators of mitophagy that regulate mitochondrial quality control play crucial role in diverse pathophysiology. Cell Biol. Toxicol. 2020, 1–34. [Google Scholar] [CrossRef]
- Pei, Y.; Cui, F.; Du, X.; Shang, G.; Xiao, W.; Yang, X.; Cui, Q. Antioxidative nanofullerol inhibits macrophage activation and development of osteoarthritis in rats. Int. J. Nanomed. 2019, ume 14, 4145–4155. [Google Scholar] [CrossRef]
- Chistyakov, V.A.; Smirnova, Y.O.; Prazdnova, E.V.; Soldatov, A.V. Possible Mechanisms of Fullerene C60Antioxidant Action. BioMed Res. Int. 2013, 2013, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Zhao, J.; Meng, H.; Hu, D.; Zhou, Y.; Zhang, X.; Wang, C.; Li, J.; Yuan, J.; Wei, Y. Carnosine-Modified Fullerene as a Highly Enhanced ROS Scavenger for Mitigating Acute Oxidative Stress. ACS Appl. Mater. Interfaces 2020, 12, 16104–16113. [Google Scholar] [CrossRef] [PubMed]
- Halenova, T.I.; Vareniuk, I.M.; Roslova, N.M.; Dzerzhynsky, M.E.; Savchuk, O.; Ostapchenko, L.; Prylutskyy, Y.I.; Ritter, U.; Scharff, P. Hepatoprotective effect of orally applied water-soluble pristine C60 fullerene against CCl4-induced acute liver injury in rats. RSC Adv. 2016, 6, 100046–100055. [Google Scholar] [CrossRef]
- Tang, Y.; Liao, S.; Liu, G.; Xiong, X.; Liu, H.; Li, F.; Tan, Z.; Kong, X.; Yin, Y.; Tan, B. Advanced single-cell pooled CRISPR screening identifies C19orf53 required for cell proliferation based on mTORC1 regulators. Cell Biol. Toxicol. 2021, 1–26. [Google Scholar] [CrossRef]
- Juan-García, A.; Carbone, S.; Ben-Mahmoud, M.; Sagratini, G.; Mañes, J. Beauvericin and ochratoxin A mycotoxins individually and combined in HepG2 cells alter lipid peroxidation, levels of reactive oxygen species and glutathione. Food Chem. Toxicol. 2020, 139, 111247. [Google Scholar] [CrossRef]
- Wu, Q.-H.; Wang, X.; Yang, W.; Nüssler, A.K.; Xiong, L.-Y.; Kuča, K.; Dohnal, V.; Zhang, X.-J.; Yuan, Z.-H. Oxidative stress-mediated cytotoxicity and metabolism of T-2 toxin and deoxynivalenol in animals and humans: An update. Arch. Toxicol. 2014, 88, 1309–1326. [Google Scholar] [CrossRef]
- Zhang, B.; Dai, Y.; Zhu, L.; He, X.; Huang, K.; Xu, W. Single-cell sequencing reveals novel mechanisms of Aflatoxin B1-induced hepatotoxicity in S phase-arrested L02 cells. Cell Biol. Toxicol. 2020, 36, 603–608. [Google Scholar] [CrossRef]
- Rizzo, A.F.; Atroshi, F.; Ahotupa, M.; Sankari, S.; Elovaara, E. Protective Effect of Antioxidants against Free Radical-Mediated Lipid Peroxidation Induced by DON or T-2 Toxin. J. Veter- Med. Ser. A 1994, 41, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, J.; Fang, H.; Yu, H.; Zhao, Y.; Shen, J.; Zhou, C.; Jin, Y. Pterostilbene inhibits deoxynivalenol-induced oxidative stress and inflammatory response in bovine mammary epithelial cells. Toxicon 2021, 189, 10–18. [Google Scholar] [CrossRef]
- Xiao, H.; Tan, B.E.; Wu, M.M.; Yin, Y.L.; Li, T.J.; Yuan, D.X.; Li, L. Effects of composite antimicrobial peptides in weanling piglets challenged with deoxynivalenol: II. Intestinal morphology and function1. J. Anim. Sci. 2013, 91, 4750–4756. [Google Scholar] [CrossRef] [PubMed]
- Holanda, D.M.; Kim, S.W. Efficacy of Mycotoxin Detoxifiers on Health and Growth of Newly-Weaned Pigs under Chronic Dietary Challenge of Deoxynivalenol. Toxins 2020, 12, 311. [Google Scholar] [CrossRef]
- Mortazavi, A.; Williams, B.A.; McCue, K.; Schaeffer, L.; Wold, B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 2008, 5, 621–628. [Google Scholar] [CrossRef]
- Ruan, Z.; Yang, Y.; Zhou, Y.; Wen, Y.; Ding, S.; Liu, G.; Wu, X.; Liao, P.; Deng, Z.; Assaad, H.; et al. Metabolomic analysis of amino acid and energy metabolism in rats supplemented with chlorogenic acid. Amino Acids 2014, 46, 2219–2229. [Google Scholar] [CrossRef]
- Liao, S.; Tang, S.; Chang, M.; Qi, M.; Li, J.; Tan, B.; Gao, Q.; Zhang, S.; Li, X.; Yin, Y.; et al. Chloroquine Downregulation of Intestinal Autophagy to Alleviate Biological Stress in Early-Weaned Piglets. Anim. 2020, 10, 290. [Google Scholar] [CrossRef] [PubMed]
- Sahu, S.C.; O’Donnell, M.W.; Wiesenfeld, P.L. Comparative hepatotoxicity of deoxynivalenol in rat, mouse and human liver cells in culture. J. Appl. Toxicol. 2010, 30, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Tang, S.; Tan, B.; Li, J.; Qi, M.; Cui, Z.; Zha, A.; Wang, Y.; Yin, Y.; Sun, P.; et al. Chloroquine Improves Deoxynivalenol-Induced Inflammatory Response and Intestinal Mucosal Damage in Piglets. Oxidative Med. Cell. Longev. 2020, 2020, 1–13. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Zhao, J.; Cao, L.; Zhu, L.; Huang, Y.; Chen, X.; Rahman, S.U.; Feng, S.; Li, Y.; et al. Deoxynivalenol Induces Inflammatory Injury in IPEC-J2 Cells via NF-κB Signaling Pathway. Toxins 2019, 11, 733. [Google Scholar] [CrossRef] [PubMed]
- Payros, D.; Ménard, S.; Laffitte, J.; Neves, M.; Tremblay-Franco, M.; Luo, S.; Fouche, E.; Snini, S.P.; Theodorou, V.; Pinton, P.; et al. The food contaminant, deoxynivalenol, modulates the Thelper/Treg balance and increases inflammatory bowel diseases. Arch. Toxicol. 2020, 94, 3173–3184. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Long, M. The biological detoxification of deoxynivalenol: A review. Food Chem. Toxicol. 2020, 145, 111649. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Li, J.; Li, F.; Hu, C.-A.A.; Liao, P.; Tan, K.; Tan, B.; Xiong, X.; Liu, G.; Li, T.; et al. Autophagy protects intestinal epithelial Cells against Deoxynivalenol toxicity by alleviating oxidative stress via IKK signaling pathway. Free Radic. Biol. Med. 2015, 89, 944–951. [Google Scholar] [CrossRef] [PubMed]

| Genes | Accession No. | Primers | Sequences (5′-3′) |
|---|---|---|---|
| β-actin | NM_007393.3 | Forward | GTCCACCTTCCAGCAGATGT |
| Reverse | GAAAGGGTGTAAAACGCAGC | ||
| A-chain | XM_003085465.1 | Forward | CGTCCAAGAATTGGATATGA |
| Reverse | AGTGACAGGCTGGGATGG | ||
| J-chain | NM_152839.3 | Forward | GAACTTTGTATACCATTTGTCAGACG |
| Reverse | CTGGGTGGCAGTAACAACCT | ||
| IL-1β | NM_008361.3 | Forward | ATGAAAGACGGCACACCCAC |
| Reverse | GCTTGTGCTCTGCTTGTGAG | ||
| IL-6 | NM_001314054.1 | Forward | GCCTTCTTGGGACTGATGCT |
| Reverse | TGTGACTCCAGCTTATCTCTTGG | ||
| lysozyme 2 | NM_017372.3 | Forward | GAATGGAATGGCTGGCTACT |
| Reverse | CGTGCTGAGCTAAACACACC | ||
| TGF-β1 | NM_011577.2 | Forward | ACTGGAGTTGTACGGCAGTG |
| Reverse | GGATCCACTTCCAACCCAGG | ||
| Muc1 | NM_013605.2 | Forward | GTAGGAGCAAGTCACCCCAC |
| Reverse | GTTGAGGCGCTTGACAAAGG | ||
| Muc3 | NM_010843.1 | Forward | GGCTTTCATCCTCCACTCCC |
| Reverse | TTGTGGTGGATGGGGAACTG | ||
| Muc4 | NM_080457.3 | Forward | TGTCATTCCACACTCCCAGA |
| Reverse | CTAGTCCTACTCTTTGCCCT | ||
| Crs1c | NM_007844.2 | Forward | AAGCGGCAACCAAATCTATG |
| Reverse | CCCGAGAGAGCAATACAGGT | ||
| IL-17 | NM_010552.3 | Forward | TACCTCAACCGTTCCACGTC |
| Reverse | TTTCCCTCCGCATTGACAC | ||
| IFN-α | NM_010502.2 | Forward | TGCCCAGCAGATCAAGAAGG |
| Reverse | TCAGGGGAAATTCCTGCACC | ||
| IFN-β1 | NM_010510.1 | Forward | CGTGGGAGATGTCCTCAACT |
| Reverse | AGATCTCTGCTCGGACCACC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, S.; Liu, G.; Tan, B.; Qi, M.; Li, J.; Li, X.; Zhu, C.; Huang, J.; Yin, Y.; Tang, Y. Fullerene C60 Protects Against Intestinal Injury from Deoxynivalenol Toxicity by Improving Antioxidant Capacity. Life 2021, 11, 491. https://doi.org/10.3390/life11060491
Liao S, Liu G, Tan B, Qi M, Li J, Li X, Zhu C, Huang J, Yin Y, Tang Y. Fullerene C60 Protects Against Intestinal Injury from Deoxynivalenol Toxicity by Improving Antioxidant Capacity. Life. 2021; 11(6):491. https://doi.org/10.3390/life11060491
Chicago/Turabian StyleLiao, Simeng, Guang Liu, Bie Tan, Ming Qi, Jianjun Li, Xiaoqing Li, Changfeng Zhu, Jiamei Huang, Yulong Yin, and Yulong Tang. 2021. "Fullerene C60 Protects Against Intestinal Injury from Deoxynivalenol Toxicity by Improving Antioxidant Capacity" Life 11, no. 6: 491. https://doi.org/10.3390/life11060491
APA StyleLiao, S., Liu, G., Tan, B., Qi, M., Li, J., Li, X., Zhu, C., Huang, J., Yin, Y., & Tang, Y. (2021). Fullerene C60 Protects Against Intestinal Injury from Deoxynivalenol Toxicity by Improving Antioxidant Capacity. Life, 11(6), 491. https://doi.org/10.3390/life11060491







